Casos Clínicos en Gastroenterología y Hepatología
Más datosEndoscopic recognition of gastric premalignant conditions (GPC) such as chronic atrophic gastritis (CAG), intestinal metaplasia (IM), and dysplasia is the first step before taking protocolized and guided biopsies during routine esophagogastroduodenoscopy (EGD). However, as some studies have pointed out, there is a lack of training on GPC recognition, as well as the diagnosis and management by European gastroenterologists vary significantly.1,2 Identifying patients with advanced GPC could improve the prognosis of gastric cancer (GC) by increasing the early diagnosis and decreasing missed cancer rates.
The European Society of Gastrointestinal Endoscopy (ESGE) has recently published a curriculum for optical diagnosis training on digestive lesions that focuses on visible gastric lesions (dysplasia/early GC). This curriculum proposes three steps: Step 1, training (using validated tools); Step 2, self-learning (by real-time assessing), and Step 3, assessing proficiency (by real-time assessing and achieving an accuracy of ≥80%).3
Although the ESGE curriculum does not address a training method that focus on recognition of GPC on a gastric mucosa without visible lesions, a positive effect of delivering training on optical diagnosis of GPC using validated classifications was found in two studies applying different strategies. In the CAG and IM/dysplasia recognition training, the Kimura-Takemoto classification (white-light) and the simplified NBI classification (blue-light spectrum), respectively, were used by the studies.4,5
Furthermore, high-definition endoscopes (HD-E) and virtual chromoendoscopy (VC), which improve the detection and characterization of GPC, are widely available in Spanish hospitals, but are not used properly.1 Because of that, we aimed to assess the applicability of the ESGE curriculum for the diagnosis of GPC in patients without visible lesions by using Sonoscape endoscopes.
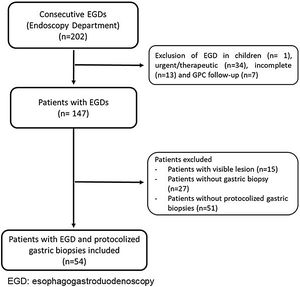
We performed a retrospective analysis of consecutive EGDs with protocolized biopsies taken in adults by a non-expert endoscopist in a community setting between July and October 2020. We excluded patients with previous diagnosis of GPC, visible lesions on the gastric mucosa, or patients with no protocolized biopsies (Fig. 1). Every EGD was performed according to daily practice and getting the informed consent from patients before the procedure. We used HD-E and VC developed by Sonoscape 550 (light-source: 2 LED). Sonoscape technology offers two modalities of VC: (i) SFI (Spectral Focused Imaging), which combines different wavelengths and light intensity increasing the contrast of lesions (useful for detection), and (ii) VIST (Versatile Intelligent Staining Technology), which uses the blue-light spectrum (useful for characterization). Protocolized biopsies were taken from antrum and corpus and were labeled in two separate vials. The biopsies were analyzed based on the clinical practice employed in the Pathology department. Every patient was categorized endoscopically and histologically into two categories: presence/absence of GPC. The GPC were CAG, IM, and dysplasia. The cases with advanced GPC were extensive IM (involving antrum and corpus) or dysplasia.
We followed the ESGE principle for optical learning of GPC in a mucosa without visible lesions by using patterns of previous validated classifications. Since there is not a validated course for the learning of GPC, we looked at the images of Kimura-Takemoto and Simplified NBI classifications (step 1), and after that we undertook a self-learning process entailing real-time assessment (step 2) and proficiency assessment (step 3).3–5
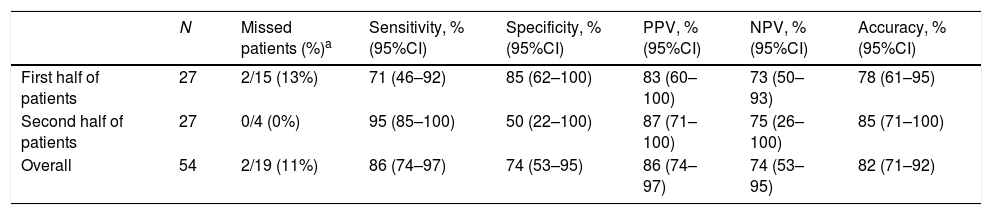
Fifty-four consecutive adult patients for all diagnostic indications were included (mean age 61±16 years old, 56% were women, and 57% had alarming symptoms). The overall diagnostic yield was: sensibility 86%, specificity 74%, positive predictive value 86%, negative predictive value 74%, and diagnostic accuracy 82%. As for diagnostic accuracy, in the first half of EGDs (step 2) it was 78%, while in the second half (step 3), it was 85%. Although this improvement was not statistically significant (p=0.48), it was over than 80% recommended by the ESGE. Moreover, two (13%) patients endoscopically classified as absence of GPC in the first part had advanced GPC in the histology; however, there was no patient in this category in the second part (Table 1).
Trend of the diagnostic accuracy of GPC during the optical diagnosis learning of a non-expert endoscopist by using Sonoscape technology.
| N | Missed patients (%)a | Sensitivity, % (95%CI) | Specificity, % (95%CI) | PPV, % (95%CI) | NPV, % (95%CI) | Accuracy, % (95%CI) | |
|---|---|---|---|---|---|---|---|
| First half of patients | 27 | 2/15 (13%) | 71 (46–92) | 85 (62–100) | 83 (60–100) | 73 (50–93) | 78 (61–95) |
| Second half of patients | 27 | 0/4 (0%) | 95 (85–100) | 50 (22–100) | 87 (71–100) | 75 (26–100) | 85 (71–100) |
| Overall | 54 | 2/19 (11%) | 86 (74–97) | 74 (53–95) | 86 (74–97) | 74 (53–95) | 82 (71–92) |
GPC: gastric premalignant conditions; PPV: positive predictive value; NPV: negative predictive value; CI confidence interval.
Based on this single experience, a non-expert endoscopist in a community setting and using Sonoscape technology could achieve a threshold for the diagnostic accuracy for identification of GPC, which would lower the risk of missing patients with advanced GPC. Learning to recognize patterns of GPC in a mucosa without visible lesion could represent the first step in the gastric mucosa optical diagnosis training.
FundingNon-financial support.
Conflict of interestThe authors do not have conflicts of interest.