INTRODUCTION
Ulcerative colitis (UC) is a chronic disease that usually alternates phases of clinical activity, usually known as «flare-up» or attack, with phases of clinical remission. This intermittent course is the most common in the natural history of UC: in fact 90% of patients show this pattern if the follow-up is long enough. About 15% of patients suffer in a given moment from a severe attack, a clinical situation which had 30% to 50% mortality in the pre-steroid era1.
In 1954 and 1955 the Oxford Group published in 2 parts a key clinical trial in the British Medical Journal. It was a randomized, controlled, and blind study that demonstrated that hydrocortisone is clearly superior to placebo in the treatment of attacks or UC2. Although ultimately between 30% and 50% of patients required surgical treatment, hydrocortisone was clearly superior to placebo both in moderate and severe cases, and the mortality rate fell to roughly 5%2. Ten years later, Truelove and Jewell3 published another key observational study: a fixed day for colectomy in steroid failures by protocol situated the final mortality in a standard rather difficult to improve: 1%3. This strategy was adopted around the world, with some local variations (5 to 14 days of steroid treatment before colectomy).
Steroids are far from perfect treatment, however. Besides common and significant side effects, colectomy is still needed in 38% (range: 25-57%) of cases, as shown in a recent meta-analysis4. Colectomy usually cures UC, but cause a definitive change in the life of a patient, and even with ileo-anal reconstruction can impair surgical treatment required in 38% (range: 25-57%). Surgery may «cure» UC but, even with ileo-anal reconstruction, decreases quality-of-life. Moreover, at least 30% of patients develop a new inflammatory disease: pouchitis, sometimes very difficult to manage.
Azathioprine and 6-mercaptopurine were introduced in the treatment of Crohn's disease and UC, but its slow onset of action limits their effectiveness in acute cases5. Cyclosporine (CyA) is a potent immunosuppresor which inhibits the production of interleukine-2 and the first evidence of the possible effectiveness of CyA in inflammatory bowel disease dates from 19846. Several subsequent studies have been published, although the data from controlled studies are quite sparse. Recently a Cochrane review has been published, but it only included the 2 randomised trials comparing CyA with placebo or not intervention in this setting7. We lack, however, an exhaustive review of the available evidence, including both controlled and observational data. With our review we try to find a response to some basic clinical questions in the setting of acute sever attack of UC: is really CyA efficacious in the treatment of acute attacks of UC?; is it a safe alternative in this context?, at what dose and by which route should it be administered?
METHODS
In searching the literature we used the key words «cyclosporine» («Cyclosporine» [MESH]) and «ulcerative colitis» («Colitis, ulcerative/Therapy» [MESH]) in the MEDLINE database, accessed via the search engine Pubmed. Using the same key words we reviewed the Cochrane Central Register of Controlled Trials. The search was conducted up to July 2004 and all papers in English, French, Spanish or German were included. From the articles obtained we evaluated the references cited, selecting those that were potentially relevant. Further, we reviewed the summaries of the last 10 years of the DDW (Digestive Disease Week) and of the UEGW (United European Gastroenterology Week).
To evaluate the efficacy of the drug, we considered data derived from clinical trials and of the observational series, whether prospective or retrospective. When reviewing safety we did also consider reports of individual cases, because infrequent side-effects can be overlooked in these relatively rare clinical situations. In some instances, when dealing with a patient series from the same authors but published at different times, we considered only the most recent reference so as to avoid duplication of patients' data. We did not include studies in children, or those in whom CyA was administered by the topical (rectal) route.
We assessed the data from studies that had used CyA via the oral route separately from those using intra-venous administration. This was for 3 reasons: a) it is possible that the effect of the drug would be slightly different; b) the doses are difficult to equate even with the new neo-oral formulations; and c) an assumption that, generally, the oral route is employed in patients with less severity of the disease.
We tabulated the data and expressed the percentage and the 95% confidence intervals (95%CI), as well as the weighted mean (to adjust for the number of patients included in each study).
RESULTS
Efficacy
The first aspect we need to highlight is that there was considerable heterogeneity among the different studies. Most papers evaluated the efficacy of CyA in patients with severe attacks of UC that were considered refractory to steroids. However, the definitions of «refractory» or «severe» were not uniform, and the doses of steroids, the accompanying therapy (for example, with antibiotics or parenteral nutrition) and, above all, the duration of the previous treatment and definitions of response were highly variable. CyA was administered by the intra-venous route in the majority of studies8-29, while sometimes the oral route was used16,30-37. Methodologically, the majority of the studies were observational series or non-controlled clinical trials; only 4 of the published studies, all using the intravenous route of administration, were controlled trials26-29, including one that had been published only as an abstract29.
TREATMENT WITH INTRAVENOUS CYCLOSPORINE
Non-controlled studies
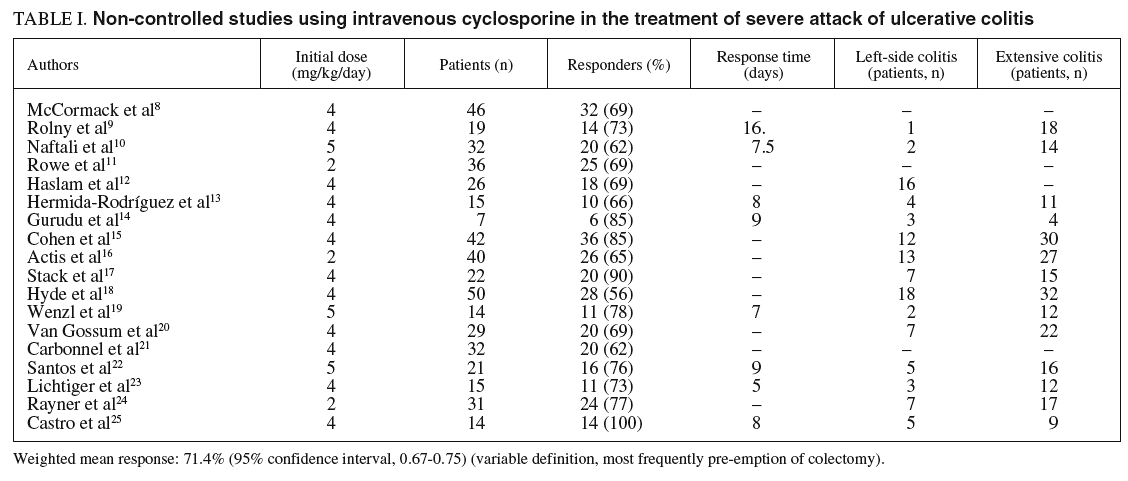
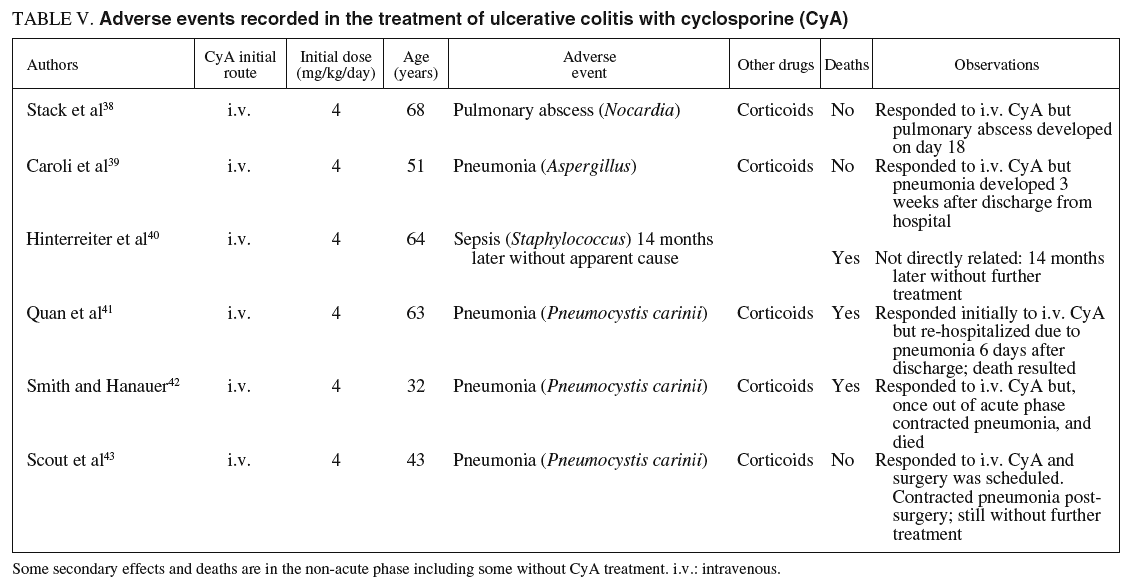
Table I summarizes the 18 non-controlled studies8-25 using intravenous CyA which had been published at the time of our analysis. The majority had been conducted in patients with severe attacks of UC refractory to intravenous steroids, but some studies included moderate cases, and the dose and time of steroid administration were highly variable, as previously stated. Any case, the total number of patients included was 491, a very important figure when comparing with controlled trials. The median initial dose used was 3.9 mg/kg/day (range: 2-5). There is o obvious relationship between dose and response as some large trials using 2 mg/kg dose show the same mean rate of response. The mean time of response, which was clearly specified only in 8 studies varied between 5.8 16 days. The rate of overall response, defined in several different ways but most frequently as the rate to avoid colectomies, was 71.4% (95% CI, 0.67-0.75), which means in real numbers avoiding colectomy in 351 of the 491 patients.
Controlled studies
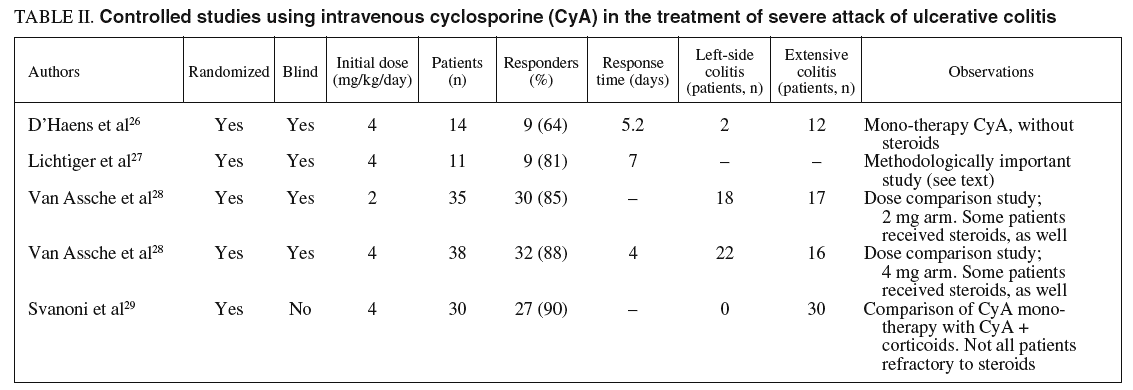
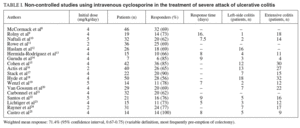
There were 4 controlled studies using intravenous CyA in the treatment of severe UC attack, 3 of them complete reports and the 4th as a summary26-29. Of the 3 complete reports published, one evaluated the efficacy of CyA in patients with severe attack of UC without response to corticoids compared to a group on placebo, the steroids being maintained in both groups26. The second study evaluated the efficacy of CyA in mono-therapy as compa red with mono-therapy with steroids27. The third study compared the efficacy of intravenous administration of 4 mg/kg against a dose of 2 mg/kg28. The study published as a summary compared mono-therapy CyA against its combination with steroids29. Table II summarizes the most important data from these studies. Overall, taking these studies together, there were 128 patients included who were treated with intravenous CyA, 35 of them at a dose of 2 mg/kg and the rest (93 patients) at 4 mg/kg. The rate of response to the CyA alone, or in combination with corticoids was 91.4% (95%CI, 0.85-0.95). Overall, in the group receiving 2 mg/kg the rate of response was 85.7% (30/35) and in the group of 4 mg/kg it was 93% (87/93). However, in the study comparing the 2 doses head to head, the tendency was the contrary, albeit not statistically significant.
Only one of this studies evaluated in a blind, randomized and placebo-controlled trial, the efficacy of CyA in patients with severe flares refractory to steroids. This was the study by Lichtiger et al27, which we will analyze in greater detail because of its importance. In this trial there were 20 patients with severe attack (not only pancolitis but also left-side colitis) who had not responded to treatment with intravenous corticoids (dose equivalent to hydrocortisone 300 mg/day) over, at least, 7 days. Excluded were the patients who had received treatment with purine analogues in the previous 2 weeks and those with toxic megacolon. The patients were randomized to receive CyA (n = 11) at a dose of 4 mg/kg/day (intravenous continuous infusion) until a response was obtained up to a maximum of 14 days, or placebo (n = 9). All the patients received, as well, hydrocortisone (300 mg intravenous daily). Mesalazine treatment was continued if previously used, but not allowed the novo. The response to the treatment was evaluated according to an index that had not been validated and was based on a modification of Truelove-Witts index. At the conclusion of day 14, out of the 11 patients in the CyA group 9 responded (82%), compared to none in the placebo group, a difference which forced the interruption of the trial after an interim analysis on ethical grounds. There was no difference in the blood concentrations of CyA between the patients who responded and those who did not. Five of the patients who did not respond in the placebo group and who did not require emergency surgery were treated with CyA (cross-over) and a response was obtained in all of them. Of the 9 patients who initially responded to CyA, one underwent an elective colectomy and the rest were treated with oral CyA at the initial dose of 6-8 mg/kg/day over 6 months. Five mantain remission without steroids at 6 months and 3 relapsed in the course of the oral CyA treatment requiring colectomy. As such, only the 45% of the patients (5/11) who were initially treated with CyA avoided colectomy in the 6 months of follow-up. In spite of the small numbers, this remains a unique trial on methodological grounds.
The second study compared steroid treatment versus CyA in mono-therapy27. Thirty patients were randomized to CyA (4 mg intravenous) or 6 methylprednisolone (40 mg) with responses being observed, after 8 days of treatment, in 64.2% (9/14) and in 53% (8/15) in the 2 treatment groups, respectively (p = 0.4). The patients included in this study were not refractory to steroids. No serious drug-related toxicity as observed with either treatment. After one year, the 66% (10/15) and 60% (9/15) of patients of CyA and steroids groups respectively avoid colectomy.
The third full study published compared the doses of 4 mg/kg versus 2 mg/kg of CyA28. Seventy-three patients were randomized to each treatment arm (38 to 4 mg/kg and 35 to 2 mg/kg). The response was 84.2% (32/38) and 85.7% (30/35), respectively; the differences not being statistically significant. The adverse events were not clearly higher in the higher-dose treatment group.
The controlled study published as a summary29 compared the treatment of CyA (4 mg/kg) in mono-therapy against CyA plus steroids. Thirteen of 15 patients in the first treatment group responded compared to 14 of 15 in the combination therapy group, all of whom with complete response.
Oral treatment
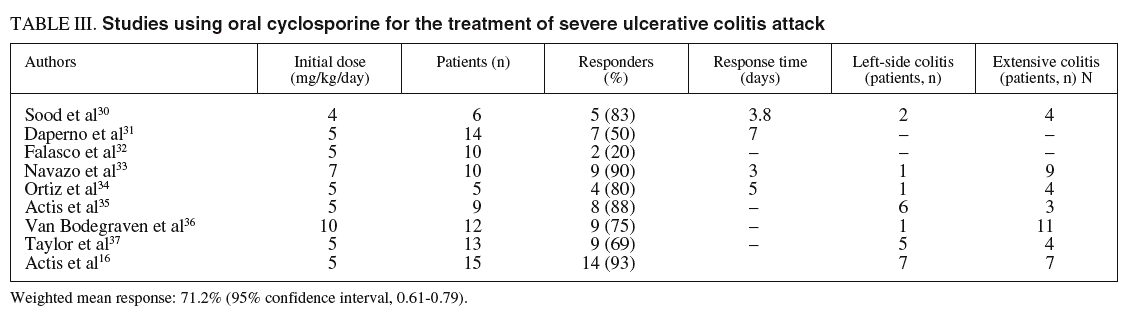
In 9 studies16,30-37, all of them non-controlled, the efficacy of oral CyA was evaluated in patients with UC. In total, there were 94 patients, some with moderate flare-ups, although usually refractory to steroids. In several studies there were no specific details on the extent of the disease (at least in 23 patients the colitis was left-sided). The doses employed varied between 4 and 10 mg/kg; with 5 mg/kg being employed in 6 of the 9 studies. The rate of response was 71.2% (95%CI, 0.61-0.79), with response being obtained in 67 of the 94 patients included. The mean time-to-response, an aspect not specified in some studies, was a weighted mean of 5.19 days (range 3.8 to 7 days). Table III reflects the more relevant aspects of the articles evaluating oral CyA.
Safety
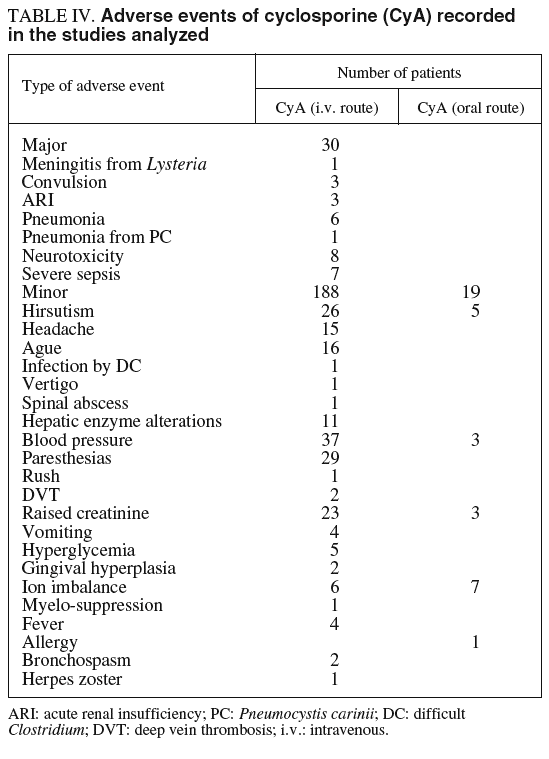
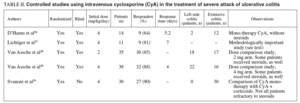
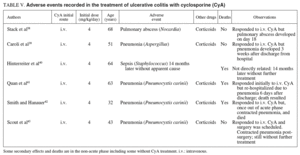
This is a very important aspect but was not always fully detailed in some studies, making difficult to extract relevant information. In some, there were no detailed descriptions of the adverse effects while, in others, the acute phase effects were mixed-in with those produced during the chronic phase and patients with UC were mixed with those with Crohn's disease. Further, the percentage of «patients with adverse effects» were not always calculated since sometimes only numbers (or percentages) were provided for each adverse effect without specifying whether some patients had more than one of these adverse effects. Table IV summarizes the side effects reported, and their frequencies.
In the studies with intravenous CyA, minor side effects were more frequent. These included headache, hirsutism, ion balance and hepatic enzyme alterations, elevation of blood pressure and paresthesias; all of which were, usually, easily controlled. The severe side effects are less frequent and, among them, those that need to be highlighted are nephrotoxicity, infections and neurotoxicity. Regarding to nephrotoxicity, although the transitory elevation of creatinine in plasma was common, in our review there was only mentioned one case of severe acute renal insufficiency, that finally was reversible12. Infections were more frequent, and not only opportunistic germs and sometimes severe, even leading to death. The first included 3 pneumonias caused by Pneumocystis carinii11,20,22, 2 of them during the acute phase and one in the remission phase, and one meningitis caused by Listeria monocytogenes12. Among the infections caused by common pathogens in the studies included in our analysis were: one sepsis by Staphylococcus aureus14, one infection by Yersinia enteritidis and another by Staphylococcus epidermidis and Haemophilus influenzae21, this one after surgery and which was fatal, and one case of pneumonia without microbiologic diagnosis12. Finally, severe neurotoxicity was reported in some trials: 2 patients had seizures15,26. Of the 5 deaths during treatment with this drug in the patient series included in the analysis, 4 of them were during the acute phase and the fifth22 during the remission phase. The causes of death during the acute phase were: one pneumonia caused by P. carinii20, one sepsis caused by S. epidermidis and Haemophilus post-surgery in a patient who did not respond to CyA (had received only 48 h of treatment)21, one subarachnoid haemorrhage12 and the last by a deep vein thrombosis16.
Although not specifically stated in detail in some studies using oral CyA, adverse events seems clearly less frequent and minor: hirsutism, ion imbalance and slight increases in plasma creatinine and blood pressure (table V).
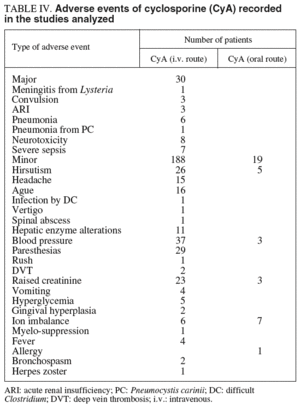
We reviewed, as well, the clinical reports published of severe secondary effects observed while using CyA in patients with UC38-43. All of these cases were infections, including one case of pulmonary abscess from Nocardia that responded well to treatment38, one case of pneumonia from Aspergillus39, 3 cases of pneumonia from P. carinii41-43, but only one of them during the acute phase. All these patients had been treated with parenteral CyA combined with steroids. Apart from these cases, there were other similar ones, mainly infections (Aspergillus, disseminated and fatal; mycotic aneurism), that had been reported in patients with Crohn's disease treated with this drug.
DISCUSSION
Medical control of severe attack of UC using steroids is not feasible in a considerable number of cases, and 30% to 50% of patients finally need colectomy4. CyA has been used in this setting, but a systematic review of evidence was not available. Although controlled data are limited, and in fact we have found only one randomized study comparing CyA to placebo26, we can conclude from our systematic review that there is compelling evidence for using CyA in the treatment of steroid-refractory acute severe attacks of UC. Data from roughly 600 patients reported in literature are very consistent, and both observational and controlled data suggest a 60-70% effectiveness, defined as avoidance of colectomy, of the drug in this particular clinical setting. Furthermore, available data from a controlled trial indicate that a 2 mg/kg dose is as effective as 4 mg/kg, previously considered as standard28. Some other data from uncontrolled studies also show high effectiveness with low toxicity with the lower (2 mg/kg/day) intravenous dose11,16,24. In our view these data support the recommendation of an initial course of 2 mg/kg that could be adjusted according to blood levels28. So, in most acute severe bouts of UC CyA should be added to steroids if clinical remission is not obtained in 3 to 7 days, and surgery is not mandatory because of complications (massive bleeding or bowel perforation).
Some data suggest that CyA is under-used and, sometimes, administered too late in the treatment of severe attack of UC45. Probably, there are several factors that can explain this restrictive policy in clinical practice: a) the possibility of important adverse events, above all, of severe infections; b) the lack of efficacy over the long-term; and probably not the least important c) the lack of experience of the gastroenterologist in the use of the CyA.
Regarding to the safety of the drug, our study shows (as do others), that the most frequent adverse effects are minor and easy to manage by decreasing the dose of the CyA. The most serious are infections, sometimes severe and occasionally even fatal. Our goal in acute severe UC should be to reach a 0% mortality46, so this point should be considered in detail. In fact we need to mention that most of severe adverse effects have been reported in individual case reports, out of the context of controlled studies. May be, the high standard of care of controlled trials could explain the low rate of complications in these clinical settings. Moreover, infections occur in patients treated with steroids at high-dose, often with a compromised nutritional status, with intravenous lines, and long hospital stays: a delay in clinical decisions (not possible in the protocol of most controlled studies) could contribute to an increase in infectious complications in some reported cases. Anyway, surgery is the other option and post-surgery infections are frequent as well, and that the mortality due to surgery is not insignificant. It is difficult to conduct comparisons in the absence of controlled clinical trials of CyA versus surgery. Further, as we commented previously, infections cannot all be attributed to CyA when practically all the patients are receiving concomitant treatment with high-dose steroids; drugs which are associated with a significant risk of infection. A final point needs to be made and that is whether an earlier introduction of CyA into the treatment scheme would offer a better control of the disease, decreasing the requirements for steroids and, as such, the risk of infection. Using 2 mg/kg/day dose may theoretically decrease adverse event without decreasing effectiveness, although data are very limited. Finally, the possibility of using mono-therapy CyA as initial treatment in some specific patients with severe attack has been suggested, but available evidence is also limited27.
Undoubtedly, CyA does not cure the disease and relapse is very common once the drug is retired, resulting in a high rate of colectomies in the subsequent year. This, as well, favors the use of surgery as the initial treatment option. However, the patient has a better quality-of-life if the colon is conserved and the activity of the disease is controlled. Although the only available data are derived from observational studies, it appears clear that when azathioprine is used to maintain remission after the sever flare-up, the rate of relapse decreases and avoid surgery in the majority of patients47. Furthermore, following surgery, 30% (or possibly more) of the patients develop pouchitis that would require medical treatment, and on occasions, surgery, later. The concept that surgery cures colitis is not true in the strict sense, at least in a third of patients. Once more the debate between surgery and treatment with CyA continues45,46.
Finally, the lack of experience of the gastroenterologist can be overcome in 3 ways: a) referring the patients with severe attack of UC to regional specialist centres (a solution that is not always possible); b) using systematic protocols and guidelines; and c) collaborating with specialists who are experts in the use of CyA (nephrologists, immunologists or hepatology colleagues experienced in liver transplantation). Different solutions would be appropriate, depending on the local circumstances. It would be easy to consult with a colleague in another centre regarding any doubts, and this would encourage safe use of the drug.
In conclusion, it is evident that CyA is not the definitive treatment for severe attack of UC, but it can be very useful in some cases and we need to consider seriously its use in all such patients. The local hospital circumstances, and that of each patient, can tip the balance towards this pharmaceutical agent or towards surgery (or other alternatives which, at the moment, are experimental), bearing in mind the arguments for and against its use. In many patients with severe attack of UC, CyA is the most reasonable therapeutic alternative, above all taking into account that its pre-operative use does not increase the risk of mortality of subsequent surgery, if needed. Elective surgery can, then, proceed with the patient in a better clinical condition.
In summary, from the data available in the literature, we suggest the following points to optimize the use of CyA:
1. Guided use. The protocol needs to be followed systematically to avoid toxic effects, or at least to minimize them. Monitoring drug levels, and active surveillance of adverse events, either those severe as renal toxicity, neurotoxicity and infections as those minor but more frequent, is essential. We recommend, in spite of other authors, to use the intravenous route to avoid problems with intestinal absorption.
2. Early administration. The decision to use CyA should not be delayed too long; after 3-5 days without response to intravenous steroids we should decide to use or not CyA. The Oxford protocol showed that with a systematic approach, the treatment clearly improves the mortality rate. Very probably, to prolong a non-effective steroid treatment is the determinant of poor prognosis in some of these patients.
3. Use of azathioprine. If remission has been achieved with CyA, the patient should receive maintenance treatment with azathioprine (or 6-mercaptopurine) to reduce the risk of relapse, usually followed by colectomy.
4. An initial dose of 2 mg/kg/day dose appears reasonable in an intent to minimize the side-effects.