The quality of colon cleansing and the tolerability of anterograde preparation are essential to the success of colorectal cancer screening.
AimTo compare the tolerability and efficacy of low-volume preparations vs the standard regimen in individuals scheduled for an early morning colonoscopy.
StudyParticipants in a population-based colorectal cancer screening program using the fecal immunochemical test who were scheduled for a colonoscopy from 09:00 a.m. to 10:20 a.m. were prospectively included and assigned to: (1) control group (PEG-ELS 4L): PEG 4L and electrolytes; (2) group AscPEG-2L: a combination of PEG and ascorbic acid 2L; and (3) group PiMg: sodium picosulfate and magnesium citrate 500mL plus 2L of clear fluids. Tolerability was evaluated with a questionnaire and the quality of bowel preparation with the Boston Bowel Preparation Scale.
ResultsA total of 292 participants were included: 98 in the PEG-ELS 4L control group, 96 in the AscPEG-2L study group and 98 in the PiMg study group. Low-volume treatments were better tolerated than the standard solution (AscPEG-2L 94.8% and PiMg 93.9% vs PEG-ELS 4L 75.5%; p<0.0001). The effectiveness of AscPEG-2L was superior to that of PEG-ELS 4L and PiMg (p=0.011 and p=0.032, respectively). Patient acceptance was higher for single-dose than for split-dose administration but efficacy was higher with the split dose than with other doses.
ConclusionsIn early morning colonoscopies, ascPEG-2L appears to be the best option, especially when administered in a split-dose.
La calidad de la limpieza del colon y la tolerancia a la preparación anterógrada son claves para el éxito de un programa de cribado de cáncer colorrectal.
ObjetivoComparar la tolerancia y eficacia de las preparaciones de volumen reducido frente a la preparación estándar en pacientes programados para colonoscopia a primera hora de la mañana.
EstudioIndividuos del programa de cribado poblacional con test de sangre oculta en heces programados para colonoscopia entre las 09:00 y 10:20 a.m fueron prospectivamente asignados a: 1) Grupo Control (PEG-ELS 4L): PEG con electrolitos 4 litros; 2) Grupo AscPEG-2L: PEG más ácido ascórbico 2 litros; y 3) Groupo PiMg: picosulfato sódico más citrato de magnesio 500ml seguido de 2 litros de líquidos claros. Se evaluó la tolerancia mediante cuestionario y la calidad mediante la Boston Bowel Preparation Scale.
ResultadosSe incluyeron 292 sujetos: 98 en el grupo control PEG-ELS 4L, 96 en el grupo a estudio AscPEG-2L y 98 en el grupo a estudio PiMg. Las soluciones de volumen reducido fueron mejor toleradas que la solución estándar (AscPEG-2L 94.8% y PiMg 93.9% vs PEG-ELS 4L 75.5%; p<0.0001). La calidad de la preparación fue superior en el grupo AscPEG-2L que en el grupo control PEG-ELS 4L y grupo PiMg (p=0.011 and p=0.032, respectivamente). Las dosis partidas fueron peor aceptadas por los sujetos pero resultaron en una mayor calidad de la preparación.
ConclusionesAscPEG-2L es la mejor opción para las colonoscopias programadas a primera hora de la mañana, especialmente cuando se administra en dosis partida.
Effective bowel cleansing is essential for a high-quality screening colonoscopy and established practice guidelines state that colonic cleansing must be considered excellent or good in at least 90% of screening colonoscopies.1–3 Poor bowel preparation is associated with longer, more complicated colonoscopies and lower cecal intubation rates.4,5 More importantly, poor colon cleansing results in missed adenomas and contributes to inappropriate surveillance intervals.6,7 Bowel preparation must be safe and well tolerated. This is especially important in the context of screening colonoscopies where patient attendance is a critical issue for the successful implementation of the program.
High-volume polyethylene glycol plus electrolytes solution (PEG-ELS) is considered the optimum bowel preparation for its efficacy, low price and safety profile.8,9 Patient tolerance is poor however, presumably due to the considerable amount of fluid that has to be consumed (4L).10 Recently, new formulations have been commercialized that appear to be equally effective and better tolerated. The addition of ascorbic acid (AscPEG-2L) has allowed halving the volume of water to be taken with PEG, and picosulfate sodium combined with magnesium citrate (PiMg) has emerged as a new low-volume alternative for bowel cleansing. These two low-volume solutions (AscPEG-2L and PiMg) appear to be as effective as the standard regimen (PEG-ELS 4L) and increase patient acceptability.11–15
The timing of bowel preparation is also critically important for effective bowel cleansing. It has been clearly demonstrated that longer time periods between the last preparation dose and the beginning of the procedure result in poorer quality bowel cleansing.16,17 In fact, the European Society of Endoscopy advises that this should not exceed 4h.8 Similarly, it has been shown that for morning colonoscopies, split-dose regimens are superior to single-dose regimens.8,11,17,18 In split-dosing, part of the preparation is administered on the evening before the procedure and the remainder on the morning of colonoscopy to avoid fecal effluent re-staining the bowel walls. Naturally, patients find this difficult when scheduled for an early morning (8:00 a.m. to 10:30 a.m.) procedure since they are obliged to wake up in the middle of the night.17 Indeed, to guarantee a minimum of 2h fasting for sedation or anesthesia, and taking into account the time needed to drink 2L of PEG, individuals need to rise at 4:00 a.m. In this context, new low-volume preparations could provide a real advantage in terms of acceptance. To date, there are no studies evaluating this clinical situation and there is a lack of data comparing the different products.
The goal of the present study was to compare the tolerability and efficacy of low-volume preparations (AscPEG-2L and PiMg) with the standard regimen (PEG-ELS 4L) in individuals scheduled for an early morning colonoscopy. We hypothesized that the two low-volume solutions would be better-tolerated and at least as effective as PEG-ELS 4L in this setting.
Subjects and methodsThis was a prospective, interventional study in adults undergoing colonoscopy in an average risk population fecal immunochemical test-based colorectal cancer screening program.
SubjectsBetween September 2011 and November 2012, individuals scheduled for an early morning colonoscopy (from 09:00 a.m. to 10:20 a.m.) at the Hospital Clinic of Barcelona as part of the Eixample Esquerre-Barcelona colorectal cancer screening program were prospectively included in the study.
Exclusion criteria were: congestive heart failure, severe or moderate kidney failure, uncontrolled hypertension and patients who declined to participate.
The study protocol was approved by Clinical Research Ethics Committee of Hospital Clínic in Barcelona (date 12/05/2011; registered 2011/657) and the Spanish Agency for Drugs and Sanitary Products (Agencia Española del Medicamento y Productos Sanitarios: registration number: SGA-EVA-2011-01; date 19/04/11).
MethodsWritten informed consent was obtained from each subject. Participants were allocated to a bowel preparation based on a random turnover of the different preparations. For instance, each specific product and timing instruction sheet was printed in batches of 20 and consecutively given until finished. Once the consent was obtained, patients and investigators were not allowed to change the product or timing dosage allocated. The following groups were established: (1) Control group (PEG-ELS 4L): PEG 4L (Solución Evacuante Bohm®, Laboratorios Bohm S.A, Fuenlabrada, Madrid, Spain) (n=120); (2) Group AscPEG-2L: combination of PEG and ascorbic acid 2L (Moviprep®, Laboratorios Norgine B.V, Madrid, Spain) (n=120); and (3) Group PiMg: sodium picosulfate and magnesium citrate 500mL plus 2L of clear fluids (CitraFleet®, Laboratorios Casen-Fleet S.L.U, Utebo, Zaragoza, Spain) (n=120).
Bowel preparationPatients were given advice by two dedicated nurses (A.S and A.P) on how to take the bowel preparation. The specific recommendations included two days low-fiber and low-fat diet, a clear fluid diet the night before and fasting for at least 2h before the colonoscopy. All subjects from the control group and half of the subjects from the low-dose study groups took the preparation from 7:00 to 11:00 p.m., the evening prior to the colonoscopy. The other half of the patients in the low-dose study group took split-dose preparation, whereby they took the first part of the product between 9:00 and 11:00 p.m. the day before the procedure and the second part 2–4h before colonoscopy. Metoclopramide was optionally provided as an antiemetic at the beginning of the treatment in every different group.
Tolerability of bowel preparationAt inclusion, the nurses delivered a questionnaire to each participant that had to be filled in at home and submitted back at the time of the procedure. This datasheet included questions about the preparation (time of initiation and finishing, volume of water and preparation intake, days of diet, other drugs), questions about adverse effects (nausea, vomiting, abdominal pain/cramps and bloating, chest pain, headache, thirst, dizziness and dysthermia) and, two literally formulated questions that were: “If you had to repeat the colonoscopy would you be willing to take the same preparation?” “...Would you be willing to take it within the same timeframe?”. The tolerability of bowel preparation was rated on a three-point scale: easy, tolerable and difficult. Later, to simplify the analysis, we grouped “easy” or “tolerable” categories together as “well-tolerated”.
Efficacy of bowel preparationAll colonoscopies were performed between 9:00 a.m. and 10:20 a.m. during standard 40-min time slots. These were performed under spontaneous breathing sedation with propofol and remifentanil controlled by a trained nurse supervised by an anesthetist (one doctor supervises three trained nurses at a time). The explorations were not systematically video-recorded but several photos documenting the landmarks and findings were routinely performed. Endoscopists were not informed of the type of bowel preparation. The quality of bowel preparation was determined at the end of the colonoscopy by the 13 endoscopists involved in the study, according to the Boston Bowel Preparation Scale (BBPS)19: 0: unprepared colon segment with mucosa not seen due to solid stool that cannot be cleared; 1: portion of mucosa of the colon segment seen, but other areas of the colon segment not well seen due to staining, residual stool and/or opaque liquid: 2: minor amount of residual staining, small fragments of stool and/or opaque liquid, but mucosa of colon segment seen well; 3: entire mucosa of colon segment seen well with no residual staining, small fragments of stool or opaque liquid. Adequate preparation was defined as a total Boston score ≥6 with no segments<2.
Statistical analysisThe primary outcome measure was the tolerability of low-volume preparations compared to PEG-ELS 4L. A sample size calculation was performed based on recent data from a study of early morning colonoscopies18 which showed that low-volume products are tolerated in 43% of subjects in relation to 23% in the control group. Considering that there were two experimental groups, 120 individuals were needed to be included in each experimental and control group, totaling 360 subjects (significance level of 5%, power of 90%, 5% drop-outs).
Numerical data with normal distribution were analyzed by Student's t-test (or ANOVA for more than two groups) and results are presented as means and Standard Deviation (mean±SD). The non-parametric tests (Mann Whitney or Kruskal–Wallis) were applied in variables with no normal distribution and results obtained as median and percentile 25 and 75 (median [P25–P75]). Categorical data were compared using Chi-Square test or Wilcoxon Rang.
All statistical tests were two-sided, and p values<0.05 were considered as statistically significant. When multiple comparisons were performed for the primary outcome, a Bonferroni correction was applied and a p value<0.025 was necessary for significance.
As a rule, intention-to-treat analyses were performed.
Analyses were performed using SPSS statistical software, version 18.0 (SPSS Inc., Chicago, IL).
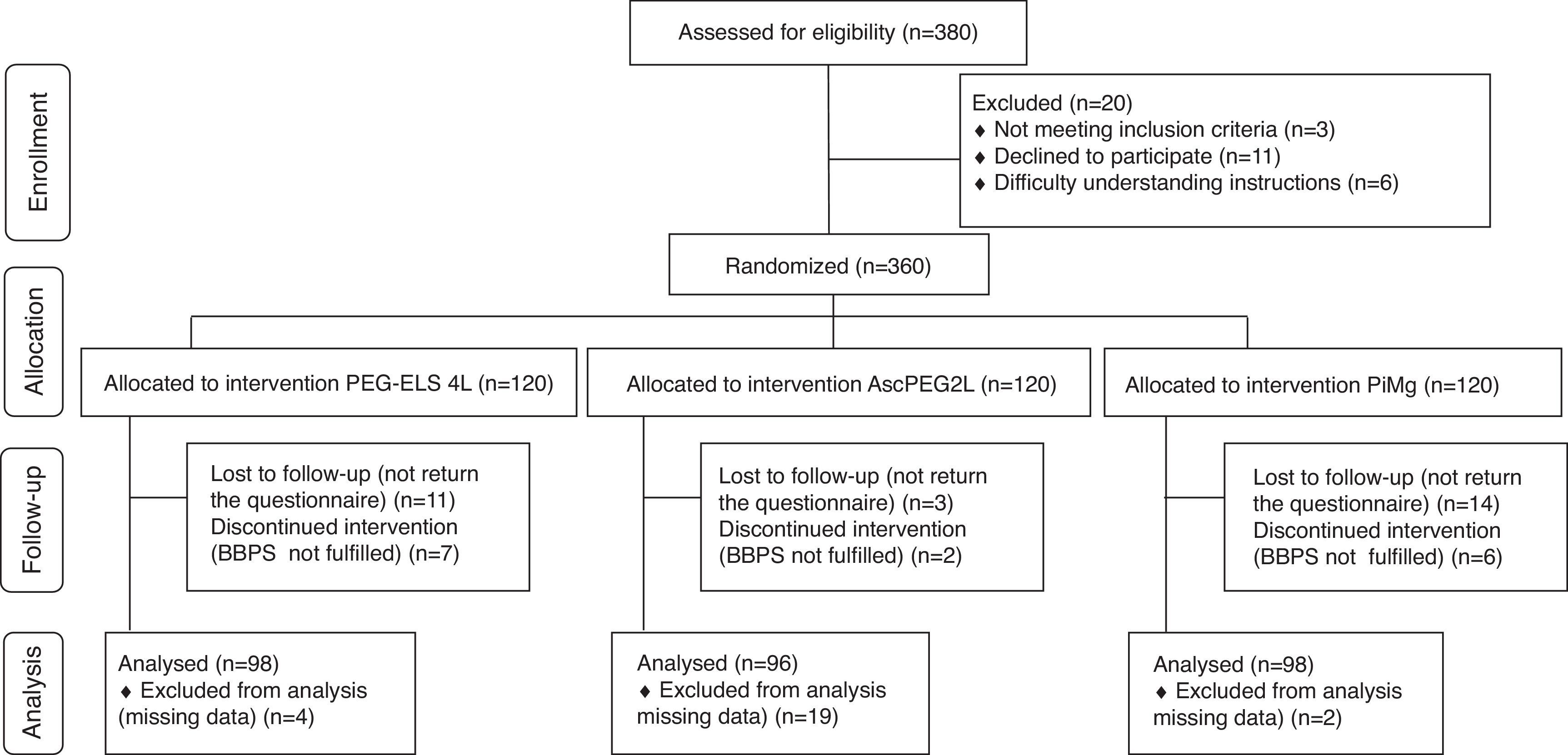
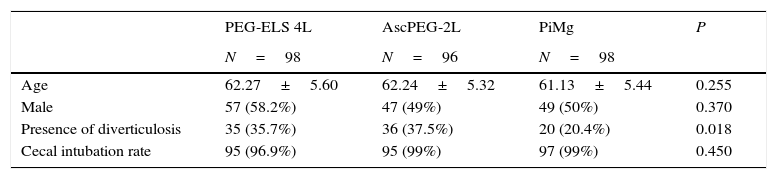
ResultsA total of 360 consecutive individuals were enrolled in the study, from which 292 subjects were included and distributed as follows: 98 in control Group PEG-ELS 4L, 96 in study group AscPEG-2L and 98 in study group PiMg (see Fig. 1). As it is shown in Table 1, demographics and baseline characteristics were similar between treatment arms. Notably, diverticulosis was less common in the PiMg group compared to the PEG-ELS 4L and AscPEG-2L groups (20% vs 36% and 37% respectively; p=0.018).
Basal patients and colonoscopy characteristics in the three groups of study.
| PEG-ELS 4L | AscPEG-2L | PiMg | P | |
|---|---|---|---|---|
| N=98 | N=96 | N=98 | ||
| Age | 62.27±5.60 | 62.24±5.32 | 61.13±5.44 | 0.255 |
| Male | 57 (58.2%) | 47 (49%) | 49 (50%) | 0.370 |
| Presence of diverticulosis | 35 (35.7%) | 36 (37.5%) | 20 (20.4%) | 0.018 |
| Cecal intubation rate | 95 (96.9%) | 95 (99%) | 97 (99%) | 0.450 |
PEG-ELS 4L – high-volume polyethylene glycol plus electrolytes solution; AscPEG-2L – low-volume polyethylene glycol plus electrolytes combined with ascorbic acid; PiMg – picosulfate sodium combined with magnesium citrate.
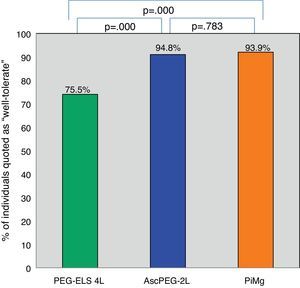
257 subjects (88.0%) described the treatment as “well-tolerated” and colonic cleansing was reported as adequate in 269 colonoscopies (92.1%). It is important to note that 17 individuals receiving PEG-ELS 4L (17.3%) did not complete the preparation because they were not able to drink the entire volume whereas in both low-volume study groups all patients completed the preparation (p<0.0001). In addition, more patients in the control group required antiemetics compared to the AscPEG-2L and PiMg groups (22.4% vs 2.1% and 8.2%, respectively; p<0.0001).
The symptoms of participants ascribed to preparation were: abdominal bloating (21.9%), dysthermia (21%), headache (17.1%), nausea (16.4%), thirst (15.1%), abdominal pain/cramps (8.9%), dizziness (6.5%), vomiting (4.5%) and thoracic pain (1%). There were no clinically significant complications related to bowel cleansing treatment or to the sedation.
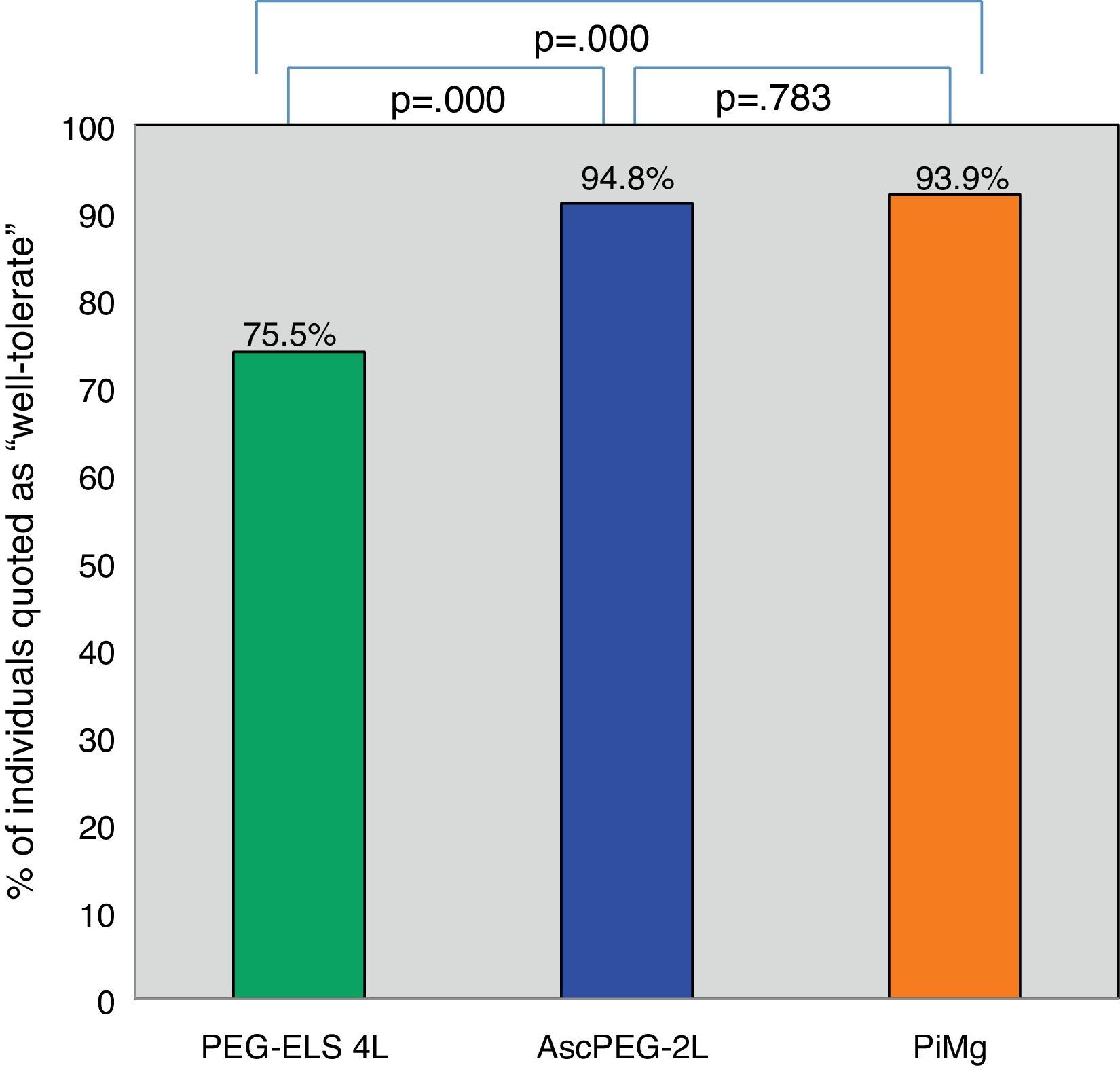
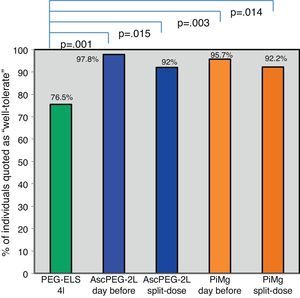
Tolerability and efficacy of low-volume solutions with respect to controlOverall, low-volume treatments were statistically significantly better tolerated than the standard solution PEG-ELS 4L (94.8% and 93.9% vs 75.5%; p<0.0001) (Fig. 2). The per-symptom analysis revealed that individuals treated with AscPEG-2L presented with less nausea, thirst and headache than those treated with PEG-ELS 4L (12.5% vs 23.5%, p=0.047; 7.3% vs 23.5%, p=0.002 and 6.2% vs 18.4%, p=0.010, respectively) whereas there were no differences in symptoms between PiMg and PEG-ELS 4L.
Tolerability of low-volume solutions with respect to control: Overall, AscPEG-2L (blue) and PiMg (orange) were statistically significantly better tolerated than the standard solution PEG-ELS 4L (green). A Mann–Whitney analysis with Bonferroni correction for multiple comparisons was applied (significant p-value<0.025). PEG-ELS 4L – high-volume polyethylene glycol plus electrolytes solution; AscPEG-2L – low-volume polyethylene glycol plus electrolytes combined with ascorbic acid; PiMg - picosulfate sodium combined with magnesium citrate.
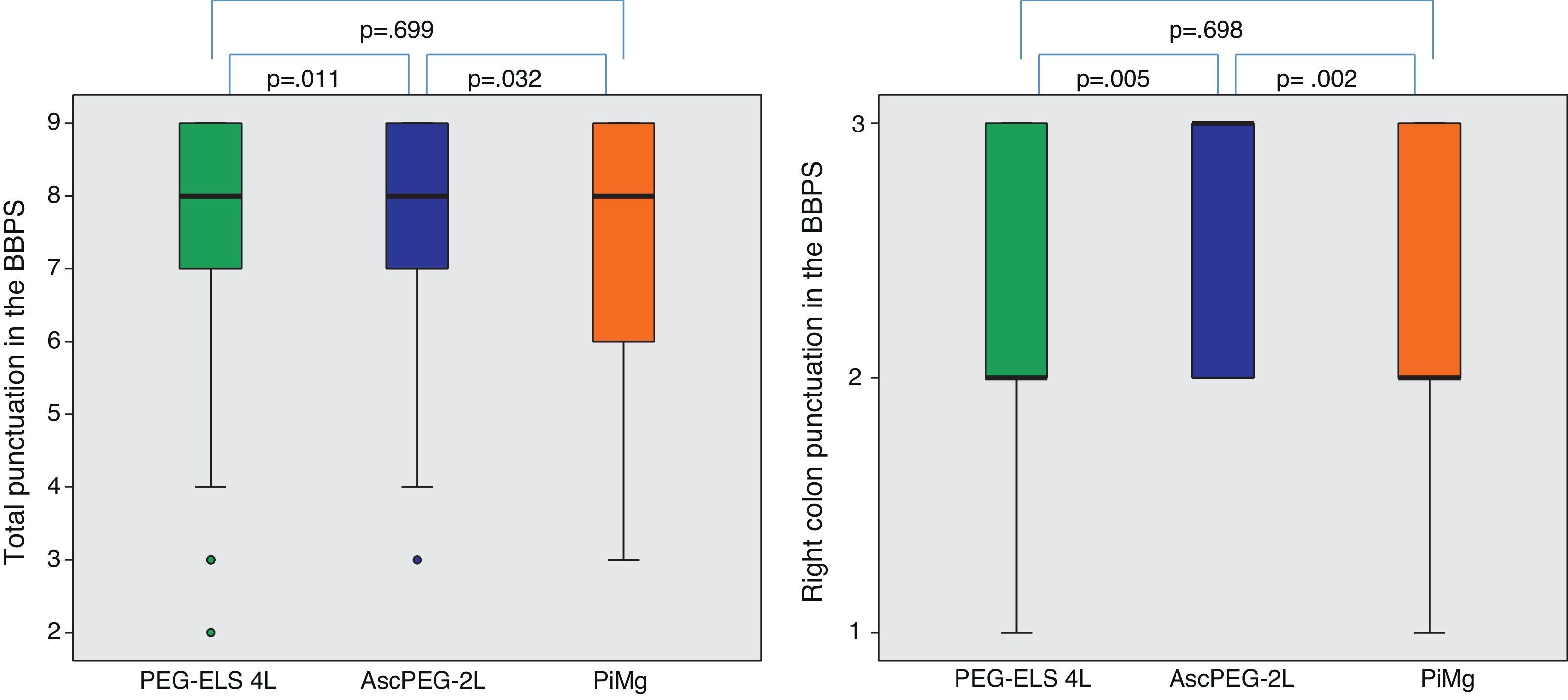
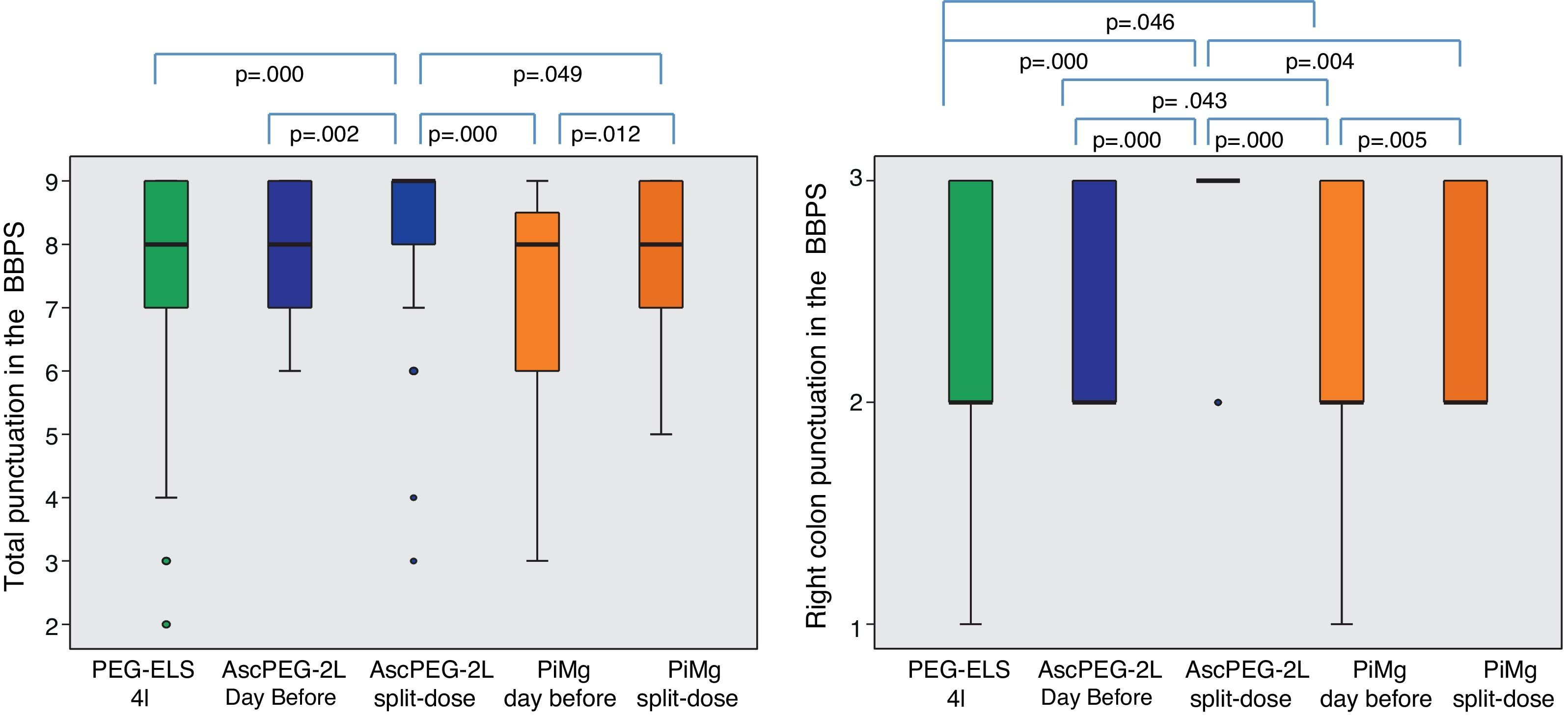
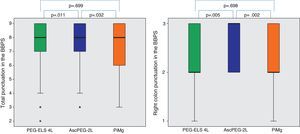
In relation to efficacy, BBPS scores were higher in the AscPEG-2L group (8 [7–9]) than in the PEG-ELS 4L control group (8 [7–9]) and PiMg group (8 [6–9]) (p=0.011 and p=0.032, respectively) (Fig. 3). Adenoma detection rate was similar in all the study arms (PEG-ELS 4L 0.63 (62/98), AscPEG-2L 0.71 (68/96) and PiMg 0.67 (66/98), p=0.532).
Efficacy of low-volume solutions with respect to control: The quality of colonic cleansing differed by preparation regimen. BBPS was better graded in the AscPEG-2L group (blue) than in the control group (green) and PiMg group (orange). These differences were more evident when looking at right colon (see right part of the figure). A Mann Whitney analysis with Bonferroni correction for multiple comparisons was applied (significant p-value<0.025). PEG-ELS 4L – high-volume polyethylene glycol plus electrolytes solution; AscPEG-2L – low-volume polyethylene glycol plus electrolytes combined with ascorbic acid; PiMg – picosulfate sodium combined with magnesium citrate; BBPS – Boston bowel preparation scale.
The per-protocol analysis depicted similar results (data not shown).
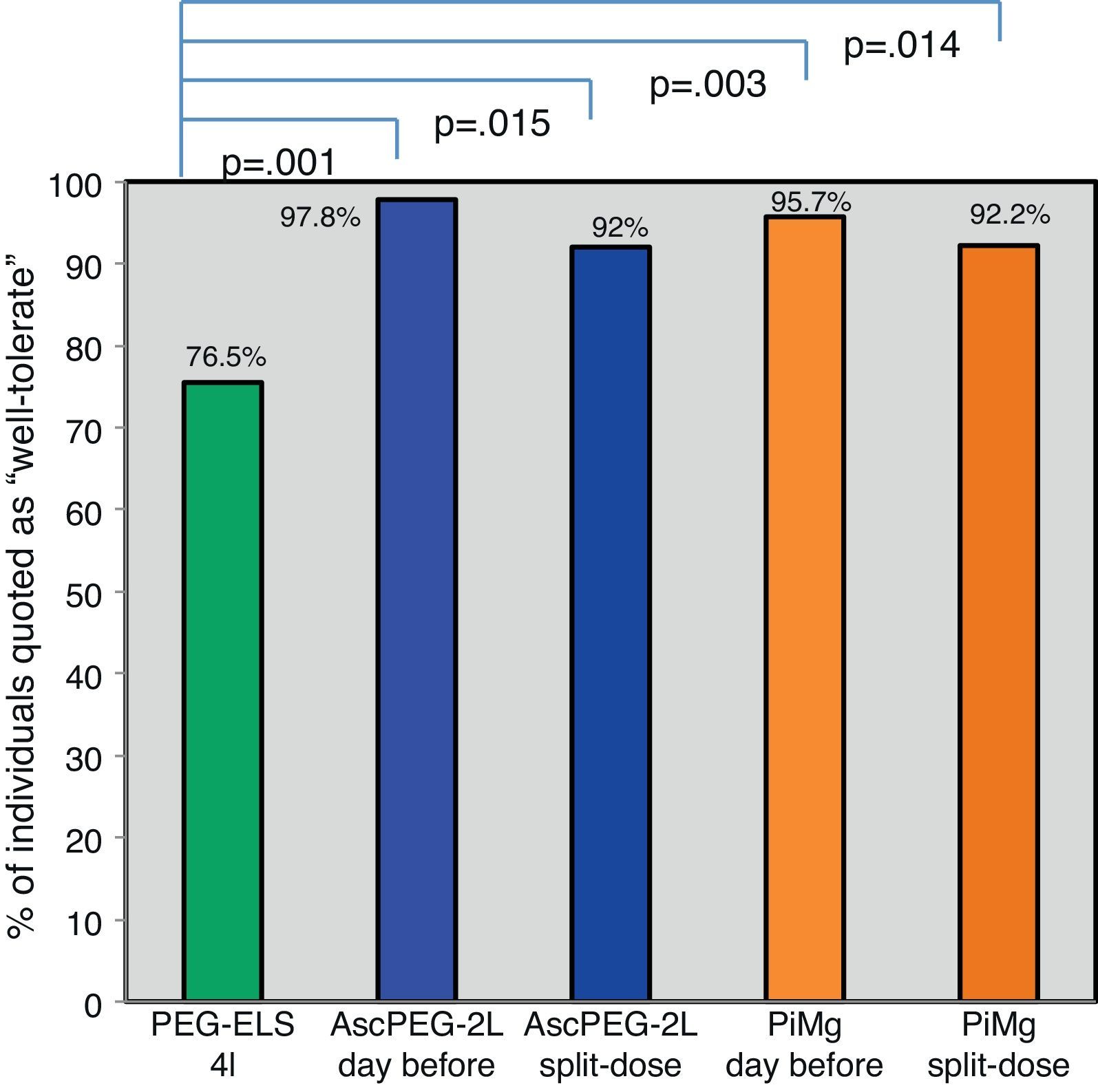
Tolerability according to dosage and productGlobally, low-volume preparations were better tolerated than PEG-ELS 4L regardless of the mode of administration (Fig. 4).
Tolerability tacking into account different products and timeframe regimens: Globally, low-volume preparations were better tolerated than PEG-ELS 4L (green) regardless of the way of administration. It seems that taking the preparation the day before (pale blue and pale orange) was better accepted than in split dose (dark blue and dark orange) but this slight difference did not reach statistical significance. PEG-ELS 4L – high-volume polyethylene glycol plus electrolytes solution; AscPEG-2L – low-volume polyethylene glycol plus electrolytes combined with ascorbic acid; PiMg – picosulfate sodium combined with magnesium citrate; BBPS – Boston bowel preparation scale.
Patient acceptance of the preparation was higher for single dose compared to split-dose administration, however this did not reach statistical significance (96.8% and 92.1% respectively p=0.158). When analyzing the answers to the question “If you had to repeat the colonoscopy would you be willing to take it within the same timeframe?”, 77% of individuals that received single dose stated that they would be willing to repeat the same dosage in the next colonoscopy compared to 46.5% in the split-dose group (p=0.001).
There were no differences in tolerability between the two low-volume solutions (AscPEG-2L 94.8% and PiMg 93.9% respectively p=0.783). However, in the per-symptom analysis, individuals treated with PiMg presented with more dizziness and headaches than patients treated with AscPEG-2L (12.2% vs 1%, p=0.002 and 26.5% vs 6.2%, p=0.000, respectively).
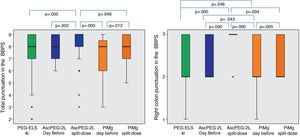
Efficacy according to dosage and productGlobally, split dosages were superior to single dosage regimes, and AscPEG-2L in split dose appeared to be the most effective. This difference was even more manifest in the right colon (Fig. 5).
Efficacy taking into account different product and timeframe regimen: globally, split dosages were superior to evening dosages (dark colors). AscPEG-2L in split dose (dark blue) appeared to be the most effective, this difference being even more manifest in right colon (see right part of the figure). PEG-ELS 4L – high-volume polyethylene glycol plus electrolytes solution; AscPEG-2L – low-volume polyethylene glycol plus electrolytes combined with ascorbic acid; PiMg – picosulfate sodium combined with magnesium citrate BBPS – Boston bowel preparation scale.
With respect to dosage, individuals who had a split-dose treatment had higher BBPS scores (9 [8–9] vs 8 [7–9]; p<0.0001). This difference appeared even clearer when targeting the right colon (3 [2–3] vs 2 [2–3]; p<0.0001).
AscPEG-2L was more effective at bowel cleansing than PiMg (BBPS 8 [7–9] vs 8 [6–9], p=0.032), which was especially evident in right colon (BBPS 3 [2–3] vs 2 [2–3], p=0.002).
DiscussionThis trial demonstrates that in early morning colonoscopies, ascorbic acid with PEG-ELS solution appears to be the best option in regard to both tolerability and efficacy, especially when administered in split-dose. The study was directed to an especially challenging group of individuals: the ones scheduled to have a colonoscopy between 9a.m. and 10:20a.m. However, although no direct conclusions can be driven for other time frames, the multiple branches of the study, and the obvious differences between groups, allow inferring that these results could be generalized to any other time frame.
Colorectal cancer screening programs are the best opportunity to achieve the goal of decreasing CRC mortality.20 However, the success of the program is dependent on subjects’ participation21,22 and achieving colonoscopy quality indicators such as adequate bowel cleansing.1–3 Bowel preparation is therefore critically important as it must effectively clean the bowel, but also be acceptable to patients. Recent data demonstrates that split-dose PEG-ELS 4L is the gold standard based on its efficacy and safety profile.23 Nonetheless, tolerability of high volume and taste of PEG-ELS can result in an unacceptable non-compliance rate. Although a previous study has suggested that after a proper explanation about 80% of patients would be willing to wake up early,24 it remains obvious that patients scheduled for an early morning procedure would prefer not to have to wake to drink a large volume of unpalatable fluid. In fact, with the widespread use of anesthesia improving procedural tolerability, bowel cleansing remains the most cited influence on patients’ acceptance and willingness to undergo colonoscopy.25 In the context of colorectal cancer screening programs, the impact of poor preparation is of utmost significance since a patient with a positive fecal occult blood test and normal colonoscopy will be considered as low risk and not be scheduled for colonoscopy for a further 10 years.22 Discomfort and the inconvenience of bowel preparation may deter compliance with colonoscopy which also carries important consequences.22,26 Taking into account these considerations, a cleansing agent that optimizes efficacy and tolerability is critical.
Alternative bowel cleansing agents to PEG have been previously limited by several concerns. Sodium phosphate, despite demonstrating equivalent efficacy to PEG-ELS,8,9 is no longer recommended due to the risk of potentially fatal electrolyte disturbances and irreversible renal damage from nephrocalcinosis (acute phosphate nephropathy).27 A number of small studies have failed to demonstrate a significant improvement in the quality of bowel cleansing with the addition of prokinetics (metoclopramide, bisacodyl, lubiprostone) to PEG or NaP.13 Moreover, the FDA has raised concerns around epithelial proliferation and ischemic colitis with high-dose bisacodyl.9 Consequently, the present study is the first study comparing both the tolerability and efficacy of commonly used preparations endorsed by experts and scientific societies.
Recent studies have shown that low-volume solutions such as ascorbic acid added to PEG-ELS and sodium picosulfate combined with magnesium citrate are better tolerated and are as effective as PEG-ELS 4L.12,14,15 Despite promising results, the majority of data is drawn from non-inferiority studies inadequately powered to demonstrate equivalence. Moreover, while the importance of colonic cleansing in screening programs is paramount, no studies were performed in a screening population. Rather participants were symptomatic patients or those seeking opportunistic screening excluding individuals with unfavorable comorbidity such as chronic constipation. The use of validated scales to evaluate the quality of colon cleansing was not widespread. Finally and most importantly, none of these studies directly compared the two low-volume products.
In the present study bowel preparation quality was assessed using the BBPS.19 This scale is well validated and assesses bowel preparation after all cleansing methods have been attempted (i.e., washing and suctioning). As preparations given in split doses tend to accumulate more fluid due to less time for the preparation to pass through the body, we consider use of a score that permits cleansing assessment vital. We propose that the BBPS is the most appropriate validated assessment scale for the study of split-dose bowel cleansing as it translates what is really meaningful, that is the ultimate view of the mucosa. Additionally the BBPS permits assessment of individual colonic segments which is of major interest given recent studies highlighting limited CRC protection in the proximal colon compared to the distal colon with multiple factors hypothesized as important including preparation.26,28 The current study showed that ascPEG-2L in split dose achieves superior cleansing of the right colon (see Fig. 4). A difference, which persisted with per-protocol analysis (see supplementary data Fig. 4 enclosed). Indeed, only 1 of the 17 individuals that did not complete the full treatment with PEG-4L presented an inadequate bowel preparation, thus, highlighting the superiority of ascPEG-2L as an effective bowel cleansing, per se.
Tolerability was assessed with a non-validated questionnaire as has been the case in other published trials.12,14,15 It is established that low-volume preparations are better tolerated15 and this point is reproduced and emphasized in our study, where PEG-ELS 4L was not tolerated in 25% of individuals, 22.4% required antiemetics and 17% did not complete all of the preparation. Contrarily, in the low-volume groups 93% found the preparation tolerable and all were compliant. Interestingly, AscPEG-2L was associated with a minor presence of symptoms than the other two preparations. Although overall tolerance was not statistically different, our results showed that screening participants preferred the evening single dosage to split-dose preparation. This may reflect reluctance to wake early as well as concerns of continence on the way to hospital.
The limitations of this study are that the sample size calculation was not performed to calculate differences in adenoma detection rate, a more clinically meaningful outcome measure. Additionally, when the study protocol was built, anesthesiologists were still reluctant to sedate patients with a large amount of fluid intake in the previous 2h. This did not allow us to include the split-dose option in the control group. Finally, treatments were not randomly assigned since there was no financial help to undertake the compulsory external monitoring required for pharmaceutical randomized controlled trials. However, product assignment was carried out in a way that neither the investigators nor the participants could choose the treatment.
In conclusion, the current study demonstrates that for early-morning colonoscopy, low-volume preparations are better tolerated than PEG-ELS 4L and more effective. Patient acceptance of the preparation was higher for single dose compared to split-dose administration, however split dosages were superior to the rest in terms of efficacy. Overall, ascorbic acid with PEG solution appears to be the best option considering both tolerability and efficacy, especially when administered in split-dose.
Financial supportNone.
Conflict of interestsCristina Rodríguez de Miguel is a research nurse supported by Olympus Medical Systems, Europe. Maria Pellisé is a consultant of Norgine, Spain.
Abiguei Torrent for her statistical assessment. Cristina Hernández for making available the data for ADR calculation. Nicholas Burgess and Nicholas Tutticci for their help with the language editing.
PROCOLON is the research group of the Barcelona's Colorectal Cancer Screening Programa, and it is currently constituted by the following members:
Cristina Álvarez, Montserrat Andreu, Josep M. Augé, Francesc Balaguer, Mercè Barau, Xavier Bessa, Felipe Bory, Andrea Burón, Antoni Castells, Xavier Castells, Mercè Comas, Rosa Costa, Míriam Cuatrecasas, Maria Estrada, Imma Garrell, Jaume Grau, Rafael Guayta, Cristina Hernández, Mar Iglesias, María López-Cerón, Francesc Macià, Leticia Moreira, Teresa Ocaña, Maria Pellisé, Mercè Pintanell, Mercè Piracés, Sandra Polbach, Àngels Pozo, Cristina Rodríguez, Maria Sala, Agustín Seoane, Anna Serradesanferm, Judith Sivilla, and Antoni Trilla.