Peripheral administration of norepinephrine is restricted due to the association of extravasation with tissue necrosis.
MethodScoping review with the objective of describing the adverse effects related to the administration of norepinephrine through short peripheral venous access and the characteristics of drug administration in patients hospitalized in ICU, surgery, and emergency services.
Results12 studies with heterogeneous characteristics by size and type of population were included. The proportion of complications associated with peripheral norepinephrine administration was less than 12% in observational studies and it was less than 2% in those that used doses less than 0.13μg/kg/min, and concentrations less than 22.3μg/mL. The main associated complication was extravasation and there were no cases of tissue necrosis at the venipuncture site, some extravasation cases were treated with phentolamine, terbutaline or topical nitroglycerin. The drug administration time ranged between 1 and 528hours with a weighted mean of 2.78h.
ConclusionThe main adverse effect was extravasation, no additional complications occurred, phentolamine and terbutaline seem to be useful, and its availability is a necessity. It is essential for the nursing staff to carry out a close assessment and comprehensive care in patients receiving norepinephrine by peripheral route.
La administración de norepinefrina por vía periférica es restringida, por la asociación de la extravasación con necrosis tisular.
MétodoRevisión de alcance con el objetivo de describir los efectos adversos relacionados con la administración de norepinefrina por acceso venoso periférico corto y las características de administración del fármaco en pacientes hospitalizados en servicios de UCI, cirugía y urgencias.
ResultadosSe incluyeron 12 estudios de características heterogéneas por tamaño y tipo de población. La proporción de complicaciones asociadas a la administración de norepinefrina por vía periférica fue inferior al 12% en los estudios observacionales y fue menor al 2% en aquellos que utilizaron dosis menores a 0.13μg/kg/min y concentraciones inferiores a 22.3μg/mL. La principal complicación asociada fue la extravasación y no se presentó ningún caso de necrosis tisular en el sitio de venopunción, el tratamiento farmacológico utilizado para su manejo fue con terbutalina o nitroglicerina tópica. El tiempo del fármaco administración osciló entre 1-y 528 horas con una media ponderada de 2,78h.
ConclusiónEl principal efecto adverso fue la extravasación, no se presentaron complicaciones adicionales, la fentolamina y terbutalina parecen ser utilices en estos casos, su disponibilidad es una necesidad para una administración periférica segura. Es necesario que la enfermera realice una valoración estrecha y un cuidado integral en los pacientes que reciben norepinefrina por vía periférica.
Hypotension is a common problem in surgical patients,1 about 40% of patients have decreased systolic blood pressure (SBP) below 80mHg, while about 90% have decreased SBP greater than 20% of baseline pressure.2 Traditionally, perioperative hypotension has been classified into preoperative hypotension, post induction hypotension, intraoperative hypotension, and postoperative hypotension.2 In general terms, an episode of sustained hypotension at any stage of the surgical procedure has been associated with deleterious effects on the function of vital organs such as heart, kidney, and brain, and with longer hospital stay, and postoperative morbidity and mortality.3 Intraoperative vasopressors are frequently used to restore blood pressure, both in rapid push-dose pressors and continuous infusion.4 However, this is not only a perioperative problem: in emergency, intensive care, and haemodynamics services, the use of norepinephrine through peripheral venous access may be necessary, especially when central venous access is not available, when the patient's critical condition cannot wait for CVC insertion and when a low dose of this drug is expected to be used for a limited time.5
Norepinephrine, an α1-β adrenergic receptor agonist, is one of the most commonly used drugs in the context of shock of various aetiologies, because it promotes an increase in systolic, diastolic, and pulse pressure and a positive inotropic effect.6,7 Because this drug has a potent vasoconstrictor effect, it can cause peripheral necrosis.8 In addition, its chemical characteristics include a pH between 3.0 and 4.0 and it belongs to the group of drugs classified as non-cytostatic vesicants; that is, those non-chemotherapeutic agents that when extravasated can cause serious damage to surrounding tissue.9
It is precisely these properties of the drug that have been related to adverse effects described in some case reports,10 identifying serious effects due to extravasation of the drug causing severe soft tissue injuries requiring flaps, grafts, or even amputation. Therefore, its use has been recommended for central venous access and restricted for peripheral venous access. However, in some circumstances, insertion of the central catheter is delayed or not feasible, requiring use of the peripheral route.
Intravenous therapy via peripheral venous access is not without risk; several complications, including phlebitis, thrombophlebitis, infiltration, extravasation, and infection, are associated with peripheral access. Among other factors, the knowledge and experience of the nurse inserting the peripheral cannula can play an important role in preventing these complications. Nurses who have the necessary skill and experience in intravenous catheter insertion, as well as knowledge of post-insertion care and maintenance, can significantly influence patient outcomes.11 However, the risks of a peripheral venous catheter are lower than those of a central catheter, whether peripherally or centrally inserted.12
Regarding these situations, observational studies have been conducted in patients who received norepinephrine through the peripheral route, which report that the use of norepinephrine at doses of 20μg/mL or less, via the peripheral route, has been safe,13,14 without finding a correlation between peripheral administration and the complications mentioned, assuming that the main risk factor for complications is the concentration of the drug and the quality of venous access. Few recent studies have been conducted regarding adverse effects of peripheral drug administration, and most of those that describe deleterious effects are case reports. This scoping review was conducted to address this gap in the literature, with a view to resolving the question in the population-concept-context (PPC) framework.
Population: Hospitalised patients in care areas (emergency, ICU, surgical, and haemodynamics); concept: adverse effects; context: peripheral administration of norepinephrine. What are the adverse effects of peripheral administration of norepinephrine in patients in care areas (ICU, emergency, surgical, and haemodynamics) and the characteristics of drug administration? The aim of this study is to describe the adverse effects related to the administration of norepinephrine through short peripheral venous access in hospitalised patients and the characteristics of administration of the drug.
MethodologyTo develop this scoping review, we conducted a reproducible search of PubMed, Scopus and Scielo, Redalyc, Cochrane and CUIDEN databases, with no database start date, with a deadline of 30 December 2021 to identify studies that investigated the use of norepinephrine through the peripheral route. The last search day was 06 June 2022. The search strategy in PubMed and Scopus databases was: ('noradrenaline' OR 'norepinephrine') AND ('peripheral infusion' OR 'peripheral cannula' OR 'peripheral administration' OR 'catheterisation, peripheral') AND ('adverse effects'), in title, keywords and MeSH terms. This search strategy was simplified for the other databases (noradrenaline OR norepinephrine) AND (adverse effects OR 'peripheral administration' OR 'catheterisation, peripheral'). All identified records were assessed by two independent investigators (AA) and (JG). According to the inclusion/exclusion criteria, a preliminary database was constructed in Microsoft Excel, with the title and abstract of the articles exported directly from the databases, based on this preliminary data record a second selection of articles was made, and before excluding an article the full text was accessed. Conflicts between two reviewers were initially resolved by consensus, and if necessary, the principal investigator (JCG) was consulted. An extended snowball search was performed by checking references of included articles.
Inclusion and exclusion criteriaRandomised clinical trials, observational, cohort or case series studies involving patients in hospital settings (ICU, surgical, emergency, and other wards) receiving norepinephrine via a short peripheral venous access, with no limit on infusion, concentration, or dose of the drug, were established as inclusion criteria for this review.
We excluded studies addressing the use of norepinephrine via midline or peripherally inserted central venous accesses, or clinical studies in non-hospitalised patients, with no language or temporal limit. Letters to the author, editorials, narrative reviews were excluded.
Results of interestThe primary outcome or main variable of interest during the review was the occurrence of any adverse events related to the administration of norepinephrine via short peripheral venous catheter, in particular extravasation, skin necrosis, limb ischaemia, compartment syndrome, infection, and any other adverse effects, as well as adverse reactions requiring treatment, the type of treatment used (phentolamine, terbutaline, or other). Secondary variables of interest included the characteristics of drug administration: peripheral treatment time, dose, concentration, diluent, and posthumous requirement of a central venous catheter.
Once the articles were selected, all data, abstracts, figures, tables and full text, were extracted independently by two members of the research team, who formed a database in Microsoft Excel from which data analysis was performed. In addition, tools were used to assess the quality of the studies: STROBE for observational studies and CARE for case reports. Two members of the research team undertook this process independently, validating whether each of the items had been met; a pooling was done to review and discuss cases where assessment did not coincide; discrepancies were resolved by consensus with the principal investigator.
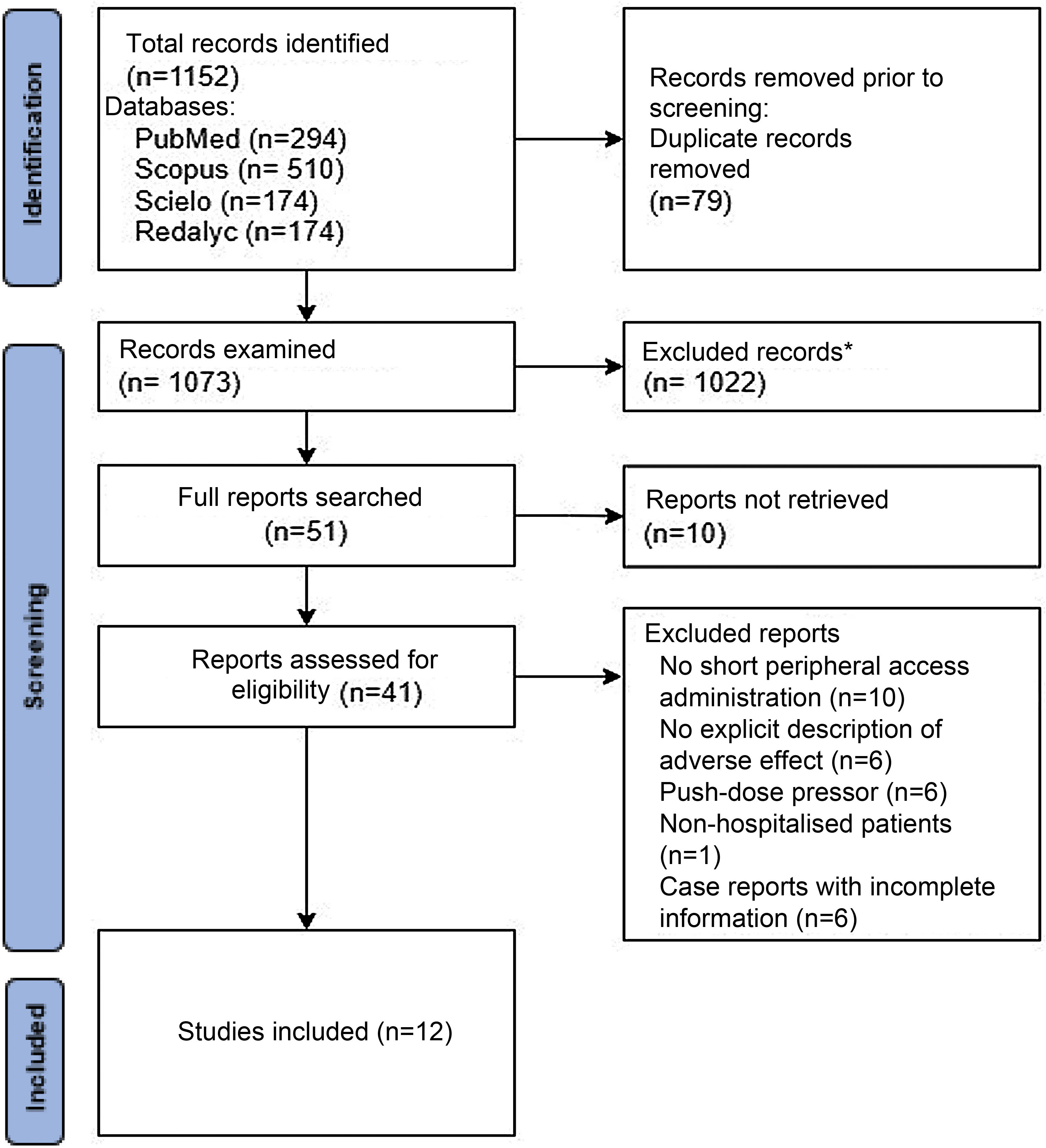
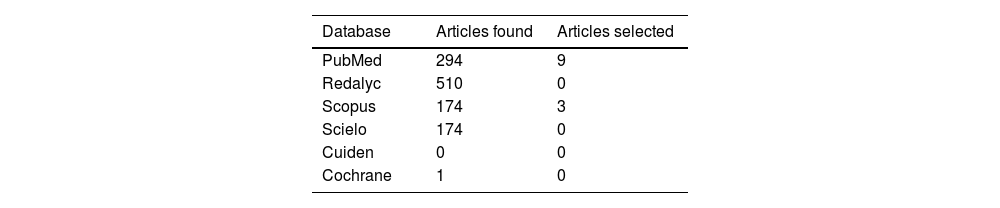
ResultsA total of 1,152 articles were retrieved in the scientific databases, after removing duplicates (79), 1,022 articles were excluded by title and abstract; it was not possible to access 10 full texts, 41 full texts were evaluated, and 29 were excluded based on the criteria mentioned above. It is worth highlighting the case reports excluded for describing incomplete information or low methodological quality. Nevertheless, a summary table of these articles, which describe cases of severe skin lesions due to peripheral administration of the drug, but with few data and low methodological quality, is attached (Appendix B Annex 1). In total, 12 studies were selected, 11 observational and one systematic review. The document selection process can be seen in the document cleansing tree (Fig. 1) and the number of articles selected for each database can be seen in Table 1.
In the studies reviewed, 16,021 norepinephrine administrations were analysed in a total of 15,874 patients. This difference lies in the administration of norepinephrine via different peripheral venous accesses in the same patient described in several studies.
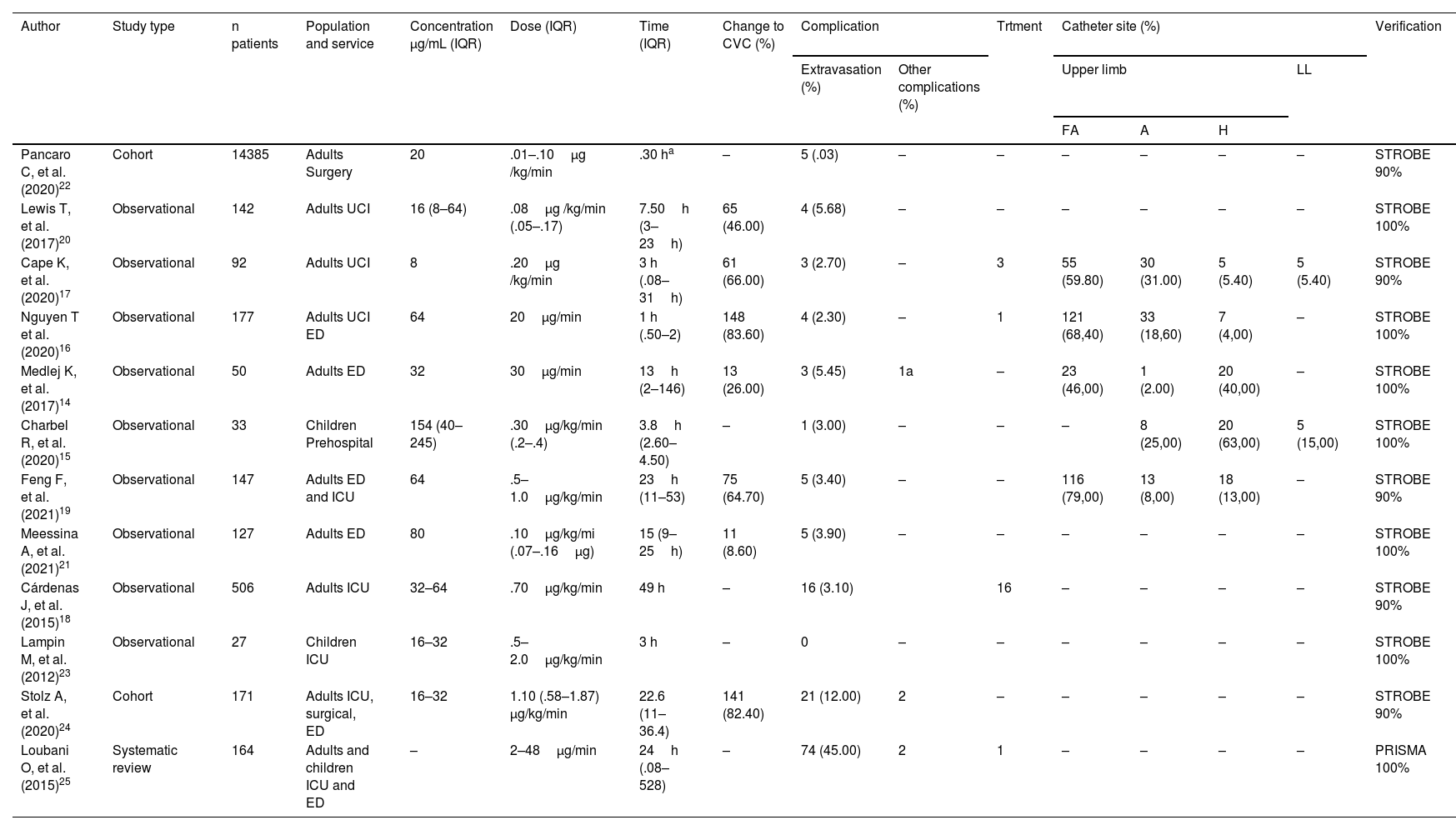
The concentration range of peripheral norepinephrine varied from 8μg /mL to 245μg/mL, with a weighted mean of 34.83μ/mL; concentration of the drug, although heterogeneous in the studies reviewed, is noteworthy in the study by Charbel et al.15 with a mean concentration of 154μg/mL, administered in times of less than 4.5h, but with a low proportion of adverse events. Saline .9% (NSS) was used as diluent in five of the studies, in the remaining studies both NSS .9% and dextrose in 5% distilled water (DDW 5%) were used; no other specific characteristics of the drug mixture are described in the studies analysed, such as post-dilution pH, drug brand, or pharmaceutical laboratory, or the frequency of the mixture change.
Dosage was another heterogeneous parameter, both in terms of measurement and ranges. In the studies by Nguyen et al.16 and Medlej et al.,14 doses not associated with patient weight were used; that is, μg/min with a range between 20 and 30μg/min, whereas in the other research studies the dosage was μg/kg /min, with a range .1 and 2μg/kg /min and with a weighted mean dose of .17μg/kg /kg/min. Administration time ranged from 1 to 528 hours, with a weighted mean of 2.78h. Peripheral administration of norepinephrine over periods longer than 24h was restricted in the studies by Cape et al.,17 in others,18 protocols for concentration, administration route, and timing were established. However, no correlations between administration time and the presence of medium and short-term complications were described. Catheter diameter and location varied from #22G to #18G, the catheter material is not described in the studies reviewed.
The site of the catheters was described in five of the reviewed studies; the main site was the forearm, followed by the arm, back of the hand, and lower limbs. In the studies by Cape and Nguyen, extravasations on the back of the hand with surrounding erythema were described, which were given phentolamine without further repercussions. The other studies do not describe complications according to catheter insertion site. The description of each of the variables analysed in each study can be seen in Table 2.
Characteristics of peripheral administration of norepinephrine and adverse effects.
| Author | Study type | n patients | Population and service | Concentration μg/mL (IQR) | Dose (IQR) | Time (IQR) | Change to CVC (%) | Complication | Trtment | Catheter site (%) | Verification | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extravasation (%) | Other complications (%) | Upper limb | LL | ||||||||||||
| FA | A | H | |||||||||||||
| Pancaro C, et al. (2020)22 | Cohort | 14385 | Adults Surgery | 20 | .01–.10μg /kg/min | .30 ha | – | 5 (.03) | – | – | – | – | – | – | STROBE 90% |
| Lewis T, et al. (2017)20 | Observational | 142 | Adults UCI | 16 (8–64) | .08μg /kg/min (.05–.17) | 7.50h (3–23h) | 65 (46.00) | 4 (5.68) | – | – | – | – | – | – | STROBE 100% |
| Cape K, et al. (2020)17 | Observational | 92 | Adults UCI | 8 | .20μg /kg/min | 3 h (.08–31h) | 61 (66.00) | 3 (2.70) | – | 3 | 55 (59.80) | 30 (31.00) | 5 (5.40) | 5 (5.40) | STROBE 90% |
| Nguyen T et al. (2020)16 | Observational | 177 | Adults UCI ED | 64 | 20μg/min | 1 h (.50–2) | 148 (83.60) | 4 (2.30) | – | 1 | 121 (68,40) | 33 (18,60) | 7 (4,00) | – | STROBE 100% |
| Medlej K, et al. (2017)14 | Observational | 50 | Adults ED | 32 | 30μg/min | 13h (2–146) | 13 (26.00) | 3 (5.45) | 1a | – | 23 (46,00) | 1 (2.00) | 20 (40,00) | – | STROBE 100% |
| Charbel R, et al. (2020)15 | Observational | 33 | Children Prehospital | 154 (40–245) | .30μg/kg/min (.2–.4) | 3.8h (2.60–4.50) | – | 1 (3.00) | – | – | – | 8 (25,00) | 20 (63,00) | 5 (15,00) | STROBE 100% |
| Feng F, et al. (2021)19 | Observational | 147 | Adults ED and ICU | 64 | .5–1.0μg/kg/min | 23h (11–53) | 75 (64.70) | 5 (3.40) | – | – | 116 (79,00) | 13 (8,00) | 18 (13,00) | – | STROBE 90% |
| Meessina A, et al. (2021)21 | Observational | 127 | Adults ED | 80 | .10μg/kg/mi (.07–.16μg) | 15 (9–25h) | 11 (8.60) | 5 (3.90) | – | – | – | – | – | – | STROBE 100% |
| Cárdenas J, et al. (2015)18 | Observational | 506 | Adults ICU | 32–64 | .70μg/kg/min | 49 h | – | 16 (3.10) | 16 | – | – | – | – | STROBE 90% | |
| Lampin M, et al. (2012)23 | Observational | 27 | Children ICU | 16–32 | .5–2.0μg/kg/min | 3 h | – | 0 | – | – | – | – | – | – | STROBE 100% |
| Stolz A, et al. (2020)24 | Cohort | 171 | Adults ICU, surgical, ED | 16–32 | 1.10 (.58–1.87) μg/kg/min | 22.6 (11–36.4) | 141 (82.40) | 21 (12.00) | 2 | – | – | – | – | – | STROBE 90% |
| Loubani O, et al. (2015)25 | Systematic review | 164 | Adults and children ICU and ED | – | 2–48μg/min | 24h (.08–528) | – | 74 (45.00) | 2 | 1 | – | – | – | – | PRISMA 100% |
A, Arm; a, Thrombophlebitis; FA, Forearm; H, Hand; IQR, Interquartile Range; LL, Lower limbs; Trtment, Treatment; (-) values not described.
Adverse events ranged from .03% to 12%. Complications described were only local: extravasation, leakage, and phlebitis. Of these, extravasation was the most frequent with 141 cases (88%), four leaks (10%), and three described as erythema and one with thrombophlebitis (1.9%). None of these had ischaemia or ulcerative lesions at the venipuncture site. The management plan for complications established in four of the studies analysed coincides in discontinuing administration, assessing the administration site, aspirating the remaining medication in the subcutaneous tissue through the catheter, elevating the limb, and applying warm compresses; in addition to this, three studies13,16,17 propose prophylactic pharmacological measures to treat extravasations. In this regard, 21 (40%) of the extravasations were treated prophylactically with phentolamine, in ranges of 5–10mg diluted in 10ml of saline solution subcutaneously at the site of extravasation; in three cases, both subcutaneous phentolamine and nitroglycerin ointment were used prophylactically; one study13 proposes the use of subcutaneous terbutaline, 1mg diluted to 10ml NSS .9% at the site of extravasation as an alternative, but its use was not necessary. The remaining cases of complications are reported in studies where there was no pre-established management of patients with extravasation, such as prophylaxis or treatment of leaks or phlebitis.
No systemic complications associated with the use of peripheral norepinephrine such as hypotension, tachycardia, rash, or deaths associated with its use were described, nor were any surgical interventions required due to administration of the drug.
The research studies where a mean dose of norepinephrine close to .7μg/kg/min was used described greater complications.18,19 However, none of the studies performed bivariate or correlational analyses comparing dose and complications. The studies with doses lower than .2μg/kg/min had a complication rate of less than 3 per 100 patients; administration time was not always restricted to 24h. In some studies19,22,24 there was no time limit and in these, the incidence of complications was higher, that is, between 5% and 12%, while in those that restricted infusion time to ranges below 24h13,20 fewer extravasations occurred. Similarly, those21 implementing a protocol for insertion, verification, and periodic assessment of venous access demonstrated safer administration. Pancaro et al.22 used hourly assessments of venous access by the nurse, while Cape et al.17 proposed two-hourly assessments by nurses. In both studies phlebitis assessment scales and extravasation management strategies were established and complications remained below 3%.
Need for central venous accessSome studies assessed patients who required central venous catheter implantation for infusion transfer, 15,379 surgical patients in particular; for short administration times no central venous catheters were implanted, while patients in ICU and EDs had a higher proportion of subsequent insertion of central accesses. A total of 568 central venous catheters (CVC) were implanted after initiation of peripheral norepinephrine, corresponding to 3% of cases; however, of these cases, 72% of insertions were performed to comply with institutional protocols, 13% were necessary due to the patient's clinical condition, and the remaining 15% due to extravasations or impossibility of cannulating a new peripheral venous access to administer concomitant treatment. Complications associated with CVC administration were not analysed, nor were the costs associated with its implantation. Table 2 gives a summary of the articles included.
DiscussionThe administration of vasopressors is a primary management strategy in haemodynamically unstable patients in different clinical areas, emergency, ICU, and surgical services. The most widely used vasopressor in different studies is norepinephrine.25 Norepinephrine can be administered either intravenously or via CVC.26 No statistically significant association has been described between administration through the peripheral route and the presence of local or systemic complications. However, in clinical practice, the use of vasopressors is still almost exclusively via the central route with high rates of central venous catheter insertion, which pose a higher risk of serious complications and infections associated with vascular devices, higher costs of care, longer insertion time and the need for expert professionals for their insertion and care,27 and close monitoring of patients by qualified personnel and in highly complex care units, although the implementation of protocols to standardise the administration of this drug via the peripheral route is recommended, defining criteria for administration, patients, professionals, assessment periods every two hours or less at doses higher than .1μg/kg/min and the management of possible extravasation of the drug. It is also necessary to consider other characteristics for peripheral administration, insertion site, catheter diameter, pH of the drug and of the mixture. Some of these variables were not described in the studies reviewed; however, administration in the lower limbs or dorsal veins of the hand, where intravascular hydrostatic pressure is higher, flow is lower, and there is less subcutaneous tissue, and therefore the theoretical risk of extravasation and ulceration is higher, should be avoided. In some case reports6,28 where it was administered in lower limbs, ulcers and necrosis occurred and required grafting.
While the rationale for avoiding the use of norepinephrine for peripheral access is mainly from case reports29,30 that describe local necrosis associated with norepinephrine administration, the characteristics of drug administration and other predisposing factors in patients are not extensively described, precautions are needed before (standardisation of practice and staff training), during (assessment of the characteristics and needs of the patient, characteristics of the drug) and after, and routes need to be established for the care of patients with extravasation, especially in centres that under certain conditions use the peripheral route to administer this drug.
Peripheral venous access cannulation has evolved to the point where various techniques are now used to obtain and assess peripheral venous access, from transillumination to ultrasound,31 and predictive scales have been designed for patient assessment. The creation and development of intravenous therapy equipment is helping to provide quality care for patients with peripheral venous access.32 These advances in peripheral vascular access have resulted in low complication rates during vasopressor administration.20 It should be noted that with vasopressor at high doses there is a risk of limb ischaemia, regardless of whether administered peripherally or locally. As such, according to the results of this review, the complications of peripheral administration are also related to high doses and concentrations, and therefore it seems reasonable to use norepinephrine peripherally at concentrations lower than .2 μg/kg /kg/min, with standardised protocols for insertion and for evaluating patients receiving norepinephrine peripherally.
The studies reviewed did not analyse the costs associated with CVC administration of norepinephrine compared to peripheral administration. According to other studies,33,34 only the insertion of a CVC results in longer hospital stays and higher health care costs. In the same vein, other authors35 analysed the net costs of CVC insertion, observing an average cost per catheter of 231,718 Colombian pesos (60 USD), of which 111,495.7 (29 USD) corresponds to the cost of the catheter, while the remainder is for the supplies and human talent necessary for its insertion. In contrast, the net cost of inserting a peripheral venous access is around 45,000 Colombian pesos. However, beyond the cost and risks of these devices, both central and peripheral venous accesses require strict care by the nurse. Although CVC is usually associated with a higher risk of infection, peripheral venous access can also lead to thrombophlebitis and septicaemia.35
LimitationsThe studies included in this review were heterogeneous, both in population, methodology, variables, and quality. Many variables that could be determinants were not included in these studies, such as staff training, education and experience, workload, additional drug and mixture characteristics (pH, osmolarity, pharmaceutical laboratory), insertion site, catheter (insertion and withdrawal time, material, diameter, length, brand), and patient characteristics or risk factors, and further studies are needed to address these gaps in knowledge.
ConclusionRegarding concentration, concentrations below 22μg/mL appear to be safe. Peripheral norepinephrine administration doses in studies where no complications occurred ranged from .02μg/mL/kg/min to .13μg/mL/kg/min. However, nurses must closely assess these patients not only by monitoring, but also by inspection, palpation, and even ultrasound assessment of the insertion site, especially in patients with neurosensory impairment. Delaying the administration of a drug such as norepinephrine can be fatal for the patient, but to administer it safely it is necessary to take into account the characteristics of the drug, the insertion site and type of catheter, the speed, the concentration, risk factors of the patient, and the setting.
In the observational studies included in this review, the main complication was drug extravasation. Although no subsequent necrosis or ulceration occurred, prophylactic interventions ranging from local heat to subcutaneous phentolamine or terbutaline and topical nitroglycerin were used. Pathways of action for extravasation should therefore be available in hospitals where peripheral administration is warranted.
FundingThis article is a product of the Family and Community Health research group of the Corporación Universitaria Remington.
Conflict of interestsThe authors have no conflict of interests to declare.