Integrins play a crucial role in the development and maintenance of the inflammatory process in patients with inflammatory bowel disease. Vedolizumab is a humanized monoclonal antibody with a predominantly gastrointestinal effect. It specifically inhibits leucocyte integrin α4β7, thus preventing its interaction with mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), which is involved in the migration of lymphocytes from the blood stream to the intestinal tissue. Vedolizumab is indicated in the treatment of moderate to severe active Crohn's disease and ulcerative colitis in adult patients with poor response, loss of response, or intolerance to conventional treatment or to tumour necrosis factor alpha (TNF-α) antagonists. This review presents the most relevant clinical outcomes of vedolizumab in the treatment of patients with ulcerative colitis.
Las integrinas desempeñan un papel clave en el desarrollo y mantenimiento del proceso inflamatorio en pacientes con enfermedad inflamatoria intestinal. Vedolizumab es un anticuerpo monoclonal humanizado con efecto predominante a nivel intestinal. Su mecanismo de acción consiste en una inhibición específica de la integrina α4ß7 de los leucocitos, lo que impide su interacción con la molécula de adhesión celular adresina de la mucosa 1 (MAdCAM-1) que participa en la migración de los linfocitos desde el torrente sanguíneo al tejido intestinal. Vedolizumab está indicado para el tratamiento de la enfermedad de Crohn y la colitis ulcerosa activas, de moderadas a graves, en pacientes adultos que hayan tenido una respuesta inadecuada, presenten pérdida de respuesta o sean intolerantes al tratamiento convencional o a un antagonista del factor de necrosis tumoral α (TNF-α). En esta revisión se presentan los resultados clínicos más importantes de vedolizumab en el tratamiento de pacientes con colitis ulcerosa.
Ulcerative colitis (UC) is a chronic idiopathic inflammatory disease of the colonic mucosa. It almost invariably affects the rectal mucosa, extending proximally in an uninterrupted pattern, and can affect the entire colonic mucosa. UC is classified phenotypically by the extent of colon involvement, from proctitis to extensive colitis.1
Both UC and Crohn disease (CD) develop from an inappropriate immune response in genetically susceptible subjects, as a result of complex interactions between environmental and microbial factors and the intestinal immune system.2 The pathophysiological mechanisms proposed in UC include epithelial barrier dysfunction, rupture of the homeostatic equilibrium between the immunity of the host mucosa and the microbiota, activation of the toll-like receptors of the dendritic cells, dysregulated immune responses at the level of the mucosa and lamina propria of the inflamed colon (with participation of T-helper and NK cells, interleukins 5, 10 and 13, Th2 cell-related cytokines and tumour necrosis factor a [TNF-α], among others), leucocyte recruitment and trafficking and, finally, various genetic factors. Better understanding of the mechanisms that induce and perpetuate inflammation of the colonic mucosa have identified new therapeutic targets and led to the development of new drugs with different mechanisms of action.3
Leucocyte trafficking and integrin antagonistsLymphocytes play a key role in the pathogenesis of UC. Integrin inhibitors are a group of drugs whose therapeutic target is to block leucocyte adhesion and trafficking systems and thereby reduce inflammation.4 The ability to alter the intrinsic mechanisms of adhesion and transmigration of the T-lymphocytes across the endothelial cells of the inflamed intestine may help resolve the existing inflammation and facilitate long-term control of UC.5,6
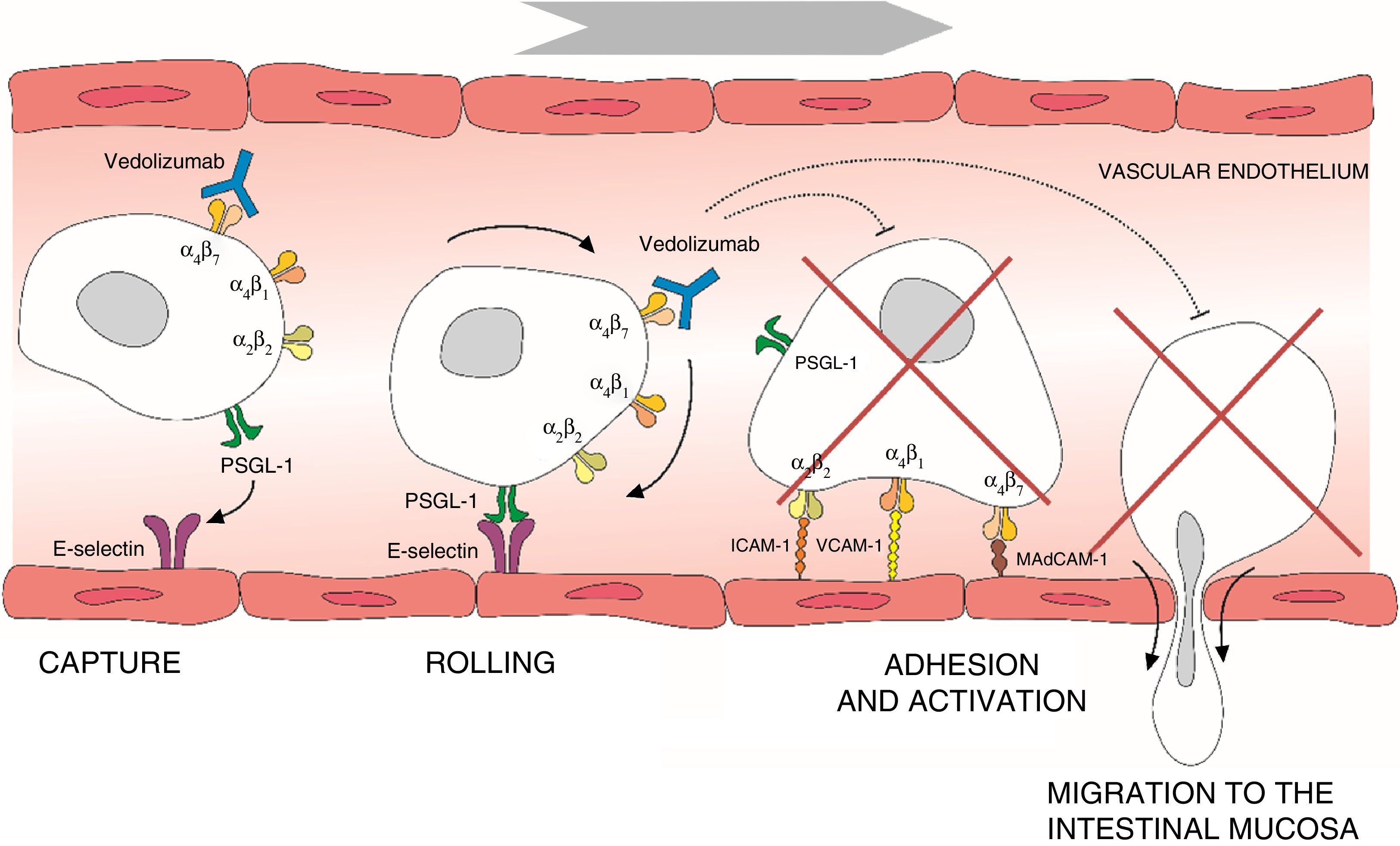
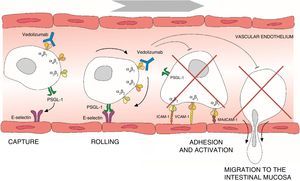
Leucocyte migration from the bloodstream across the intestinal endothelium occurs through a series of mechanisms based on adhesion systems involving integrins α4β1 and α4β7 as well vascular cell adhesion molecule 1 (VCAM-1) and mucosal addressin cellular adhesion molecule 1 (MAdCAM-1).6–8 Integrin α4β7 is expressed in a specific subtype of memory T helper cells that mainly migrate to the gastrointestinal (GI) tract and cause the inflammation characteristic of UC.9 Once transendothelial leucocyte migration has taken place, the inflammatory process spreads as a result of local proliferation and activation of lymphocytes and reduction of lymphocyte output from the mucosa. Adhesion molecules also participate in the interaction between T cells and the dendritic or mesenchymal cells of the intestinal mucosa and submucosa, facilitating T cell activation.10 The α4 integrins also play an important role in the inflammatory process by interacting with various tissue ligands which, in turn, induce co-stimulatory signals that lead to T lymphocyte proliferation and adhesion, cytokine production and increased T cell expression of the matrix metalloproteinases that degrade the extracellular matrix and facilitate cell migration.11 Thus, interruption of adhesion molecule interactions may be effective in decreasing T cell recruitment, inhibiting local co-stimulation signals, or a combination of both (Fig. 1).
Process of lymphocyte migration from the blood vessels to the intestinal mucosa and mechanism of action of vedolizumab. ICAM-1, intercellular adhesion molecule 1; MAdCAM-1, mucosal vascular addressin cell adhesion molecule 1; PSGL-1, P-selectin glycoprotein ligand 1; VCAM-1, vascular cell adhesion molecule 1.
Treatment of UC essentially depends on the severity of the flares and response to corticosteroids. In fact, half of patients with UC will never require corticosteroid treatment and, therefore, will not require any other drugs apart from aminosalicylates.12 Among patients who require oral or intravenous corticosteroids, half will require immunosuppressive treatments, either for corticosteroid refractory- or dependent-disease.13 Vedolizumab, like infliximab, adalimumab and golimumab (the 3 anti-TNF-α drugs approved in Spain for the treatment of UC), is approved for the treatment of patients with active UC when conventional treatment (understood as treatment with corticosteroids or thiopurine immunosuppressants) has failed. Apart from this indication, vedolizumab could be a very interesting therapeutic alternative in primary failure or in secondary loss of response to anti-TNF-α treatment.
Vedolizumab is a recombinant humanised monoclonal IgG1 antibody that acts as a selective inhibitor of the α4β7 heterodimer, thus inhibiting adhesion and migration of leukocytes towards the GI tract.14 The drug is an integrin α4β7 antagonist with no identified immunosuppressant activity. By binding to α4β7 on certain T lymphocytes, vedolizumab inhibits adhesion of these cells to MAdCAM-1, but not to VCAM-1.15 The adhesion molecule MAdCAM-1 is expressed in the intestine and in gut-associated tissues (such as the pancreas) and lymphoid tissue (such as Peyer's patches, or intestinal lamina propria).16 It is not generally detected in most extra-intestinal tissues (normal or inflamed), including those with mucosal surfaces, or in the endothelium of the veins of the lung, liver, kidney, heart, salivary glands, uterus, ovary, skeletal muscle, skin or brain parenchyma.16 However, MAdCAM-1 has been detected in liver tissue after prolonged inflammatory bowel disease-associated inflammation.17 Adhesion molecule MAdCAM-1 expressed in the endothelial cells of the intestine plays a critical role in T lymphocyte migration to the tissues of the intestinal tract. Unlike natalizumab, vedolizumab does not bind to or inhibit the function of integrins α4β1 and αEβ7, and because it does not induce alterations in systemic immunosuppression, it does not affect the immune surveillance mechanisms of the central nervous system (CNS). Therefore, although natalizumab treatment is associated with an increased risk of progressive multifocal leukoencephalopathy (PML), a progressive demyelinating disease of the CNS caused by an opportunistic human polyomavirus (the John Cunningham or JC virus), during the clinical development of vedolizumab, which included approximately 3000 patients (mean exposure 19 months), no cases of PML were reported in patients treated with vedolizumab, despite the fact that more than 80% of patients had received immunosuppressive treatment prior to inclusion in the study.18–20
In May 2014, the United States Food and Drug Administration and the European Medicines Agency gave marketing approval for vedolizumab (Entyvio®, Takeda Pharmaceuticals International GmbH) for the treatment of moderately to severely active CD and UC in adults who have had an inadequate response with, lost response to or are intolerant to conventional treatment or to a TNF-α antagonist.21
Efficacy of vedolizumab in patients with ulcerative colitisPrevious versions of vedolizumab were called LDP-02, MLN02 and MLN0002. For clarity, in this review we will refer to all versions as “vedolizumab”. Phase 1 studies in healthy volunteers have confirmed the gut-selective mechanism of action of vedolizumab,22 absence of effect on the lymphocytes in the cerebrospinal fluid (CD4+: CD8+ ratio)23 and similarity in the pharmacokinetic parameters in patients with UC and CD, with no need for dose variation in the presence of covariables such as age, sex, weight, presence of anti-vedolizumab antibodies and albumin.24
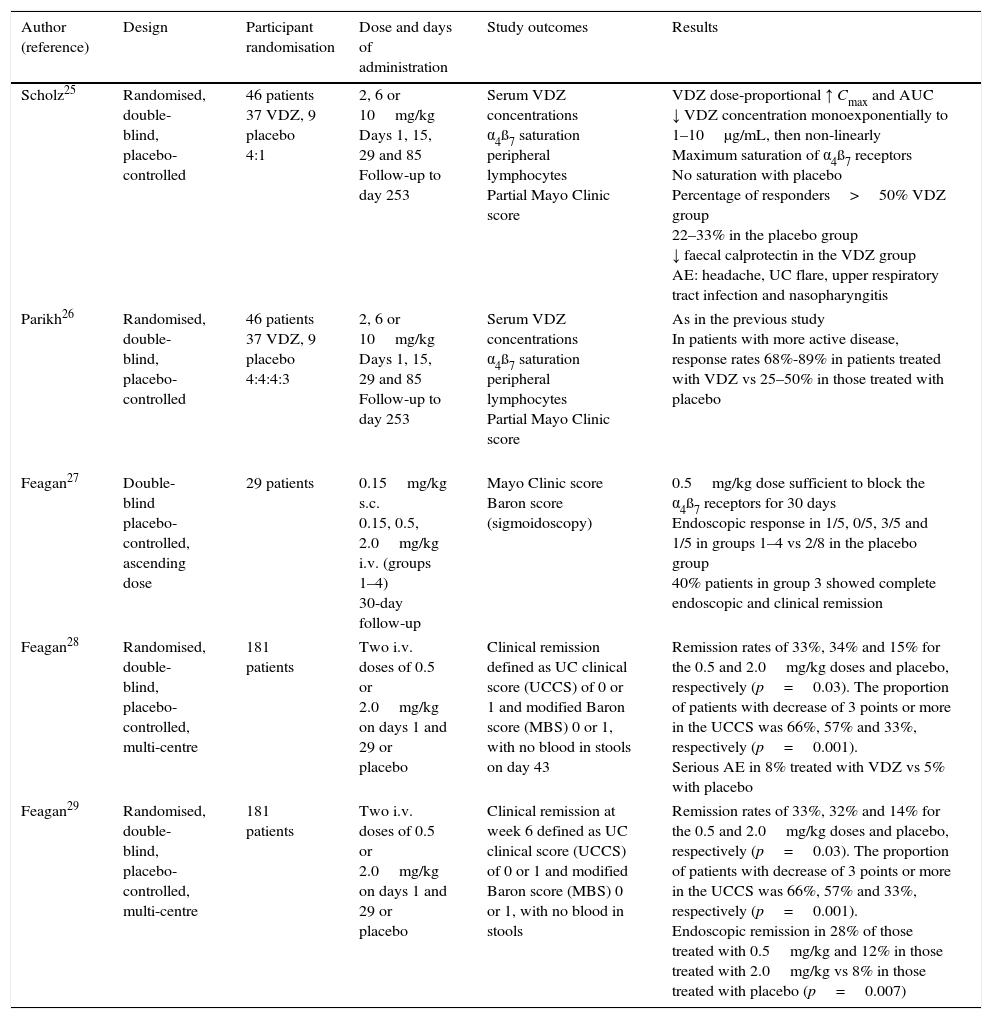
Similarly, different phase 2 studies in patients with active UC have provided efficacy, safety, tolerability and pharmacokinetic data (Table 1). In 46 patients included in a randomised, double-blind, placebo-controlled study treated with vedolizumab (2, 6 or 10mg/kg) or placebo on days 1, 15, 29 and 85, and monitored for 8 months, vedolizumab showed dose-proportional pharmacokinetics, with saturation of the α4β7 receptors on peripheral blood lymphocytes.25,26 Another study with 29 patients with moderate-severe UC compared various doses of vedolizumab (0.15mg/kg subcutaneously or 0.15, 0.5 or 2mg/kg intravenously [i.v.]) with placebo. The 0.5mg/kg dose was sufficient to achieve saturation of the α4β7 receptors of the circulating lymphocytes for 30 days.27 In a double-blind, placebo-controlled multicentre study that included 181 patients with active UC randomised to treatment with 0.5 or 2mg/kg (or placebo) on days 1 and 29, the rates of remission were significantly higher in the active treatment group, and at week 6 were 33%, 32% and 14% for the groups treated with 0.5mg/kg, 2mg/kg and placebo, respectively (p=0.002), with endoscopic remission rates of 28%, 12% and 8%, respectively.29
Phase 2 clinical trials comparing vedolizumab against placebo in patients with active ulcerative colitis.
| Author (reference) | Design | Participant randomisation | Dose and days of administration | Study outcomes | Results |
|---|---|---|---|---|---|
| Scholz25 | Randomised, double-blind, placebo-controlled | 46 patients 37 VDZ, 9 placebo 4:1 | 2, 6 or 10mg/kg Days 1, 15, 29 and 85 Follow-up to day 253 | Serum VDZ concentrations α4ß7 saturation peripheral lymphocytes Partial Mayo Clinic score | VDZ dose-proportional ↑ Cmax and AUC ↓ VDZ concentration monoexponentially to 1–10μg/mL, then non-linearly Maximum saturation of α4ß7 receptors No saturation with placebo Percentage of responders>50% VDZ group 22–33% in the placebo group ↓ faecal calprotectin in the VDZ group AE: headache, UC flare, upper respiratory tract infection and nasopharyngitis |
| Parikh26 | Randomised, double-blind, placebo-controlled | 46 patients 37 VDZ, 9 placebo 4:4:4:3 | 2, 6 or 10mg/kg Days 1, 15, 29 and 85 Follow-up to day 253 | Serum VDZ concentrations α4ß7 saturation peripheral lymphocytes Partial Mayo Clinic score | As in the previous study In patients with more active disease, response rates 68%-89% in patients treated with VDZ vs 25–50% in those treated with placebo |
| Feagan27 | Double-blind placebo-controlled, ascending dose | 29 patients | 0.15mg/kg s.c. 0.15, 0.5, 2.0mg/kg i.v. (groups 1–4) 30-day follow-up | Mayo Clinic score Baron score (sigmoidoscopy) | 0.5mg/kg dose sufficient to block the α4ß7 receptors for 30 days Endoscopic response in 1/5, 0/5, 3/5 and 1/5 in groups 1–4 vs 2/8 in the placebo group 40% patients in group 3 showed complete endoscopic and clinical remission |
| Feagan28 | Randomised, double-blind, placebo-controlled, multi-centre | 181 patients | Two i.v. doses of 0.5 or 2.0mg/kg on days 1 and 29 or placebo | Clinical remission defined as UC clinical score (UCCS) of 0 or 1 and modified Baron score (MBS) 0 or 1, with no blood in stools on day 43 | Remission rates of 33%, 34% and 15% for the 0.5 and 2.0mg/kg doses and placebo, respectively (p=0.03). The proportion of patients with decrease of 3 points or more in the UCCS was 66%, 57% and 33%, respectively (p=0.001). Serious AE in 8% treated with VDZ vs 5% with placebo |
| Feagan29 | Randomised, double-blind, placebo-controlled, multi-centre | 181 patients | Two i.v. doses of 0.5 or 2.0mg/kg on days 1 and 29 or placebo | Clinical remission at week 6 defined as UC clinical score (UCCS) of 0 or 1 and modified Baron score (MBS) 0 or 1, with no blood in stools | Remission rates of 33%, 32% and 14% for the 0.5 and 2.0mg/kg doses and placebo, respectively (p=0.03). The proportion of patients with decrease of 3 points or more in the UCCS was 66%, 57% and 33%, respectively (p=0.001). Endoscopic remission in 28% of those treated with 0.5mg/kg and 12% in those treated with 2.0mg/kg vs 8% in those treated with placebo (p=0.007) |
AE, adverse events; UC, ulcerative colitis; VDZ, vedolizumab.
The efficacy and safety of vedolizumab has been studied in a phase 3 clinical trial in patients with UC (GEMINI 1).20,30
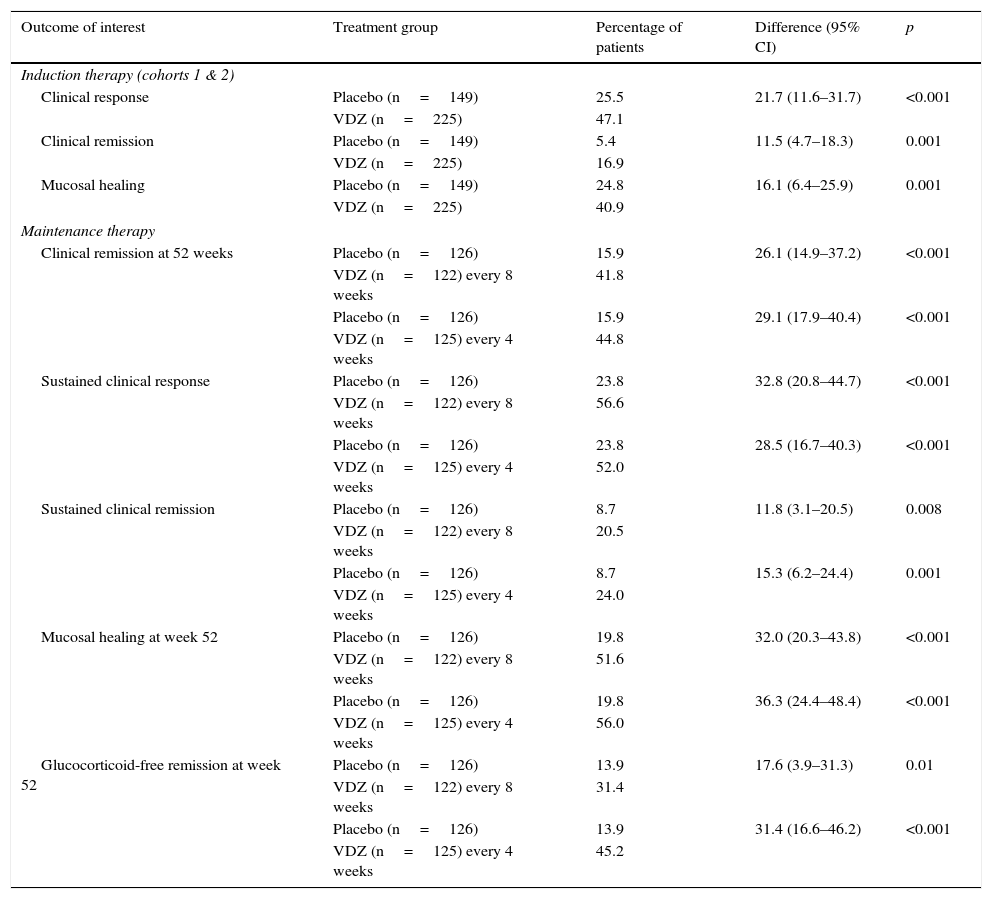
This trial integrated the results of 2 randomised, double blind, placebo-controlled studies. It included patients with active UC, defined as a Mayo Clinic score of 6–12, with a minimum sigmoidoscopy subscore of 2, and failure of previous treatments (lack of response or adverse events), including glucocorticoids, immunosuppressants or anti-TNF-α. The induction therapy study included 2 cohorts: cohort 1 was made up of 374 patients who were treated with 300mg of vedolizumab i.v. or placebo at weeks 0 and 2, while cohort 2 (open study) was made up of 521 patients treated with vedolizumab also at weeks 0 and 2. The clinical response was evaluated in both cohorts at week 6. In the maintenance therapy study, patients from both cohorts who showed a response to vedolizumab at week 6 were randomised to continue treatment with vedolizumab every 4 or 8 weeks, or placebo up to 52 weeks. The primary outcome in the induction phase was clinical response at week 6, defined as a decrease in the Mayo Clinic score of at least 3 points and a 30% or more reduction with respect to the baseline score, with a decrease of at least 1 point in the rectal bleeding score or an absolute rectal bleeding score of 0 or 1. The secondary outcomes included clinical remission (Mayo Clinic score of 2 or lower with no rectal bleeding subscore higher than 1 and mucosal healing, defined as an endoscopic subscore of 0 or 1). The primary outcome for the maintenance phase was clinical remission at week 52, and the secondary outcomes were durable clinical response and clinical remission (at weeks 6 and 52), mucosal healing at week 52 and glucocorticoid-free remission at week 52 in patients who received this medication at baseline. Quality of life (QoL) was measured using a specific questionnaire for patients with inflammatory bowel disease (IBDQ).
In both the induction and maintenance treatment trial, the differences between the vedolizumab and placebo treatment groups were statistically significant for all the primary and secondary outcomes of interest (Table 2). Patients treated with vedolizumab also experienced more significant improvements in the partial Mayo Clinic score, IBDQ score, faecal calprotectin concentration and glucocorticoid use than patients treated with placebo. In a post hoc analysis, no clear differences were observed in the efficacy of vedolizumab administered every 4 or every 8 weeks. No differences in the most common adverse events (headache, nasopharyngitis, upper respiratory tract infection, arthralgia, nausea, abdominal pain, fatigue, anaemia and cough) were observed between vedolizumab and placebo groups, although serious infections were more common in the active treatment group (2.9% vs 1.9%). No cases of PML were observed.
Main outcomes of the GEMINI 1 phase 3 trial20,30: vedolizumab (VDZ) vs placebo.
| Outcome of interest | Treatment group | Percentage of patients | Difference (95% CI) | p |
|---|---|---|---|---|
| Induction therapy (cohorts 1 & 2) | ||||
| Clinical response | Placebo (n=149) | 25.5 | 21.7 (11.6–31.7) | <0.001 |
| VDZ (n=225) | 47.1 | |||
| Clinical remission | Placebo (n=149) | 5.4 | 11.5 (4.7–18.3) | 0.001 |
| VDZ (n=225) | 16.9 | |||
| Mucosal healing | Placebo (n=149) | 24.8 | 16.1 (6.4–25.9) | 0.001 |
| VDZ (n=225) | 40.9 | |||
| Maintenance therapy | ||||
| Clinical remission at 52 weeks | Placebo (n=126) | 15.9 | 26.1 (14.9–37.2) | <0.001 |
| VDZ (n=122) every 8 weeks | 41.8 | |||
| Placebo (n=126) | 15.9 | 29.1 (17.9–40.4) | <0.001 | |
| VDZ (n=125) every 4 weeks | 44.8 | |||
| Sustained clinical response | Placebo (n=126) | 23.8 | 32.8 (20.8–44.7) | <0.001 |
| VDZ (n=122) every 8 weeks | 56.6 | |||
| Placebo (n=126) | 23.8 | 28.5 (16.7–40.3) | <0.001 | |
| VDZ (n=125) every 4 weeks | 52.0 | |||
| Sustained clinical remission | Placebo (n=126) | 8.7 | 11.8 (3.1–20.5) | 0.008 |
| VDZ (n=122) every 8 weeks | 20.5 | |||
| Placebo (n=126) | 8.7 | 15.3 (6.2–24.4) | 0.001 | |
| VDZ (n=125) every 4 weeks | 24.0 | |||
| Mucosal healing at week 52 | Placebo (n=126) | 19.8 | 32.0 (20.3–43.8) | <0.001 |
| VDZ (n=122) every 8 weeks | 51.6 | |||
| Placebo (n=126) | 19.8 | 36.3 (24.4–48.4) | <0.001 | |
| VDZ (n=125) every 4 weeks | 56.0 | |||
| Glucocorticoid-free remission at week 52 | Placebo (n=126) | 13.9 | 17.6 (3.9–31.3) | 0.01 |
| VDZ (n=122) every 8 weeks | 31.4 | |||
| Placebo (n=126) | 13.9 | 31.4 (16.6–46.2) | <0.001 | |
| VDZ (n=125) every 4 weeks | 45.2 | |||
95% CI, 95% confidence interval; VDZ, vedolizumab.
The results of the GEMINI 1 trial showed that vedolizumab was more effective than placebo as induction and maintenance treatment in patients with active UC. Similarly, additional analyses in other patient subgroups in this trial, including previous failure of anti-TNF-α or immunomodulatory treatment31 or patients from cohorts who were non-responders at week 6,32 also showed the superiority of vedolizumab compared to placebo at weeks 6 and 52. QoL measurement using generic instruments, such as the short form SF-36 health questionnaire and the EQ-5D questionnaire visual analogue scale, as well as the IBDQ specific questionnaire, also showed that vedolizumab has beneficial effects on patient QoL.33
Data on the long-term efficacy of vedolizumab are also available, derived from the open-label extension study of the GEMINI 1 trial (GEMINI LTS), which includes results on clinical remission up to week 104.34 Thus, in a total of 275 patients who completed the GEMINI 1 trial and received any dose of vedolizumab during the GEMINI LTS, clinical remission was obtained in 73% of cases. Similarly, in the subgroup of patients with previous failure of anti-TNF-α treatment, 65% maintained clinical remission at week 104.35 Apart from the clinical activity, preliminary findings are also available for mucosal healing at week 52, which was 28% in the vedolizumab group compared to 8.7% in the placebo group (p<0.001), with rates of endoscopic improvement and clinical remission of 31% and 13%, respectively (p<0.001).36 These studies also showed that the efficacy of vedolizumab does not differ according to age,37 baseline faecal calprotectin levels38 or concomitant use of corticosteroids or immunomodulators.39 Finally, an extension study of the GEMINI 1 trial showed the safety of re-administering vedolizumab every 4 weeks after 1 year with no treatment.40
Two systematic reviews have analysed the efficacy of vedolizumab in the treatment of UC.41,42 In one of these,41 which included 8 randomised controlled trials with biological agents (vedolizumab, abatacept, visilizumab, golimumab), vedolizumab was significantly more effective than placebo in achieving clinical response, clinical remission and mucosal healing in the induction phase, with relative benefits of 82%, 166% and 75%, respectively (p<0.001 for all comparisons); in the maintenance phase, vedolizumab achieved a significantly higher percentage of clinical remission and mucosal healing with respect to placebo (p<0.01). In the other systematic review that included 4 randomised controlled clinical trials comparing vedolizumab with placebo in a total of 606 patients, aggregated analyses showed the statistically significant superiority of vedolizumab compared to placebo for inducing remission, clinical response, endoscopic remission and maintenance of remission.42
Safety of vedolizumab treatmentThe adverse events most commonly observed in patients treated with vedolizumab include nasopharyngitis, headache, nausea, arthralgia, pyrexia, fatigue, upper respiratory tract infections, cough and abdominal pain.43 The safety data described in a recent systematic review41 suggested that vedolizumab was as safe as placebo in terms of risk of adverse events, serious adverse events or deaths in the induction phase. In the maintenance phase, the risk of adverse events was also similar between patients treated with vedolizumab or placebo, but the differences between groups for the risk of serious adverse events was marginally significant (p=0.05). In a systematic review of 4 clinical trials with a total of 606 patients,42 no statistically significant difference was found between vedolizumab and placebo in terms of risk of any adverse event (relative risk [RR] 0.99, 95% confidence interval [95% CI]: 0.93–1.07) or serious adverse events (RR 1.01; 95% CI: 0.72–1.42). However, a statistically significant difference was observed in patients who withdrew due to an adverse event: 6% for vedolizumab vs 11% in the case of placebo (RR 0.55; 95% CI: 0.35–0.87; 2 studies, 941 patients). Nonetheless, no severe infections, including PML, were reported in patients with UC in the GEMINI 1 trial.20
Finally, an immunogenicity rate of 4% has been described in the GEMINI 1 and 2 controlled studies (56 of 1434 patients).30,44,45 Anti-vedolizumab antibodies were detected in approximately 10% of patients 16 weeks after the last dose of vedolizumab.30,44 In these trials, 5% of patients who presented an adverse event considered by the investigator to be infusion-related also tested positive for anti-vedolizumab antibodies. Nevertheless, in general, there was no correlation between the development of anti-vedolizumab antibodies and the clinical response or adverse events.
ConclusionsVedolizumab has been shown to be more effective than placebo for inducing and sustaining clinical remission in UC in a manner similar to anti-TNF-α agents approved for the treatment of UC (infliximab, golimumab and adalimumab). Vedolizumab, like infliximab, adalimumab and golimumab (the 3 anti-TNF-α drugs currently approved in Spain for the treatment of UC), has been approved for the treatment of patients with UC in whom conventional treatment has failed. Beyond this approved indication, one of the most attractive scenarios concerns patients with previous exposure to an anti-TNF agent. The secondary loss of response to anti-TNF-α is usually managed by escalation of the same drug or by switching to another anti-TNF, strategies that usually increase costs and have shown acceptable short- but not long-term efficacy. In the 25% or so of patients who do not present a primary response to a first anti-TNF,46,47 a second anti-TNF is even less likely to be effective.48,49 It therefore seems reasonable to consider vedolizumab as the drug of choice in both situations (primary and secondary failure of an anti-TNF-α), and it can also be used as a first-line drug in case of failure or intolerance to conventional immunosuppressants (thiopurines or methotrexate).
Moreover, vedolizumab has been shown to have a good safety profile, with so far none of the neurological complications (PML) reported with other drugs that share a similar mechanism of action (integrin inhibition), which is probably explained by the gut-selectivity of vedolizumab. It is precisely this selectivity that could give it an advantage over other drugs with a more non-specific or systemic effect, such as thiopurines, calcineurines or anti-TNF-α agents. Finally, immunogenicity (i.e. the formation of antibodies against vedolizumab) seems to be rare, possibly lower that that described with anti-TNF-α drugs.
In summary, vedolizumab can be considered a promising option for the treatment (induction, and above all, maintenance) of moderate or severe UC. Its position in the therapeutic arsenal in relation to other drugs — especially anti-TNF-α agents — is not yet established. This will depend on accumulated experience with the efficacy (including more patients and longer follow-up) and safety (if the favourable safety profile described to date is confirmed) of vedolizumab. Finally, some important aspects should be clarified in the future, such as the efficacy of vedolizumab in patients with severe UC refractory to corticosteroids, or the possible synergy between this drug and traditional immunosuppressants.
Conflict of interestsThe authors have received no remuneration for writing this article and have acted completely independently in its preparation, with no employee of Takeda Farmacéutica España having participated in its drafting. Takeda Pharmaceuticals International had the opportunity to review the manuscript.
E. Domènech: scientific consultancy, conferences, support for research or training activities: MSD, AbbVie, Takeda, Hospira, Kern Pharma, Ferring, Faes Farma, Shire Pharmaceuticals, Chiesi, Otsuka Pharmaceuticals, and Gebro Pharma.
J.P. Gisbert: scientific consultancy, conferences, support for research or training activities: MSD, AbbVie, Takeda, Hospira, Kern Pharma, Pfizer, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Chiesi, Laboratorios Casen Fleet, Otsuka Pharmaceutical, Uriach and Dr. Falk Pharma and Gebro Pharma.
The authors would like to thank Dr Marta Pulido and Nature Publishing Group Iberoamérica for their assistance in writing this article, with editorial support funded by Takeda Farmacéutica España.
Please cite this article as: Domènech E, Gisbert JP. Eficacia y seguridad de vedolizumab en el tratamiento de la colitis ulcerosa. Gastroenterol Hepatol. 2016;39:677–686.