The identification of findings that suggest a unique dysbiotic microbial signature in Autism Spectrum Disorders (ASD), has drawn the attention towards promising therapies for ASD targeting gut-microbiota. In order to help physicians to make clinical decisions based on significant evidence, this work offers a systematic review of original peer-reviewed studies focused on microbiota-targeted treatments in ASD children.
MethodsThe systematic review was conducted following the PRISMA guidelines. Quality of research was assessed using the National Health and Medical Research Council (NHMRC). Of 110 potential records initially identified, only 9 articles accomplished our inclusion criteria.
ResultsA decrease in specific Clostridiales species and/or an increase in Bacillales was consistent in several studies after the microbiota-targeted interventions, whereas mixed results were seen in other phyla, congruent with different baseline trends in their ASD samples. Behavioral and GI function responses varied across interventions.
ConclusionPreliminary data show microbiota-based therapies to have a positive effect on ASD patients. However, further well-designed, large-scale randomized controlled trials with standardized protocols are needed to support the effectiveness and safety of these treatments.
Autism spectrum disorders (ASDs) is an umbrella term that incorporates a set of early onset neurodevelopmental disorders previously referred to as Autism, Asperger syndrome, Pervasive Developmental Disorder-Not Otherwise Specified and Childhood Disintegrative Disorder.1 Over the past decades, the reported incidence of ASD has been steadily increasing, from 1 every 150 children in 2000,2,3 and 1 in 88 births in 20084,5 to 1 every 68 in 20106–8 and 1 in 37 in 2016 in the United States,3 which has led to growing concern among the scientific community.1
Although majority of research has focused on the genetic etiology of ASD,9 inherited single chromosomal or gene defects can account for few ASD cases, thereby evidencing the role of acquired mutations as a result of an interplay with environmental factors.9,10 In this regard, frequent comorbidity between ASD and gastrointestinal (GI) symptoms11–15 and mounting evidence underpinning the role of gut microbiota on several neurobehavioral disorders6,16–20 has drawn attention to the contribution of gut metabolome and gut microbial pattern to ASD etiopathogenesis.6 In fact, dysbiosis in the gastrointestinal tract of ASD children has been consistently found in some studies, with different trends in the relative abundances of Firmicutes and Bacteroidetes compared to neurotypical (NT) children,21,22 increased Proteobacteria, Clostridium and Candida species, and a decreased amount of beneficial bacteria such as Actinobacteria and specifically, the anti-inflammatory family Bifidobacterium.23–26
Microbial colonization in offspring occurs at birth and achieves its stability between 6 and 36 month of life,27 an age in which diagnosis of ASD is usually made.1–6 During this period, exposure to detrimental factors has been proved to increase long-term susceptibility to ASD onset3 through alteration of microbial balance, which is known as dysbiosis.28
Interest in therapies targeting ASD dysbiotic microbiota has gradually ballooned. In this sense, diet,29,30 probiotics,31,32 prebiotics,33 antibiotics,34 antifungal supplementations,24 fecal microbiota transplantation (FMT)35,36 and microbiota transfer therapy37 have been put forward as possible therapeutic options for ASD, some of which have already been proved useful in certain psychiatric disorders, such as depression or anxiety.27 For ASD, however, evidence on these interventions is limited33,38 and cumulative narrative reviews rely on original studies conducted on non-specific ASD animal models or studies about the effect on gut microbial-dependent alterations common to ASD patients, rather than on ASD children themselves.
Regardless of the lack of clinical studies, methodology limitations, and the low number of consistent studies evidencing improvement of symptoms in ASD patients after these therapies, almost one-fifth of the physicians promote the use of probiotics in ASD children, and close to 60% consent to its usage when parents are already employing them.39 This issue was covered by a review published on 2015 by Srinivasjois et al.,40 who discouraged the use of probiotics as adjuvant treatments for ASD given the low applicability and methodological limitation of the reviewed studies. To date, the most complete overview on the state of evidence regarding microbiota-targeted treatments in ASD is the review carried out by Rosenfeld et al. in 2015.7 However, in this review the search strategy and selection of studies was not systematized and authors did not include a critical evaluation of strengths and weaknesses of the reviewed work. On top of that, new research has been performed on this matter after the above-mentioned studies were published. Altogether, an updated systematic review should be useful for physicians to make clinical decisions based on significant evidence.
Our aim in this systematic review is to describe evidence from original research focusing on the effects of modulation of gut microbiota in ASD patients. In addition, inclusion of clinical improvements in behavioral and core ASD symptomatology, GI concomitant conditions, and other ASD-related manifestations (i.e. sleep patterns) as result variables have been considered and reviewed.
MethodsThis systematic review followed the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).41
Search strategy and study selectionWe conducted a search of intervention studies describing the effects of modulation of gut microbiota in ASD patients. The search was undertaken in May 2018 using MEDLINE via PubMed by 2 researchers (IL and PS) independently. A third researcher (JS) reviewed a random sample of 10% of the studies to assess agreement and reviewed all included studies to approve eligibility.
The Pubmed search strategy was: (autism OR "Autism spectrum disorder" OR autistic OR "pervasive developmental disorder" OR "Asperger") AND ("gut microbiota" OR “microbiome” OR “intestinal microbiota” OR “fecal microbiota” OR “microflora”) AND “treatment”.
In addition, the reference lists of selected publications were also screened for potentially eligible studies. The same authors retrieved and assessed potentially relevant articles. The inclusion criteria were as follows:
- •
Original published, peer-reviewed study.
- •
Intervention on patients diagnosed or suspected of having ASD.
- •
The effect of the intervention on gut microbiota and/or metabolome is studied.
- •
Full article available in English.
Conversely, review articles and meta-analyses, studies based on ASD-animal models and those that did not study the effect of the intervention on the gut microbiota or metabolome of patients were excluded from the qualitative analysis.
Data extractionTwo reviewers (IL and PS) independently extracted data for included studies. We used predesigned data extraction form to extract information on study design and level of evidence, type of analysis, Gut-microbial/Metabolome changes, clinical (behavioral and GI) outcomes and limitations.
Quality assessmentLevel of evidence was graded based on the National Health and Medical Research Council (NHMRC) levels of evidence42 by JS.
ReliabilityA fourth reviewer (EL) blinded to the primary reviewer’s (IL, PS and JS) decisions checked the article selection, data extraction, and risk of bias assessment stages of the review. Any differences of opinion were discussed.
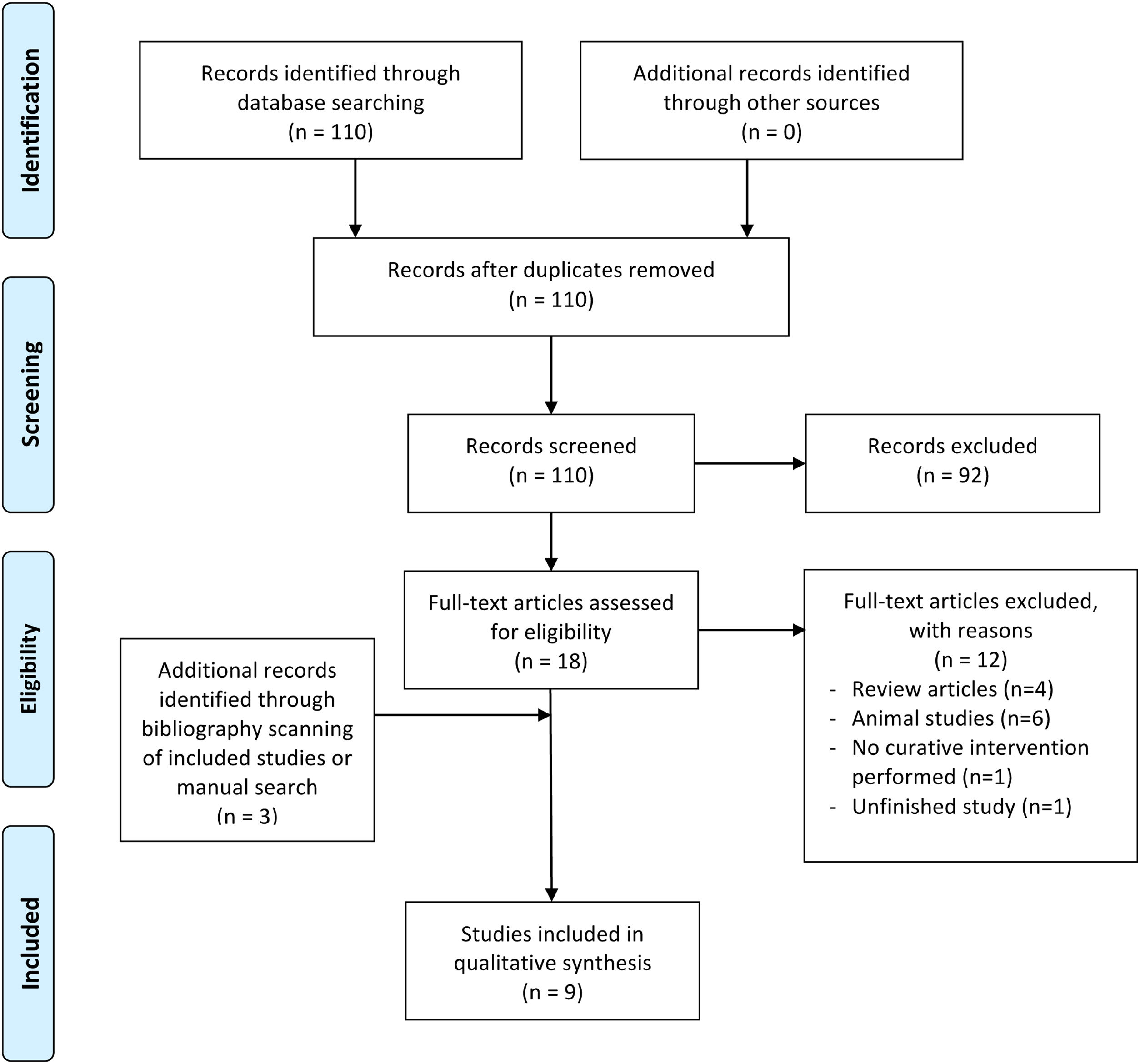
ResultsStudy selectionFig. 1 presents the results of the literature search and study selection process. The primary search yielded 110 potential records. At the first stage, the titles and abstracts of 110 articles were read and 92 articles were excluded. After full-text reading of the 18 remaining articles, a further 12 were excluded. Finally, 3 articles were added by hand search and 9 were included in the final review of this report.
PRISMA Flow chart showing selection of included studies. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visitwww.prisma-statement.org.
Included studies are shown in Table 1.8,20,31,33,43–46
Study characteristics of human reports.
| Author/year | Study group and intervention | Study design (level ofevidence) | Type of analysis (M: microbial/ metabolome; G: Gastrointestinal function; P: psychological) | Microbial/ Metabolome (M), Psychological (P) and GI (G) outcomes | Limitations | |||
|---|---|---|---|---|---|---|---|---|
| Probiotics | ||||||||
| Parracho et al.,2010 | n = 17 ASD children (m:f = 20:2; aged 6−11y).Controls: crossover placebo feeding.Intervention: Lactobacillus plantarum WCSF1 (4.5 × 1010 CFU/g) for 3 weeksFollow up: 3 w (washout) | Randomized controlled trial (II) | M. Stool samples were examined by microscopy using FISH.P. Behavior scores were assessed using the DBC.G. GI characterized through parental diary report. | M. Significantly higher lactobacilli and enterococci, and significantly lower Clostridium cluster XIVa counts after probiotic feeding;. No significant differences on Chis150 (Clostridium clusters I and II).P. Both placebo and probiotic feeding decreased TBPS scores, but only probiotics lowered it below the clinical threshold (p > 0.05). Scores for disruptive behavior, anxiety, self-absorbed behavior and communication disturbances were lower than baseline during probiotic feeding.G. Improved stool consistency during probiotic feeding.Effects on M., P., and G. reversed after cessation. | Sex bias.Small sample size.No control group.Low compliance assessment of pP. and G, symptoms..Possible short feeding period to note clinically significant resultsShort follow-up period | |||
| Kaluzna- Czaplinska and Blaszczyk 2012 | n = 22 ASD children with GI dysfunction (m:f = 18:2; aged 4- 10y).Intervention: oral Lactobacillus acidophilus (strain Rosell; 11.5 × 109 CFU/g) twice a day for 2mo. | Case series (IV) | M. Concentrations of DA, LA, and ratioDA/LA in urine were determined by capillary GC/MS..P. Observation of behavioral outcomes.G. GI dysfunction assessed by individual questionnaires at baseline. | M. Lower DA, and decreased DA/LA ratio in urine after probiotic supplementation.P. Improvement in concentration and ability to carry out orders on probiotic therapy; but no difference in behavioral responses to other people’s emotions or eye contact.G. GI function not studied after treatment. | Sex bias.Small sample size.No control group.No follow-up period.High risk of selection and performance bias.Probiotic/prebiotic intake prior to enrollment not assessed. | |||
| Tomova et al.,2015 | n = 10 ASD children (m:f = 9:1; aged 2−9y).Controls: 9 non-ASD siblings (m:f = 7:2), 10 unrelated controls (m:f = 10:0).*Intervention: oral capsule (“Children Dophilus”) of 3 Lactobacillus strains + 2 Bifidobacterium + 1 Streptococcus, 3 times a day for 4mo. | Non- randomized experimental trial (III-2) | M. Fecal samples analyzed by bacterial DNA concentration and DNA amplification with specific PCR primers.P. Severity of ASD, evaluated using CARS and ADI.G. GI condition evaluated based on parental questionnaires. | M. Significant decrease in Firmicutes, which results in the increase of the Bacteroidetes/ Firmicutes ratio to the level of healthy individuals. Levelling of the amount of Bifidobacterium, high in ASD children at baseline, to that of healthy controls. Duplication of Lactobacillus and decrease of Desulfovibrio. Decrease of TNFα levels.P. CARS and behavior not studied after probiotic supplementation.G. GI scores were not provided after probiotic supplementation,although a strong correlation was found between GI symptoms and TNFα levels, which decreased after probiotic intake. | Sex bias.Small sample size.No follow-up period.High risk of selection bias.Probiotic/prebiotic intake and diet prior to/during enrollment not assessed.Effect of probiotics was not studied on siblings and healthy controls. | |||
| Vamcomycin + probiotics | ||||||||
| Xiong et al., 2016 | n = 62 ASD children (m:f = 48:14; aged 1.5-7y).Controls: n = 62 age/gender- matched non-ASDs.Intervention (1 therapeutic course): oral vancomycin (50 mg/kg/d) for 30 days + subsequent Bifidobacterium animalis (BB-12, 2 pills/day) + 15 day-discontinuance.Follow up: 3−6mo (only M). | Controlled before-and- after study (III-i) | M. Urine samples were pretreated and analyzed by GC/MS in order to achieve metabolomics profiling, which was normalized using a creatinine internal standardP. Behavior assessed employing the ABC score.G. GI condition measurement method was not provided.. | M. Significant decreases and complete elimination in some cases of HPHPA, 3HPA and 3HHA, which regressed rising up to the initial levels in 3 patients after treatment discontinuance.P. Significant decrease in ABC mean value from 73 to 59 after two therapeutic course treatments. Amelioration of communication and eye contact in 90% of ASD children; but no improvement in stereotyped behavior.G. Improvement of constipation in all 22 children who displayed this symptom. | Only HPHPA-positive ASD selected.Lack of positive control group.Potential confounding of dietary status. | |||
| Antifungals | ||||||||
| Shaw et al.,2000 | n=23 ASD children (m:f = 21:2; aged 2−12y).Controls: n = 37 unrelated controls (m:f = 20:17, aged 3−12y).Intervention: 10 × 104 CFUs of oral nystatin suspension, 4 times a day for 10 days (1st course). Additional 60 day- administration if persistent abnormalities (2nd course).Follow up: up to 2y (only P, some patients). | Controlled before-and- after study (III-i) | M. Organic acid analysis in urine by GC/MS at baseline and after therapy, to test for 10 metabolites of possible fungal origin.P. Assessment of the severity of behavioral traits using CARS. | M. Among the 8 metabolites significantly increased in ASD children, 3 were significantly lowered after 1 st course of treatment, and only Hydroxymethylfurancarboxylic Acid, and Furandicarboxylic Acid) remained stable after the 2nd course. Phenylcarboxylic Acid significantly raised after the 1 st course, although no significance was found after the 2nd.P. Significant mean decrease in CARS score, specifically: lower hyperactivity, increased eye contact and vocalization, better sleep patterns and concentration, increased imaginative play, reduced stereotypical behaviors, and better academic performance. Possitive effects reversed after cessation. | Sex bias.Small sample size.High risk of selection and performance bias.. High dropout rates,.Low adherence to protocol, since only 18 children completed the CARS evaluation at baseline, and 9 were reevaluated after therapy. | |||
| Kantarcioglu et al., 2015 | n = 1555 stool samples from ASD suspected/ diagnosed children (m:f = 879:676, aged 9mo-17y). Intervention: nystatin and fluconazole in vitro. | Case series (IV) | M. Yeast isolation and antifungal susceptibility. | M.Candida species, Trichosporon mucoides and S. cerevisiae were found in ASD samples at a notably higher rate than in healthy subjects. The vast majority of yeast (except for Candida krusei and C. glabrata) were inhibited by nystatin and fluconazole. | In vitro studyLack study on bacterial communitiesNo control group. No follow-up period.No verification of ASD diagnosis | |||
| Vitamin A | ||||||||
| Liu et al., 2017 | n= 64 ASD children (m:f = 55:9, aged 1−8y), subset of n = 20 for microbial analysis.Intervention: vitamin A (20 × 104 IU) for 6mo. | Case series (IV) | M. CD38 and ROPA mRNA levels.P. SRS, ABC and CARS to measure ASD symptoms and behaviors. | M. Significant Bacteroidetes increase, driven by a higher amount of Bacteroides, and Bifidobacterium decrease, which lowered the levels of Actinobacteria (p > 0.05). Proportions were higher for Prevotella, and lower for Peptostreptococcaceae_incertae_sedis, Enterobacter, Escherichia-Shigella and Clostridium (unknown p value). Increased plasma retinol, CD38 and RORA mRNA levels. No significant differences on richness and diversity.P. Autism symptom scale scores showed no differences. | Small sample size for microbial analysis.No control group.No follow-up period. | |||
| MTT | ||||||||
| Kang et al.,2017 | n = 18 ASD children aged 7- 17y.Intervention: 10 week of MTT treatment.Follow up: 2mo. | Case series (IV) | M. Parental collection of stool samples, microbial DNA extraction and amplification with archaeal and bacterial 16S rRNA genes.P. PGI-III to assess parental impression on improvements in ASD symptoms. CARS, SRS and ABC at baseline and at the end of treatment and monitoring periods. VASB-II was performed only at baseline and after the monitoring period.G. Assessment of GI symptoms by GSRS. Daily Stool Records at baseline for 2 weeks, and the last weeks of the follow up. | M. Increase in variety and abundance of Bifidobacterium (x4), Prevotella and Desulfovibrio. Higher overall bacterial diversity (OTUs and phylogenetic-wise) by the end of treatment and during the 2-month follow-up.P. CARS scores decreased by 22% from baseline scores, and remained 24% lower over the 2-month follow-up. PGI-III, SRS and VABS-II showed improvements in social skill deficits, irritability, hyperactivity, aberrant speech, stereotypy and lethargy, communication, socialization and daily living skills. The VABS-II showed an increase by 1.4y in average of developmental age.G. GSRS score decreased 82% from baseline and remained improved (77%) at follow-up. Significant improvements were seen in abdominal pain, indigestion, constipation and diarrhea; patients presented abnormal stool or constipation less frequently than in the past. | Small sample size.Unknown gender representation in sample.High risk of selection and performance bias..No control group. | |||
| Prebiotics | ||||||||
| Grimaldi et al., 2016 | n = 3 male ASD children aged 5−10 years.Controls: 3 male non-ASDs. Intervention: prebiotic galactooligosaccharide (B- GOS). | Controlled before-and- after study(III-i) | M. Fecal samples were analyzed using flow cytometry combined with fluorescence in situ hybridization and metabolic activity by HPLC and H- NMR. | M. B-GOS administration significantly increased bifidobacterial populations in both ASD and controls, and lactobacilli in controls. Changes in Clostridium, Roseburia, Bacteroides, Atopobium, Faecalibacterium prausnitzii, Sutterella spp, and Veillonellaceae. Significantly altered short- chain fatty acid production in both groups and increased ethanol and lactate in ASD. | In vitro studySmall sample size.Sex bias.No follow-upNot details about dosage. | |||
All findings are statistically significant unless otherwise stated
3HHA: 3-Hydroxyhippuric Acid; 3HPA: 3-Hydroxyphenylacetic Acid; 4EPS: 4-ethylphenylsulfate; ABC: Autism Behavior Checklist; ADI: Autism diagnostic interview; CARS: Childhood Autism Rating Scale; CBCL 1.5−5: Child Behavior Checklist 1.5–5; CFU: colony-forming units; DA, LA:D-arabinitol, L-arabinitol; DBC: Development Behavior Checklist; GI: Gastrointestinal; GSRS: Gastrointestinal Symptom Rating Scale; HPHPA: 3-(3-Hydroxyphenyl)-3-hydroxypropionic Acid; IU: international units; LC: liquid chromatograph; Mc Arthur-CDIs: MacArthur-Bates Communicative Development Inventories; MIA: Maternal immune activation; n0: initial sample; nf: number of subjects completing the study/ sample size after withdrawal or selection is completed; NT: neurotypical; PGI-III: Parent Global Impressions- III; PSI: Parenting Stress Index; RBS-R: Repetitive Behavior Scale-Revised; RORA: acid-related orphan receptor alpha; SCQ: Social Communication Questionnaire; SRS: Social Responsiveness Scale; TBPS: overall indicator of behavioral/emotional disturbances; VABS-II: Vineland Adaptive Behavior Scale-Second Edition..
Most of them had limited sample size, sometimes due to low adherence to protocol or lost follow-up.20,45 Moreover, some suffered from sex bias, as female representation in sample was low.8,20,45 Nearly all of the studies suffered from limitations related to methodology and patient enrollment, as verification of ASD diagnosis was rarely carried out. For instance, Kantarcioglu recognized inclusion of subjects suspected of having ASD in their sample, besides those with confirmed diagnosis.24 In some cases, medication-taking, concomitant pathologies, dietary status and/or prior intake of probiotics/prebiotics was not taken into consideration in the exclusion criteria.5,8,31,45 In addition, some of the studies did not contrast the outcomes of interventions with a control group.24,44,46
Microbiota outcomes were assessed by quantification of targeted microbial or fungal species described as being involved in manifestations of ASD,20,24,43 detection of their related metabolites5,31,45, or both.8,33,44 One third of studies analyzed fungal profile exclusively24,31,45 and the remaining two thirds studied bacterial populations only,8,20,33,43,44,46 with the exception of Kang, who also studied archaeal genes.43
Among all reports, two were in vitro studies,24,33 which impeded them from assessing clinical outcomes. Likewise, another study did not report behavioral effects after treatment.8 Those which did, relied on several autism behavioral scales performed by the clinician and/or parents in clinical samples. More than half of the studies analyzed baseline GI conditions,8,20,31,43,46 using parental diary reports and questionnaires8,20,31,43 in all cases but one,5 whose measuring method was not provided. However, only a third provided GI outcome after treatment.20,43,46
The supplementation period lasted approximately 80 days on average. In most cases, the measurements were carried out during or immediately after treatment cessation, except in four studies,20,43,45,46 with a follow-up period ranging from 3 weeks to 2 years.
Only one study reported adverse effects after probiotic intake in 3 out of 17 children.20
Major findings of included studiesThe main microbial alterations are shown in Table 2.
Microbial changes in ASD children and ASD animal models following microbiota-targeted interventions.
Green arrows indicate findings not reaching statistical significance (p > 0.05), black arrows indicate significant findings, brackets indicate sensitive sub-population (if brackets are not used, changes occur in all sample sub-groups, i.e. cecal, fecal, clindamycin-treated hamsters, PPA-treated hamsters), V stands for “vessel” and indicates the replication of psychochemical conditions in proximal (V1), transverse (V2) and distal (V3) colon in Grimaldi ‘s in vitro study.
Among them, significant changes on the Firmicutes phylum were reported on 4 out of the 6 studies analyzing bacterial populations, specifically those testing for probiotic,8,20 prebiotic33 or Vancomycin + probiotic intake.5 The 2 studies which examined the total amount of Firmicutes in ASD children8,44 found a decrease after intervention, which was significant in only one of them,8 consistent with higher baseline levels of Firmicutes in the ASD sample related to NT controls. In particular, all studies showing changes in this phylum agree upon a decrease in specific Clostridiales species5,20,33 and/or an increase in Bacillales8,20,33 after treatment, that could equilibrate opposite trends in these two classes of bacteria in ASD children before the intervention. Results have been inconsistent for Lachnospiraceae33 and seem to be species-dependent for Clostridium.
Other mixed results after the microbiota-targeted interventions were seen in the phyla Bacteroidetes33,43,44 and Actinobacteria,8,33,43,44 congruent with different baseline trends in their ASD samples. Desulfovibrio, a genus belonging to the plylum Proteobacteria, showed opposite trends in human studies according to the type of treatment performed (decrease after probiotic intake8; vs. increase post-MTT43). The reduction of Enterobacteriaceae was either non-significant (Vitamin A),44 or inconclusive (probiotic).33 Regarding fungal populations, hyperproliferation was inhibited by antifungals24,45 and some probiotics containing L. Acidophilus.31 However, antifungal susceptibility tests carried out by Kantarcioglu et al. revealed two resistant non-albicans Candida species, C. krusei and C. glabrata.24
Overall, only 2 out of 3 reviewed studies on probiotic intake studied behavior after supplementation.20,31 The use of species from Lactobacillus, alone or combined with Bifidobacterium, was the most common intervention. After Lactobacillus acidophilus supplementation for 2 months, the ability to carry out orders and concentration improved, but no difference could be seen in empathy responses or eye contact,31 while provision of Lactobacillus plantarum did have significant effects on sociability and communication.20 Similarly, both nystatin therapy and MTT improved repetitive behaviors.43,45 Nystatin therapy also showed gains on hyperactivity, eye contact, vocalization, sleep patterns, imaginative play, concentration and academic performance.45
Regarding GI condition, MTT exerted a prominent and lasting effect, evidenced in improvements in GI symptoms.43 Amelioration of GI dysfunction was also seen following vancomycin + Bifidobacterium animalis5 and Lactobacillus plantarum,20 but regressed soon after treatment cessation in the last case.
Among the 4 studies reporting on clinical status after treatment cessation, half showed reversion of microbial and clinical changes (Lactobacillus plantarum and Vancomycin followed by a probiotic supplement).20,46 MTT’s improvements persisted during the follow up and nystatin effects persisted only as long as adherence continued.
Adverse outcomes such as skin rash, diarrhea and weight loss have been reported in one study.20
DiscussionIn the present systematic review, we survey the original literature on therapeutic approaches targeting gut microbiome for ASD, providing a resource to guide treatment based on evidence. There is growing evidence that the gut microbiome might influence ASD outbreak and progression. However, the lack of consistent knowledge implicit in the novelty of these considerations, and the still poor understanding of the complex microbial and metabolome distinctive signature in ASD patients, often translates onto difficulties in microbiota-based therapy planning, which is usually performed on a trial and error basis.
Among the multiple possible microbiome-targeted intervention strategies which have been postulated in the published literature, we found that only certain probiotic, antifungal, vancomycin, prebiotic supplementation and MTT have been tested in ASD children.
Overall, the findings of our review support the ability of probiotics to mitigate gut dysbiosis,8,20 in some cases increasing the Bacteroidetes/Firmicutes ratio to the level of healthy individuals,8 reducing the growth of Candida, Desulfovibrio and Clostridia species8,20,31 and increasing beneficial bacteria such as Lactobacilli and Enterococci.8,20 Prebiotic supplementation with galactooligosaccharide B-GOS was also shown to raise Lactobacilli species.33 Contradictorily, MTT appeared to increase Desulfovibrio species.8,43
A study using probiotics showed a decrease in short chain fatty acids (SCFAs),11 which are fermentation products of dietary carbohydrates produced, among others, by Clostridium, Ruminococcaceae, Lachnospiraceae and Desulfovibrio. SCFAs have been found to be increased in ASD.47–50 However, since KD implementation in ASD children, which increased SCFA-producing species,22 translated onto significant improvement of autistic core symptoms30,51,52 their role in ASD etiopathogenesis remains questionable.
Likewise, the role of Bifidobacterium species is controversial. Whereas the majority of studies report a lower baseline relative abundance of Bifidobacterium in ASD children compared to control children,33,43,50,53,54 which is supported by findings on the ASD-specific BTBR mouse model,55 two studies have not found significant differences.8,56 Notice that these studies did not consider special diets, prebiotic or probiotic intake prior to/during enrollment as exclusion criteria. In further contradiction, two of the analyzed studies reported a significant and strong decrease in Bifidobacterium levels after probiotic intake8 and Vitamin A (VA) supplementation.44 However, the study using probiotics did not study associated clinical outcomes,8 and the one using VA reported no significant changes on autism symptom scores after 6 months of intervention,44 which might be explained by the decrease in bifidobacterial species, together with the absence of reduction of Firmicutes and Clostridium. A study conducted by Weston57 actually found that decreasing Bifidobacteria growth rate resulted in a significant higher risk for developing ASD. In fact, Bifidobacteria levels appear to be inversely correlated through feedback interactions with Desulfovibrio and Clostridium, two of the suggested most relevant bacteria strains in ASD etiopathogenesis.57 Along these lines, our review supports that increasing Bifidobacterial populations (MTT, B-GOS supplementation) or decreasing Desulfovibrio and Clostridium growth rates could be feasible targets in microbiota-based ASD therapies when baseline counts are abnormal.
In an attempt at lowering the growth rates of Clostridia and Desulfovibrio populations, Vancomycin, which selectively targets Clostridia, has been shown to temporarily relieve ASD symptoms.34,46 However, the recurrence of symptoms after treatment withdrawal attributed to Clostridium surviving spores shows the requirement of a long-term antibiotic therapy not feasible for ASD patients, which could pose a risk due to possible adverse effects and the development of drug-resistant bacteria. In an attempt to prevent recurrence of symptoms, Xiong et al.46 used a short-term probiotic (Bifidobacterium animalis) supplement therapy, which resulted in a less pronounced rate of symptom recurrence compared with a previous study by Sandler, who used only Vancomycin.34 However, results are not conclusive, since Xiong’s study46 does not include a control group solely on Vancomycin to allow direct comparison of results.
A further line of investigation could be supplementation with Saccharomyces boulardii, proposed by L. Linday58 due to its efficacy in Clostridium difficile colitis. Overall, in the reviewed articles, Clostridia species decreased after Lactobacillus plantarum20 and B-GOS in ASD children.33
Comprehensively, our review suggests that clinical benefits of probiotic use in ASD children remain controversial. Whereas some human studies reported improvements in disruptive behaviors, self-absorbed behavior, communication, anxiety, concentration, ability to carry out orders and social affect13,20,31,59; findings regarding sociality have yielded contradictory results on mice,4,59 perhaps due to the difficulty of extrapolating findings in animal models. Some results suggest a possible specificity of the bacterial species-mediated behavioral modulation. Indeed, the most effective microbiota therapy on sociability appeared to be Lactobacillus plantarum, whose action was reversed once intake was stopped after 3 weeks.20 Conversely, a study conducted by Grossi et al.13 found that administration of an oral probiotic mixture containing 5 Lactobacilli, 3 Bifidobacterial and 2 Streptococcal strains for 4 months had significant and lasting effects on social affect and communication. It should be noted that results were referred to a single child suffering from pervasive developmental disorder (PDD), hence larger studies are needed to underpin this hypothesis. Likewise, analysis of impact of this mixture on the microbiota of ASD individuals should be performed, since its effect has only been studied on patients with irritable bowel syndrome or functional diarrhea.60 The lack of response of repetitive behaviors to probiotics and current treatments recommended by clinical guidelines61 is consistent with the postulated preponderance of a genetic etiology for this particular symptom.62 For this reason, it is of special interest results supporting the effect of nystatin therapy and MTT to ameliorate this trait.43,45 However, safety and tolerance of these treatments needs to be further studied.43
Since nystatin was found to inhibit the overgrown Candida species in the gastrointestinal tract of ASD children,24,25,63,64 the ability of L. acidophilus23,31 to decrease the growth of Candida species in animal models of ASD could result in a reduction of stereotypical behaviors. However, further studies are needed to support this hypothesis. Regarding GI condition, the most effective microbiota-targeted method seems to be the MTT.43 However, improvements were also seen in the remaining 2 studies that addressed comorbid GI symptoms.20,46
In considering other clinical symptoms, only Shaw et al. 2000 reported significant improvement in sleep patterns after nystatin supplementation.45 Even so, non- pharmacological approaches should be considered prior to its usage.61
LimitationsAn important limitation of the current review is the use of only one database (Pubmed) in the search strategy. In addition, the methodology limitations and small sample size of the analyzed studies often leaps to tentative conclusions that can’t always be extrapolated to the whole ASD population. Finally, the majority of studies targeting microbiota in ASD were focused on probiotic administration, whereas few research is available on the effects of Vitamin A, MTT and KD on ASD children, which hinders cross-study comparisons within these therapies.
ConclusionsThe results of this systematic review suggest that more well-designed, large-scale randomized controlled trials with standardized protocols are needed before definitive conclusions are drawn regarding the clinical benefits of microbiota-targeted therapies in ASD children through mitigation of gut dysbiosis, and their possible side-effects in a short and extended period of time. While research showed significant changes in the microbial profile of ASD children following implementation of these therapies, these were inconsistent across studies and a clinical improvement was not always reported. Likewise, assumption of tolerance and safety based on the non-pharmacological nature of these therapies should be questioned, since the risks of MTT are still unknown, and long-term effects of probiotics consumption remain unclear to date remain unclear to date.12
Moreover, because microbiota is subject to dietary and pharmacological changes, novel research should take into consideration dietary status, and prebiotic/probiotic/antibiotic intake prior to enrollment in the study.
Additionally, given the wide variety of clinical and microbial phenotypes in ASD patients, and the specificity of these still-developing therapies on certain traits and microbial populations, it is likely that treatment approach should be tailored to meet the patient needs.
Nevertheless, given the limited effectiveness of available drug treatments on nuclear symptoms of ASD and their undesirable side effects, microbiota-targeted interventions such as probiotic, prebiotic, MTT, KD and antifungal use are particularly attractive as non-pharmacological approaches in ASD subjects.
Ethical considerationsNone.
FundingNo financial support was provided for the making of this review.
Conflict of interestP. Gracia-García has received honorarium from Servier and non-financial support to attend scientific meetings from Pfizer, Lundbeck y Nutrición Médica. I. Lasheras and J. Santabárbara have no competing interest to declare. On behalf of all authors, the corresponding author states that there is no conflict of interest regarding the content and writing of this paper.
Authors acknowledge Paloma Seral (PS) and Eva Latorre (EL) for their contribution in the search of eligible papers.