Breast cancer consists of a heterogeneous group of tumors with different features, biology and treatments. Cancer stem cells (CSCs) have been associated with an aggressive cellular behavior, resistance to chemotherapy and radiotherapy in many types of neoplasms, and a strong correlation between prominin-1 (CD133) expression in cancer stem cells from different types of cancer exist. A discussion is presented on recent immunotherapeutic strategies that target CD133 in breast CSCs. Furthermore, it is suggested that immunotherapy targeting CD133 breast CSCs and/or in combination with other current treatments result in a better outcome.
La neoplasia de mama consiste en un grupo heterogéneo de tumores con características, biología y tratamientos diferentes. Las células madre del cáncer se han asociado con un comportamiento agresivo celular, resistencia a la quimioterapia y la radioterapia en muchos tipos de tumores y existe una fuerte correlación en la expresión de prominin-1 (CD133) en las células madre de cáncer de diferentes tipos de cáncer. En este artículo se discuten las estrategias recientes de inmunoterapia que se dirigen a CD133 de las células madre cancerosas de mama. Además, mostramos que la inmunoterapia dirigida a CD133 y/o en combinación con otros tratamientos actuales podría tener un mejor resultado.
Despite considerable advances in early detection, diagnosis, and treatment, breast cancer (BC) is the most frequently diagnosed cancer in women.1 To date, there are many therapies that in its majority are non-specific toward cells that cause cancer, such as cancer stem cells. Considering that cancer cells escape to our immune detection, the new idea is to try to use the weapons of the immune system against these cancer cells. Immunotherapy is a fast advancing methodology involving one of two approaches: (1) Stimulating our own immune system to work harder or smarter to attack cancer cells and (2) Giving you immune system components, such as man-made immune system proteins.2 Breast cancers express multiple putative tumor-associated antigens, such as human epidermal growth factor receptor 2 (HER-2) and Mucin 1 (MUC1), which have been the successful focus of vaccine development over the past decade, translating into tumor-specific immune responses and, in some cases, clinical benefit. These successes observed with novel immunotherapeutic strategies, such as immune checkpoint blockade and adoptive T-cell therapies in other malignancies, combined with other strategies have the potential to revolutionize the treatment of breast cancer, specially targeting cancer stem cells.1,3,4
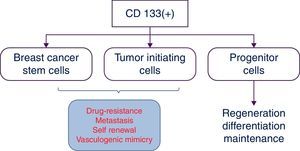
CD133 or prominin-1, a five transmembrane domain cell-surface glycoprotein, initially associated with cholesterol and later described as a specific biomarker to select human hematopoietic progenitor cells. CD133 is recognized as an important biomarker to identify and isolate the specific cell subpopulation named “cancer stem cells” in many types of neoplasms including breast cancer. CD133+ cells have stemness properties such as drug-resistance, self-renewal, differentiation ability; high proliferation (Fig. 1) and they are able also to form tumors in xenografts. These cells with CD133+ are more resistant to radiation and standard chemotherapy than CD133(−) cells.5,6 In this review we discussed CD133 in breast cancer stem cells and the current advances as a target of immunotherapy. Furthermore, we considered the possible combination of other anticancer therapies for a better outcome.
CD133 in triple-negative breast cancerTriple-negative breast cancer (TNBC) to date is the breast neoplasia with highest risk, primarily affecting young women. Defined on the basis of immunohistochemistry, negative for estrogen receptor (ER), progesterone receptor (PR) and HER2 (member of the epidermal growth factor receptor), it represent approximately 20% of all breast tumors with a considerable clinical relevance due to be resistant to conventional chemotherapy, poor prognosis and a significantly worse clinical outcome than other cancers.7,8 Cantile et al.9 suggest that this poor prognosis is probably due to a nuclear mislocalization of CD133, which normally shows membrane localization and more sporadically cytoplasmic localization. Furthermore as it is known that surface molecules, when are moving into the nucleus, can act as transcriptional regulators by interfering with molecular pathways directly connected to the proliferation and differentiation of tumor cells.
Low expression of CD133 characterizes cells with larger adhesion area, lower proliferation rate and reduced migration speed, indicative of a less undifferentiated phenotype. Conversely, when compared with high CD133 expression, the cells show higher invasive capability and increased expression of proteins involved in metastasis and drug-resistance of breast tumors.10,11 For example, expression of signaling protein known as phospholipase Cβ2 (PLC-β2) correlates with the levels of CD133 and has a role in inducing the CD133high cells to CD133low cells conversion. This mean that, in TNBC cells, the de-regulation of PLC-β2 is responsible of the switch from an early to a mature tumoral phenotype also by reducing the expression of CD133.7 However, Twist1 (transcription factor), which induced by hypoxia accelerate vasculogenic mimicry by increasing population of CD133(+) cells is responsible for the regrowth of TNBCs.12
Epigenetic changes (DNA methylation, acetylation, chromatin modification, microRNA, etc.) also have a correlation with CD133 in breast cancer stem cells (BCSCs) in TNBC, implicated in the progression and recurrence. Methylation regulates specific BCSC-related genes [CD44, CD133, CD24, MSH1 (Musashi-1), and ALDH1], epigenetic profile that can identify aggressive subtypes, such as TNBC.13
The primo vascular system (PVS)The PVSs consider is distributed throughout the entire body. The system is composed of nodes storing many small cells and thin vessels branching out from the nodes. Inside the vessel there are multiple sub vessels. The PVS is found in and on most organs, including the brain, and interestingly inside some lymph and blood vessels. The PVS is normally difficult to visualize due to its semitransparent optical property and its small size. The diameter of primo vessels (PVs) is in the range of 20–50μm and the size of a primo node (PN), 100–1000μm. Its outermost layer is more porous than that of blood or lymph capillary vessels, and the nuclei of the PVS endothelial cells are rod shaped. Inside the fluid of PVS, there are cells presenting stem cell markers CD133, Oct4, and Nanog, which may imply that this system has a role in regeneration and potential relevance to breast cancer. According to results from an animal study using xenografts of various cancer types (lung, ovarian, skin, gastric cancer, and leukemia), as the tumor grows, the PVS is formed in a high density in the vicinity of the tumor. In addition, it was shown that PVs connect the primary and secondary tumors and that cancer cells were transported via the PVs in an active manner.2
Breast cancer stem cellsRecently has emerged the concept of tumor control probability (TCP), formalism derived to compare various treatment regimens of radiation therapy, defined as the probability that given a prescribed dose of radiation, a tumor has been eradicated or controlled. In the traditional view of cancer, all cells share the ability to divide without limit and thus have the potential to generate a malignant tumor. However, a different approach arise considering a sub-population of cells, the so-called cancer stem cells (CSCs), responsible for the initiation and maintenance of the tumor. A key implication of the CSC hypothesis is that these cells must be eradicated to achieve cures, thus is possible to define TCPs as the probability of eradicating CSCs for a given dose of radiation. A proteins expression profile, such as CD44high/CD24low/CD133(+), is often used as a biomarker to monitor CSCs enrichment. However, it is increasingly recognized that not all cells bearing this expression profile are necessarily CSCs, and in particular early generations of progenitor cells may share the same phenotype.14,15 We need to take in account that CD133 expression is heterogeneous in different carcinomas but was strikingly hyperexpressed in a tubulolobular variant of breast cancer, generally with a good prognosis.16 Moreover, CD133 along with epithelial mesenchymal transition proteins and N-cadherin it could be explained the metastatic events in breast cancer.17,18
The traditional view of cancer asserts that all cells in a malignant tumor are clonogenic, with genetic and epigenetic differences. The existence of CSCs has been firmly identified in leukaemia19 and more recently in many solid tumors including breast cancer.20 As few as ∼200 cells of this CSCs populations are capable of generate tumors in animals, whereas the bulk of the tumor population is tumorigenic only when implanted at high numbers. Like their normal counterparts, the cancer stem cells have the ability to self-renew, driving tumorigenicity and possibly recurrence and metastasis, and have the ability to differentiate, generating the heterogeneity of the tumors. In these cells, cell surface and transmembrane proteins such as CD44, CD47, CD123, EpCAM (CD326), CD133, IGF receptor I, and proteins of the Notch and Wnt signaling pathways are expressed.21,22 The presence of many of these proteins determine the heterogeneity of CSCs, a critical factor for use customized therapies. Mukhopadhyay et al.23 studied the proportion of breast cancer cells expressing CD44(high) CD24(low/neg), ALDH1(+), CD49f (high), CD133(high), and CD34(high) differed, suggesting heterogeneity. However CSCs from breast cancer expressed only CD133 in high proportion in comparison to the other proteins.
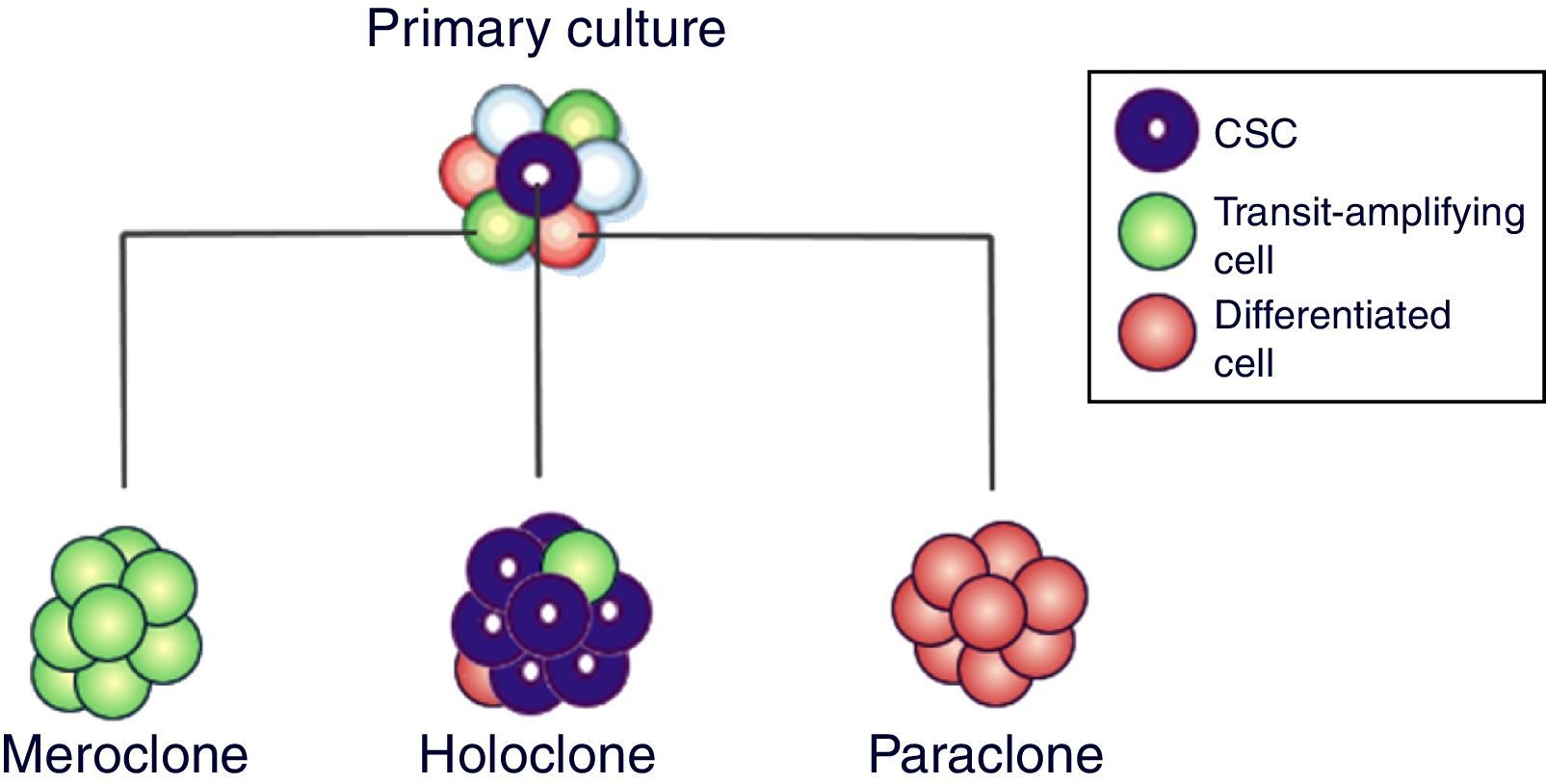
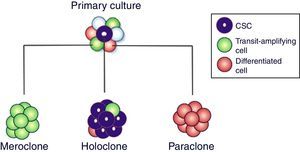
When primary cultures of normal cells are cloned, three types of colony grow, called holoclones (derived from stem cells), meroclones (transit-amplifying cells) and paraclones (differentiated cells)10 (Fig. 2). Holoclone cells are capable of forming more colonies on soft agar than meroclone cells and paraclone cells, suggesting that holoclone cells had higher self-renew potential and might harbors cancer stem cells (CSCs) subpopulation. Strikingly, holoclone display CD133(+) phenotype and formed vascular malformations (VM). In addition, holoclone acquire endothelial cell marker vascular endothelial-cadherin expression and upregulated matrix metalloproteinase (MMP)-2 and MMP-9 expression. The subpopulation with holoclone morphology, CD133(+) phenotype and CSCs characteristics might have the capacity of transdifferentiation and contributed to VM in triple negative breast cancer.10,11
Current models of stem cell biology assume that normal and neoplastic stem cells reside at the apices of hierarchies and differentiate into non-stem progeny in a unidirectional manner. Chaffer et al.24 reported a subpopulation of basal-like human mammary epithelial cells that spontaneously could converted into stem-like cells. Moreover, oncogenic transformation enhances the spontaneous conversion, so that nonstem cancer cells give rise to cancer stem cell (CSC)-like cells in vitro and in vivo. These findings demonstrate that normal and CSC-like cells can arise de novo from more differentiated cell types and that hierarchical models of mammary stem cell biology should encompass bidirectional interconversions between stem and nonstem compartments.
Contradictory, others investigations demonstrated that also CD133(−) cells can show the same characteristics of those positive for CD133+. Hence, some inconsistencies among published data on CD133 function can be ascribed to different causes questioning the main role as specific marker of cancer stem cells.24 Indeed, it is still a matter of debate whether CD133+ cells truly represent the ultimate tumorigenic population. However, the belief that CD133 may act as a universal marker of CSCs has been met with a high degree of controversy in the research community.6 On the basis of the involvement of CSCs in tumorigenesis and treatment resistance, it is conceivable that only eradication of CSCs can lead to a cancer cure.24,25
Recently, Shi et al.25 purified exosomes (family of bioactive vesicles that are secreted from various types of cell, including tumor cells) in 4T1 mouse breast cancer cells and mouse mammary gland epithelial cells with phenotype CD133(+). Exosomes derived from breast cancer cells have been demonstrated to perform important functions in tumor progression in vitro and in vivo. Exosome uptake by CD133+ and CD133-4T1 cells indicates that the proliferation of CD133+ cells increase and the apoptosis is suppressed.
Malignant papillary lesions are rare malignant tumors in the breast. Differentiation between benign or atypical and malignant papillary lesions is difficult. Accordingly to Lin et al.26 while the expression of CD133 in papillary carcinomas is significantly lower than in benign and atypical papillomas, CD133 expression in invasive carcinoma is significantly higher than that in papillary carcinomas. In infiltrating ductal carcinoma (IDC) tissues, CD133, CD44, and CD82 have positive relationship in this carcinoma especially in its expression in CSCs.27
Targeting breast cancer stem cells may improve cancer therapy. To immunologically target CSC phenotypes, innate immune responses to CSCs have been reported using Natural killer cells and γδ T cells. To target CSC specifically, in vitro CSC-primed T cells, CSC-based dendritic cell vaccine have demonstrated significant induction of anti-CSC immunity both in vivo in immunocompetent hosts and in vitro as evident by CSC reactivity of CSC vaccine-primed antibodies and T cells.28 In addition, CD133 along with ALDH,29 CD44 and HER2 have served as markers to isolate CSCs from a number of tumor types in animal models and human tumors. Therefore targeting CD133, others markers and elements involved in the CSC niche (myeloid-derived suppressor cells, and cytokines, immune checkpoint-PD1/PDL1)30 may provide additional novel strategies to enhance the immunological targeting of CSCs.28,31
Diagnostic approachBeyond its possible correlation with stemness of tumor cells, CD133 is considered as an important biomarker in breast cancer, since it correlates with tumor size, metastasis and clinical stage, and it could be used in diagnosis. Breast tumor cells have often already been disseminated from the primary site and can be detected in the bone marrow, where hematopoietic progenitor cells (HPCs) are also resident.32,33
In ductal breast carcinomas, epithelial specific antigen (ESA), one of the breast CSC markers, is an indicator of tumor recurrence, while GPR30 is associated with hormone receptors. Despite the correlation between GPR30 and the nuclear estrogen receptor, the expression in many patients is dependent.34 However, currently CD133 and Her-1 is reported as important markers of CSCs for the prognosis of triple-negative breast cancer. The expression of CD133 with Her-1 corresponded to tumor size, clinical stage and lymphatic metastasis, but not to age and histological grade.35
Several studies have suggested that Geminin expression is a marker of the S/G2/M phase of the cell cycle.36 Geminin is frequently overexpressed, in vivo, in a variety of human tumors (kidney, colon, breast, lung cancer and lymphoma) and its expression rises with increasing tumor grade, which correlates to a poor prognosis.37 Therefore, CD133 expression is associated to high Geminin expression. This association between the expression of CD133 and Geminin will indicate the molecular stratification of breast tumors and in particular triple-negative breast cancers.38
In addition to tests of self-renewal, migration and vasculogenic mimicry, potentially involved in generation of circulating tumor cells (CTCs), CD133 expression in CTCs of nometastatic breast cancer (BC) patients is another option for diagnosis in early stages of the disease. Nadal et al.39 isolated CTCs by immunomagnetic techniques using magnetic beads. They identified CTCs positive to CD133 in 65% of patients at baseline and 47.8% after systemic therapy. Before any treatment, CTCs positive to CD133 are more frequently isolated in patients with luminal BC subtype. No statistically significant differences are found between proportion of, CTCs positive to CD133 and BC subtypes after systemic therapy, implying a relative enrichment of CTCs CD133(+) in triple negative and HER2-amplified tumors. While CTCs decreases after chemotherapy when analyzing the population CTCs positive to CD133 is enriched in post-treatment samples in non-luminal BC subtypes. Therefore CD133 has a potential role as a promising marker of chemoresistance in non-luminal BC patients.
The pathologic complete response (pCR) rate with neoadjuvant chemotherapy remains at 30%. CD133 before neoadjuvant chemotherapy might be a useful marker for predicting the effectiveness of this care and recurrence of breast cancer after chemotherapy.40 Oct-4 with CD133 is also observed in tumors obtained after neoadjuvant chemotherapy. Moreover, the breast CSCs profile CD44(+)/CD24(−/low) and CD133(+) are more frequently observed in hypoxic regions of tumor, whereas ALDH-1(+) cells more commonly co-localized to tumors with high microvessel density. Also, the CD326(−)CD45(−) fraction of patients with elevated SNAIL1 (involved in embryonic mesoderm formation) and ZEB1 (zinc finger E-box-binding homeobox 1) transcripts had a higher percentage of ALDH(+)/CD133(+) cells in their blood than patients with normal SNAIL1 and ZEB1 expression.41
Immunotherapeutic strategyMany common cancer therapies such as chemotherapy fail to eliminate completely CSCs, leading to cancer recurrence and progression, selective targeting of CSCs with monoclonal antibodies represents a novel therapeutic strategy against cancer. Monoclonal antibodies are used against CSCs proteins21 showing efficacy reducing cancer in mice, and some of them have demonstrated antitumor activity in clinical settings. For example, DDX3X (DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 3, X-linked) is suggested as an immunogenic protein preferentially expressed in CD133(+) breast tumor cells. Vaccination with DDX3X primed specific T cells, resulting in protective and therapeutic antitumor immunity. The DDX3X-primed CD4(+) T cells produced CD133(+) tumor-specific IFNγ and IL-17 mediating potent antitumor therapeutic efficacy.42
Although antibodies against CD133 are commercially available, there are many hurdles. The most widely used anti-CD133 monoclonal antibodies recognize what was initially thought to be poorly-defined glycosylated epitopes,43 but more recently reported to be non-glycosylated epitopes that are lost during differentiation, perhaps due to epitope masking.44 In either case, these antibodies do not detect cells expressing certain post-translationally modified CD133 epitopes and therefore cannot be used for such purposes. Second, commercially available anti-CD133 antibodies that target an unmodified CD133 epitope are often polyclonal. Third, most of the currently available antibodies are only suitable for use in limited biological assays. However, Swaminathan et al.45 have generated a novel anti-CD133 monoclonal antibody, using a recombinant protein consisting of highly immunogenic amino acid residues selected from the native CD133 protein as an immunogenic that specifically recognizes a non-glycosylated epitope of CD133 and is useful in multiple biological assays.
To eliminate cells with phenotypic markers of CSC-like, Mine et al.46 characterized cell populations with the breast luminal CSC phenotype [epithelial specific antigen(+) (ESA) CD44(hi) CD24(lo), CD44(hi) CD133(+), and CD133(+) CD24(lo)]. Once characterized it, they targeted with cytotoxic T lymphocytes (CTL), Numb and Notch, under the hypothesis that both antagonistic proteins prevent the metastases in patients whose tumors are resistant to conventional treatments.
Recently Huang et al.47 generated an anti-CD3/anti-CD133 bispecific antibody (BsAb) and bound it to the cytokine-induced killer (CIK) cells as effector cells (BsAb-CIK) to target CD133(high) CSCs. The killing of CD133(high) cancer cells by the BsAb-CIK cells was significantly higher than the killing by the parental CIK or by CIK cells bound with anti-CD3 (CD3-CIK) without CD133 targeting. Therefore, a similar investigation could be made in breast cancer stem cells positive to CD133.
Immunotherapy in combination with other therapeutic strategiesMonoclonal antibodies are very specific to their targets and have a relatively short lifespan inside the organism. This limits the undesirable side effects, while potentiating the anti-cancer capabilities of the therapy. Unfortunately, while this means that monoclonal antibody immunotherapy is considerably safer than other forms of anti-cancer therapy – namely small molecules – it is precisely due to their short lifespan that the efficacy of the treatment is limited. This drawback might be overcome through the simultaneously use of monoclonal antibodies paired up with chemotherapy48 (Fig. 3).
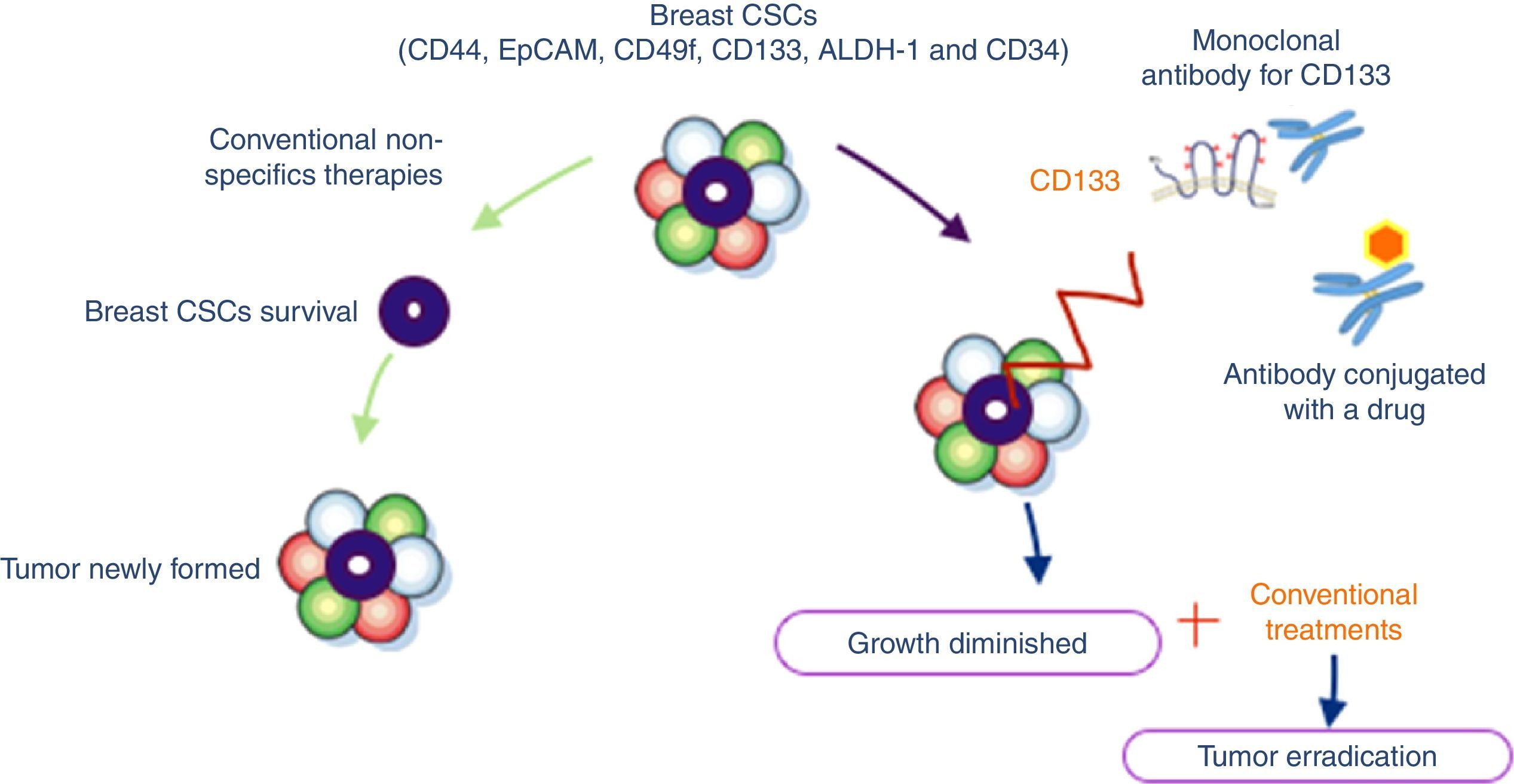
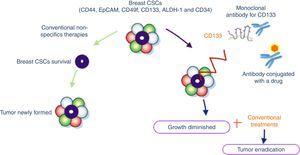
Breast cancer stem cells with specifics antigens: CD44, EpCAM (epithelial cell adhesion molecule), CD49f, ALDH-1 (aldehyde dehydrogenase), CD34 and CD133. Conventional non-specifics therapies allow newly tumor formation due to they do not attack cells responsible for tumor survival (CSCs). Accordingly to literature CD133 could be used as a target for immunotherapy giving tumor growth diminished and possibly upon combine conventional treatments we could obtain better outcomes in clinical settings.
Relying on immunotherapy studies and other types of treatments, the combination of different treatment could be successful against breast cancer. For example, the chemotherapy drug, Paclitaxel (microtubule-stabilizing anticancer agent) is used for the treatment of cancer but not is very effective in decreasing the TIC population. However polymeric nanoparticles targeting CD133 by conjugating an anti-CD133 monoclonal antibody to nanoparticles (CD133NPs) loaded with paclitaxel formulated using poly (d, l lactide-co-glycolide) polymer has demonstrated good results. These CD133-targeted nanoparticles are efficiently internalized by cancer cells, which abundantly express CD133 (>9-fold higher uptake than non-targeted control nanoparticles). CD133NPs also decreased effectively the amount of mammospheres and colonies formed.49 Along with NPB304, a novel derivative of Sinenxan A, significantly might sensitize resistant breast cancer cells to paclitaxel.50
Circulating endothelial cells (CECs) as well as bone-marrow-derived endothelial precursor cells (EPC) play an important role in neovascularization and tumors growth. Fürstenberger et al.51 found that CECs are significantly elevated in breast cancer patients and decreased during chemotherapy, whereas EPC (CD34+/VEGFR-2+) as well as their progenitor cell population CD133+/CD34+ and the population of CD34+ stem cells increased. Concomitantly with the increase of progenitor cells an increase of VEGF, erythropoietin and angiopoietin-2 is observed. Chemotherapy can only reduce the amounts of mature CEC, probably reflecting detached cells from tumor vessels, whereas the EPC and their progenitors are mobilized by chemotherapy. Since this mobilization of EPC may contribute to tumor neovascularization an early antiangiogenic therapy in combination with chemotherapy could be beneficial for the success of cancer therapy.
Given their intrinsic ability to home to tumor sites, endothelial progenitor cells (EPCs) are attractive as cellular vehicles for targeted cancer gene therapy. However, collecting sufficient EPCs is one of the challenging issues critical for effective clinical translation of this new approach. Purwanti et al.52 used an embryoid body formation method to derive CD133+CD34+ EPCs from human iPS cells. The generated EPCs expressed endothelial markers such as CD31, Flk1, and vascular endothelial-cadherin without expression of the CD45 hematopoietic marker. After intravenous injection, the iPS cell-derived EPCs migrated toward orthotopic and lung metastatic tumors in the mouse 4T1 breast cancer model but did not promote tumor growth and metastasis. The systemic injection of the CD40 ligand-expressing EPCs stimulated the secretion of both tumor necrosis factor-α and interferon-γ and increased the caspase 3/7 activity in the lungs with metastatic tumors, leading to prolonged survival of the tumor bearing mice.
Sodium butyrate (NaBu) is regarded as a potential reagent for cancer therapy. 40% of the NaBu resistant cells express the cancer stem cells marker, the CD133, whereas only 10% intact cells present the CD133 antigen. Furthermore, the endogenous expressing c-MET contributes to the survival of cancer stem ion from the treatment of NaBu. The CD133+ group also presents a higher level of c-MET. A combination treatment of MET siRNA and NaBu efficiently prohibited the breast cancer progression, and the incident rate of the tumor decreased to 18%.53
A major challenge when targeting CD133-expressing CSCs is to prevent depletion of the normal stem cell pool. Photochemical internalization (PCI) is a drug delivery technology for local, light-controlled cytosolic release of drugs entrapped in endosomes and lysosomes. It includes drugs that are too large or too hydrophilic to penetrate the cell membrane.54 PCI of antibody–drug conjugates such as immunotoxins provides high selectivity against specific receptors up-regulated in cancer and have been used as model drugs for the development of the PCI method since they are sequestered in endo/lysosomal vesicles. Immunotoxins based on type 1 ribosome-inactivating protein toxins, such as saporin and gelonin, are taken up by receptor-mediated endocytosis. Thus, combining CD133-targeting therapeutics with the PCI technology, where light-activation of the drug is constrained to the tumor, should be particularly beneficial as this minimizes the targeting of distant normal stem cells and potentially provides a wider therapeutic window of the CSC-targeting drug. Despite PCI of CD133-targeting toxins may be used as a minimal invasive strategy in the treatment of sarcomas, this can be used for other solid tumors expressing CD133 as breast cancer.33
In human breast tissues, CD133 expression is lost in tumor-associated endothelial cells; also conversely, CD49b was strongly stained in the tumors, associated vessels and ducts but was weakly stained in the background epithelia. q-PCR analysis revealed that CD44 and PSCA (prostate stem cell antigen) are reduced in patients with poor outcome (metastatic disease and death from breast cancer), with a marked reduction in ductal carcinoma, particularly with metastasis to bone although these did not reach significant difference. CD133 was significantly reduced in patients with metastatic disease and was also significantly reduced in patients with ductal carcinoma/bone metastasis. Conversely, CD49F was increased in patients with a poor outcome and those with ductal cancer and bone metastases. Such differential expression may play a part in breast cancer disease progression, and suggests that the current stem cell theory may not hold true for all cancer types.55
Ligand of CD133 has been identified as LS-7 (amino acid sequence: LQNAPRS), a specific binding peptide targeting mouse CD133, was screened and identified for the first time by phage-displayed peptide library technology. High-affinity binding of the peptide to CD133 in vitro. Confocal microscopy confirmed the co-localization of LS-7 positive cells and CD133-positive cells. Migration and wound-healing assays showed that LS-7 significantly inhibited the migration of colon and breast cancer cells in a concentration-dependent manner. In vivo experiments also confirmed the high specificity and affinity of LS-7 to CD133. RT-PCR and Western blot showed that the expressions of only c-Met and STAT3 decreased obviously in colon and breast cancer cells exposed to LS-7.56
Many chemotherapeutic regimens trigger cancer cell death while inducing dendritic cell maturation and subsequent immune responses. However, chemotherapy-induced immunogenic cell death (ICD) has thus far been restricted to select agents. In contrast, several chemotherapeutic drugs modulate antitumor immune responses, despite not inducing classic ICD. Docetaxel treatment of tumor cells did not induce ATP or high-mobility group box 1 (HMGB1) secretion, or cell death. However, calreticulin (CRT) exposure was observed in all cell lines examined after chemotherapy treatment. Killing by carcinoembryonic antigen (CEA), MUC-1, or PSA-specific CD8(+) CTLs was significantly enhanced after docetaxel treatment. The killing is associated with increases in components of antigen-processing machinery, and mediated largely by CRT membrane translocation, as determined by functional knockdown of CRT, PERK, or CRT-blocking peptide. In the treatment docetaxel-resistant cells with phenotype (MDR-1(+), CD133(+)) were found. These cells, while resistant to direct cytostatic effects of Docetaxel, are not resistant to the chemomodulatory effects that resulted in enhancement of CTL killing. Here appears the term “immunogenic modulation,” where exposure of tumor cells to nonlethal/sublethal doses of chemotherapy alters tumor phenotype to render the tumor more sensitive to CTL killing. Docetaxel alter the phenotype of human tumor cells and increase their susceptibility to CD8+ CTL-mediated killing. These observations are distinct and complementary to ICD and highlight a mechanism whereby chemotherapy can be used in combination with immunotherapy.57
An approach different to antibodies are the aptamers, a class of small nucleic acid ligands that are composed of RNA or single-stranded DNA oligonucleotides and have high specificity and affinity for their targets. Similar to antibodies, aptamers interact with their targets by recognizing a specific three-dimensional structure and are thus termed “chemical antibodies.” In contrast to protein antibodies, aptamers offer unique chemical and biological characteristics based on their oligonucleotide properties.58 Shigdar et al.59 isolated and characterized two RNA aptamers, including the smallest described 15 nucleotide RNA aptamers, which specifically recognize the AC133 epitope and the CD133 protein with high sensitivity. As well, both these aptamers show superior tumor penetration and retention when compared to the AC133 antibody in a 3-D tumor sphere model.
In endocrinotherapy, acquired tamoxifen (TAM) resistance is the main reason for failure during such therapy. Breast CSCs played an important role in TAM-induced resistance during breast cancer therapy. Consistently, qRT-PCR revealed that TAM-resistant (TAM-R) MCF7 cells expressed increased mRNA levels of stem cell markers including SOX-2, OCT-4, and CD133 also these cells expressed increased mRNA levels of Snail, vimentin, and N-cadherin and decreased levels of E-cadherin, which are considered as EMT characteristics.11,60 Therefore targeting CD133 could be used for the successful of endocrinotherapy.61,62
Bostad et al.63 demonstrated laser-controlled targeting of CD133 in vivo. They used photochemical internalization (PCI) for the endosomal escape of the novel CD133-targeting immunotoxin AC133-saporin (PCIAC133-saporin). PCI employs an endocytic vesicle-localizing photosensitizer, which generates reactive oxygen species upon light-activation causing a rupture of the vesicle membranes and endosomal escape of entrapped drugs. This strategy blocked cell proliferation and induced 100% inhibition of cell viability and colony forming ability at the highest light doses, whereas no cytotoxicity was obtained in the absence of light. Efficient PCI-based CD133-targeting was in addition demonstrated in the stem-cell-like, triple negative breast cancer cell line MDA-MB-231 and in the aggressive malignant melanoma cell line FEMX-1, whereas no enhanced targeting was obtained in the CD133-negative breast cancer cell line MCF-7. PCIAC133-saporin induced mainly necrosis and a minimal apoptotic response.
Accumulating evidence has shown inhibitory effects of vitamin D and its analogs on the cancer stem cell signaling pathways.64 A study published by Wahler et al. and So & Suh in this year show that Vitamin D is a potential preventive/therapeutic agent against CSCs that have CD133 and CD44, EpCAM, CD49f, CXCR4, ALDH-1, and CD24.65
ConclusionCD133 are adequate biomarker of breast cancer cells and breast CSCs that might be useful as another surrogate marker for therapeutic selection and monitoring the heterogeneity of cancer and as a target of immunotherapy with antibodies. Further exploration of the association between this marker and specifics stages of the disease is needed for efficient anti-cancer immunotherapy using CD133. Therefore, conventional treatments along with immunotherapy against CD133 and other specifics markers of CSCs are a promising treatment option in efforts to eradicate breast cancer in the clinical settings.
Conflict of interestThe authors have no conflicts of interest to declare.
To CONCYTEC through CIENCIACTIVA for its financial support.