Onchocerciasis is caused by Onchocerca volvulus and mainly leads to pruritus and skin and visual disorders, including blindness. Seventeen million people are infected in 38 countries; 31 of these are in sub-Saharan Africa, six in Latin America and one on the Arabian Peninsula. More than 99% of cases occur in sub-Saharan Africa where 120 million people are at risk of infection. Eye disorders have been well-documented; however, skin disorders have not been described accurately. The objective of our study was to describe the epidemiology, main skin manifestations and treatment of imported onchocerciasis.
Material and methodsA retrospective study was thus conducted by analysing the main demographic, clinical and treatment data regarding a cohort of 400 patients attending a reference clinical unit over a 17-year period.
ResultsMost patients were female (55%) with mean age 37.5±16.7 years. All the migrants came from sub-Saharan countries. The most frequently occurring dermatological symptom was pruritus. Ivermectin had been used as first-line therapy and adverse reactions had been described in 11 patients (3.2%).
ConclusionsThe results indicate the fact that there should be a clinical suspicion of onchocerciasis regarding immigrants from endemic areas having skin lesions compatible with the disease's profile or asymptomatic patients having eosinophilia or unexplained high IgE. Moreover, skin snips from the buttocks region were very fruitful and treatment with ivermectin was seen to be safe. This is the largest case series regarding imported onchocerciasis described up to the present time.
La oncocercosis está causada por Onchocerca volvulus que produce fundamentalmente trastornos cutáneos, prurito y alteraciones visuales. Diecisiete millones de personas están infectadas en 38 países; 31 de ellos en África subsahariana, 6 en América Latina y uno en la península arábiga. Más del 99% de los casos se producen en el África subsahariana, donde 120 millones de personas están en riesgo de infección. Mientras los trastornos oculares han sido bien documentados, los trastornos cutáneos no se han descrito con precisión. El objetivo de nuestro estudio es describir la epidemiología, las principales manifestaciones cutáneas y el tratamiento de la oncocercosis importada.
Material y métodosEstudio retrospectivo de una cohorte de 400 pacientes atendidos en una unidad de referencia a lo largo de un período de 17 años con los principales datos demográficos, clínicos y de tratamiento.
ResultadosLa mayoría de pacientes eran mujeres (55%) con una edad media de 37,5±16,7 años. Todos los migrantes procedían de países subsaharianos. El síntoma dermatológico más frecuente fue el prurito. La ivermectina fue el fármaco de elección, describiéndose reacciones adversas en 11 pacientes (3,2%).
ConclusionesLos resultados señalan de que se debe mantener una sospecha clínica de oncocercosis en inmigrantes procedentes de áreas endémicas y lesiones cutáneas sugerentes o en pacientes asintomáticos con eosinofilia o IgE inexplicada. Además, los pellizcos cutáneos de glúteos fueron altamente rentables. El tratamiento con ivermectina es seguro. Esta es la mayor serie de casos de oncocercosis importada descrita hasta la fecha.
Onchocerciasis affects around 17 million people in 38 countries in the world.1 Thirty-one of them are located in sub-Saharan Africa, six in Latin America and one in the Arabian Peninsula. More than 99% of cases occur in sub-Saharan Africa where 120 million people live at risk of infection.2,3
Different programmes have been done to control of onchocerciasis as “Onchocerciasis Control Programme” (OCP) (1974–2002), “Onchocerciasis Elimination Programme in the Americas” (OEPA) (1991–2012) and “African Programme for Onchocerciasis Control” (APOC) (1995–2015). OCP covered 52.5 million people in 11 West African countries. Continued monitoring ensures that onchocerciasis does not reinvade the countries covered by the programme. OEPA began in 1992 with the objective to eliminate the disease throughout the Americas by 2012 through biannual ivermectin treatment.4–6 APOC coordinated mass treatment with ivermectin in 16 sub-Saharan countries.7,8 The aim was to eliminate onchocerciasis in these countries by 2015 via annual ivermectin treatment.
Onchocerca volvulus is a major cause of chronic skin and eye lesions, often progressing to blindness in endemic areas and leading to serious socioeconomic consequences. The main clinical manifestations of onchocerciasis are pruritus, subcutaneous nodules, onchocercal skin disease, ocular changes (punctate and sclerosing keratitis, uveitis, optic atrophy, etc.) and some systemic features as fever or Nodding syndrome. Ocular pathology has been well-documented for a long time.9–12 However, skin disorders have not been accurately described and these lesions need to be clearly and practically classified. Murdock et al., classified skin lesions in 1993.13
The present study has examined a cohort of 400 patients having imported onchocerciasis who were diagnosed at the Carlos III Hospital's Tropical Medicine Unit in Madrid, Spain, over a 17-year period. Data were analysed regarding demographics, clinical and biological patterns, diagnosis, co-infection, treatment and outcome.
MethodsEthics statementThis was a retrospective analysis of data obtained over a 17-year period; such data were collected anonymously to ensure impartial analysis. Written informed consent therefore was not obtained from individual participants. The research was approved by the La Paz–Carlos III Hospital's Ethics Committee.
StudyLa Paz-Carlos III Hospital in Madrid, Spain, is a tropical disease referral unit. Most patients voluntarily attend the emergency unit or are referred from primary care or general hospitals in Madrid. A very small percentage of patients come from other regions.
A retrospective study was made of data regarding immigrants diagnosed as having onchocerciasis between January 1st 1993 and June 31st 2009. Pertinent medical files were reviewed; the data recorded there included demographics (age, gender, nationality, country where infected, time to first consultation) and clinical characteristics (symptoms, type of dermatosis first appeared). Eye examination results and analytical data regarding serologic tests for syphilis, HIV, hepatitis B and C, eosinophil count, IgE levels and stool test results regarding ova and parasites were reviewed. Other laboratory test results were also recorded.
The skin snips involved taking 3-mm punch biopsies from multiple sites (buttocks and back), placing them in normal saline solution and incubating them at room temperature for 24h to facilitate microscopic observation of the microfilariae emerging from the skin samples. The Mazzotti test consists of a 50mg oral dose of diethylcarbamazine (DEC) leading to the death of the microfilariae and associated symptoms of worsening pruritus about 20–90min later. The symptoms usually peak 24h later and then subside over the next 48–72h. The Mazzotti test had been used when direct diagnosis proved negative. Systematically ophthalmology exploration was done in patients with clinical suspicion of onchocerciasis and negative skin snips. Eosinophilia had been defined as an increase in peripheral blood eosinophilic leukocytes to more than 0.450×109eosinophils/L of blood. Relative eosinophilia had been defined as an elevated percentage of eosinophils (>5%) in individuals having <0.450×109eosinophils/L. Hyper-IgE had been defined as an increase in peripheral blood IgE more than 200U/mL, classified as being mild (>200–399U/mL), moderate (>399–999U/mL) and/or severe (>1000U/mL).
Immigrants having a diagnosis of onchocerciasis met the present inclusion criteria; diagnosis was established by the presence of one of the following: microfilariae in the skin and/or onchocercomata, microfilariae in the eyes on ophthalmologic examination or positive Mazzotti test. Exclusion criteria referred to unspecified diagnosis methods (i.e. specifying serological test and clinical data only) and medical records having missing data.
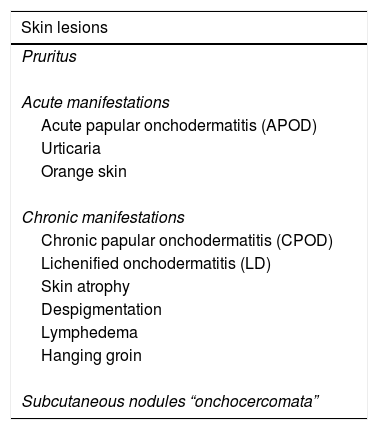
A modified Murdoch skin assessment system (i.e. clinical classification and grading of cutaneous changes in onchocerciasis) (Table 1)7 was used for current data analysis.
The classification of skin lesions Murdoch modified.7
| Skin lesions |
|---|
| Pruritus |
| Acute manifestations |
| Acute papular onchodermatitis (APOD) |
| Urticaria |
| Orange skin |
| Chronic manifestations |
| Chronic papular onchodermatitis (CPOD) |
| Lichenified onchodermatitis (LD) |
| Skin atrophy |
| Despigmentation |
| Lymphedema |
| Hanging groin |
| Subcutaneous nodules “onchocercomata” |
Categorical variable results were expressed as percentages and as the mean and standard deviation (SD) for continuous variables. A chi-square test was used for comparing the association between categorical variables (i.e. clinical and demographics variables); measured outcomes were expressed as the odds ratio (OR) together with 95% CI for OR. Continuous variables were compared by Student's t-test or Mann–Whitney test for two groups, depending on their normal or non-normal distribution. The corresponding regression models were also used for multivariate analysis, considering p<0.05 for statistically significant difference arising from chance. The Statistical Package for the Social Sciences (SPSS 23) was used for analysing all data.
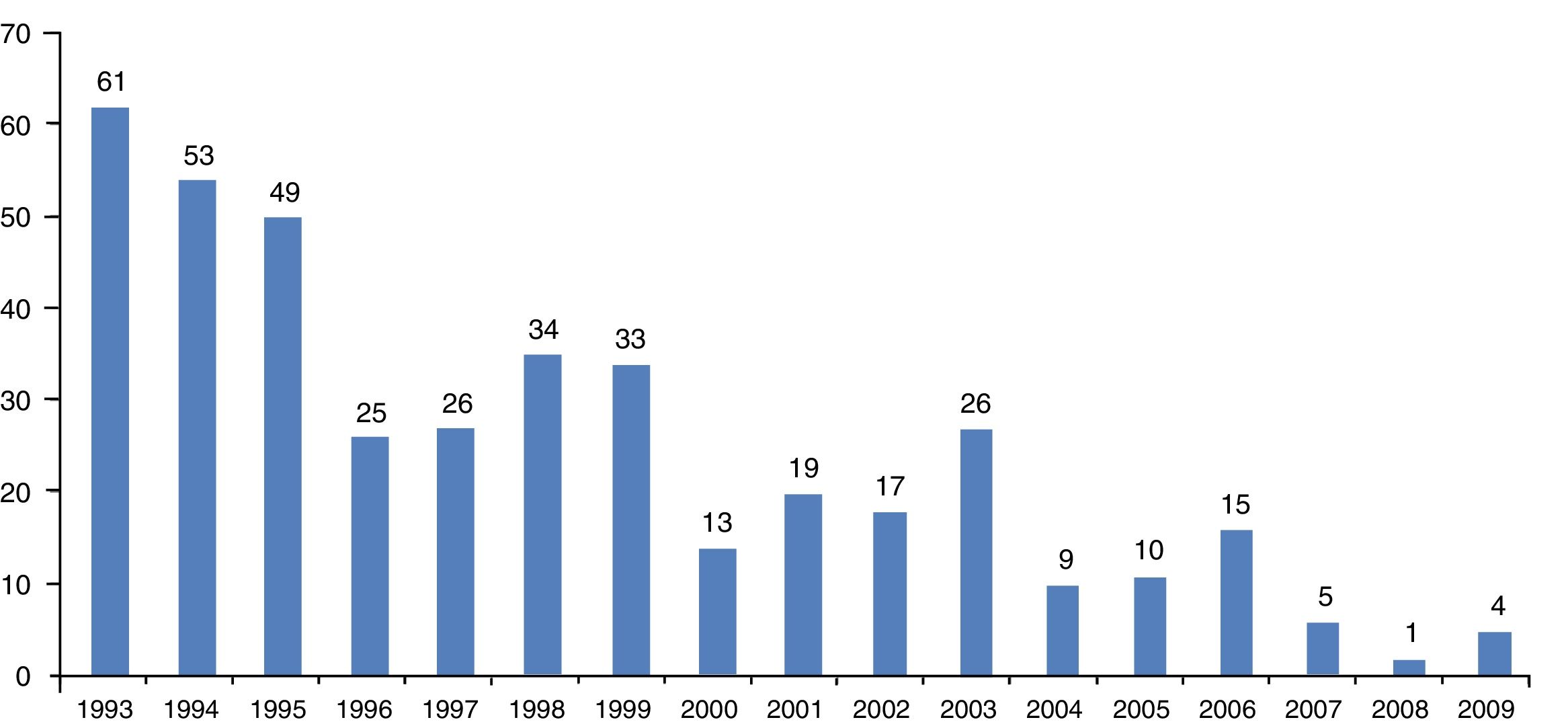
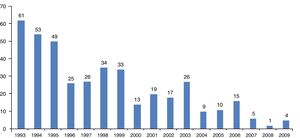
ResultsFour hundred cases of immigrants from tropical and subtropical areas diagnosed as suffering onchocerciasis were identified over the 17-year period at the Carlos III Hospital. A decrease in onchocerciasis cases had been observed during 1993–2009 (Fig. 1).
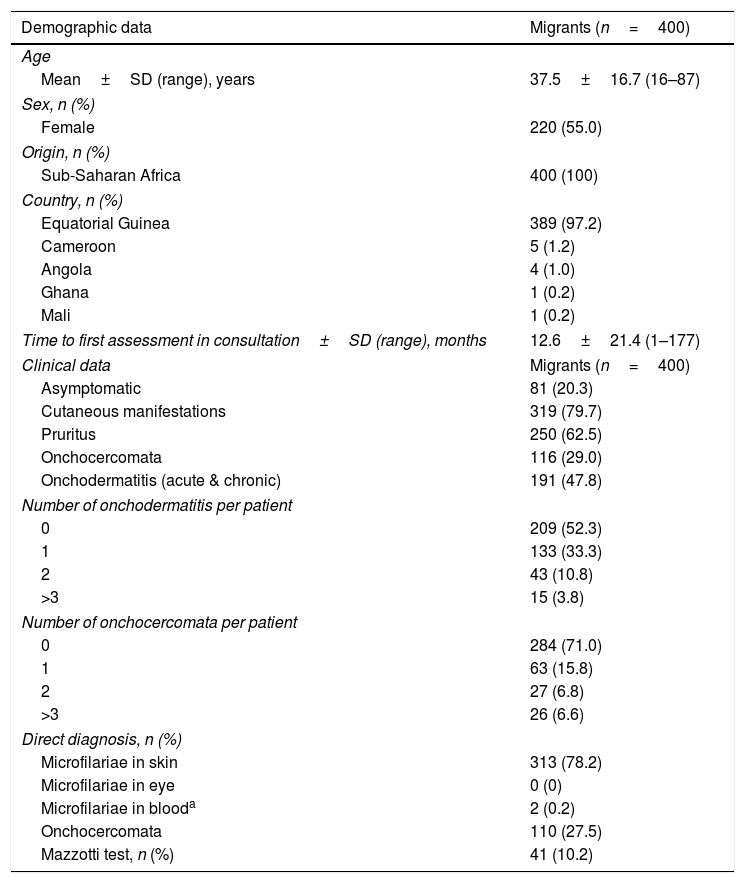
Epidemiological dataOne hundred and eighty patients were male (45%), 220 female (55%). Migrants’ mean age (±SD) was 37.5±16.7 years (range, 16–87), median (25th, 75th percentiles) value being 31 years (24, 52). All the migrants came from sub-Saharan countries and all patients were infected in their country of origin. Table 2 gives the baseline demographic features for the cohort of 400 patients having imported onchocerciasis. The time between arrival in Spain and the first consultation ranged from less than 1 month to 177 months (mean±SD, 12.6±21.4).
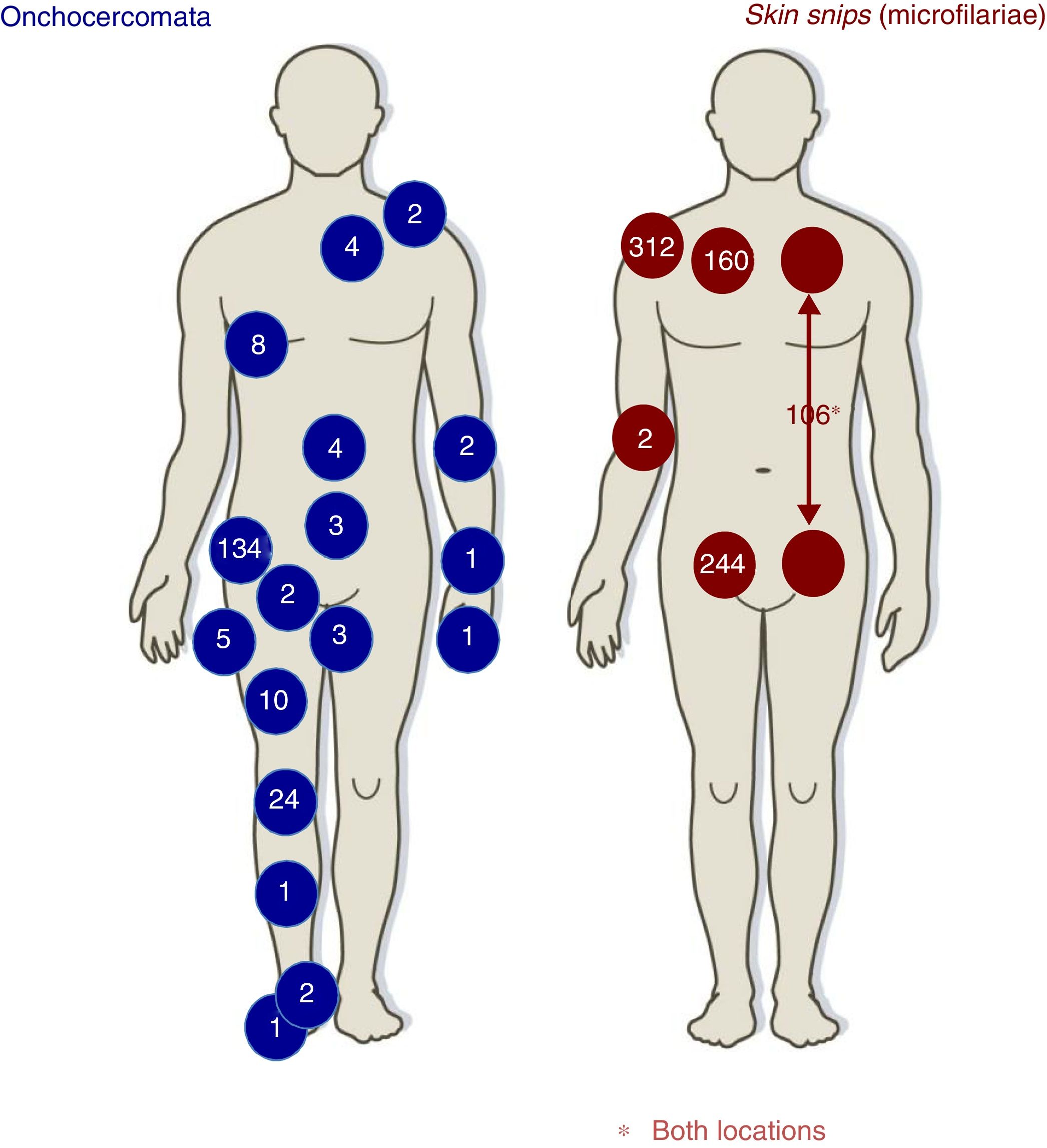
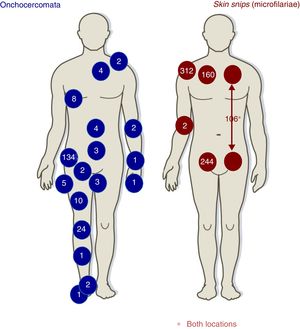
DiagnosisTable 2 lists the main forms of diagnosing onchocerciasis. Skin snips had been positive 313 (78.2%) patients; Fig. 2 describes skin snip distribution. Diagnosis made just by skin snip was 188/313 (60.0%), diagnosis by skin snip and onchocercomata was 64/400 (16.0%) and diagnosis just by onchocercomata was 46/110 (41.8%). Fig. 2 shows onchocercomata distribution.
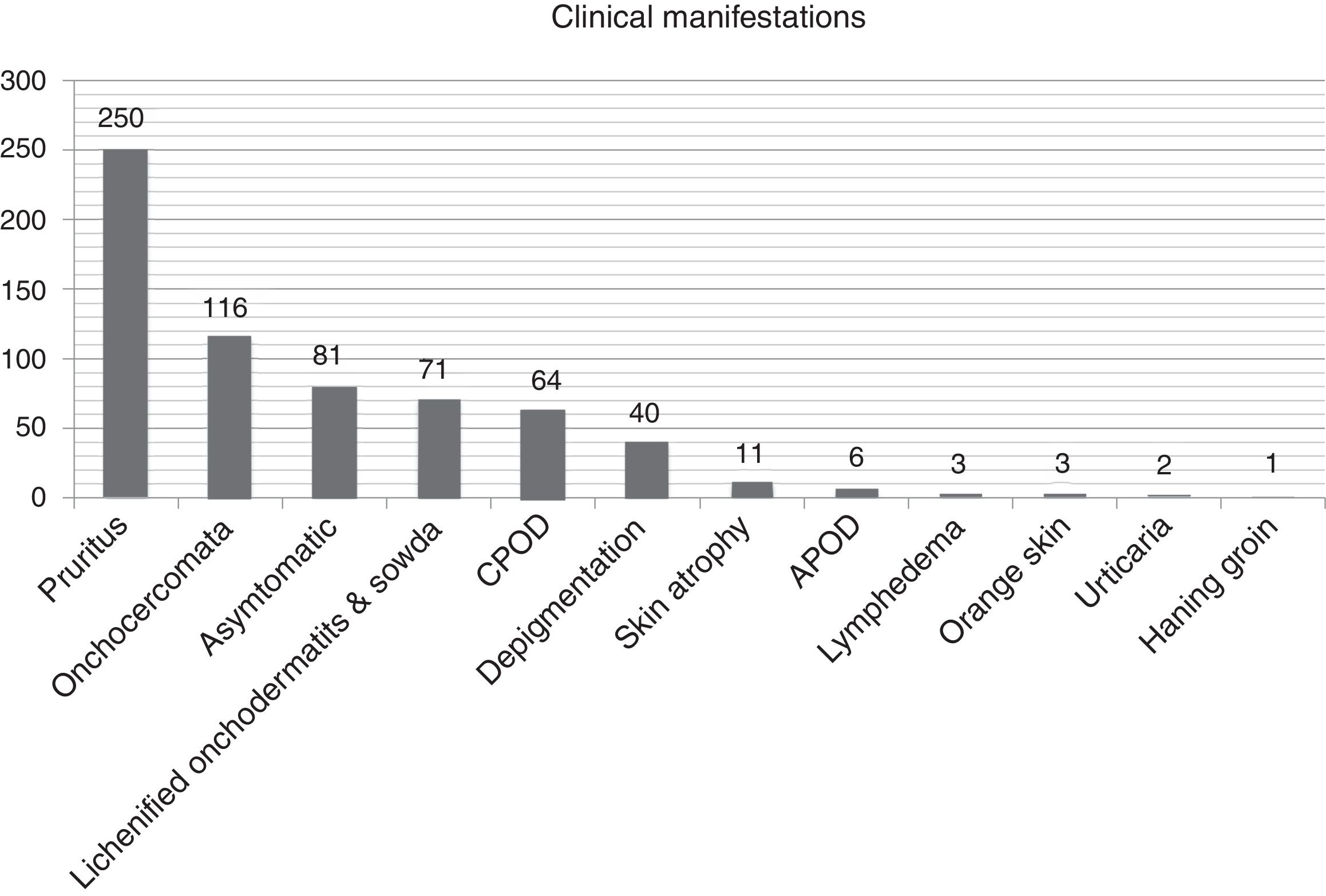
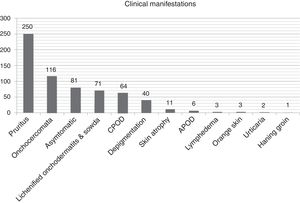
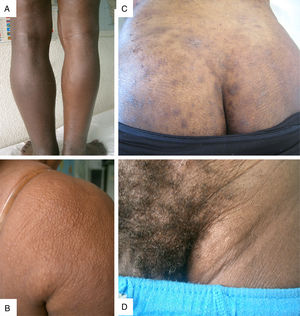
Clinical and biological patternTables 2 and 3 list the 400-patient cohort's main analytical and clinical features. The most frequently occurring dermatological symptom was pruritus (250 patients, 62.5%); Fig. 3 gives the main clinical forms of onchocerciasis. Fig. 4 shows representative clinical pictures of onchocerciasis. Isolated clinical manifestations occurred in 125 patients (31.3%); 194 (48.5%) patients had combined clinical manifestations, 150 (37.5%) had two symptoms and 44 (11.0%) three symptoms whilst 20.3% of the cohort (81 patients) were asymptomatic.
Main demographic and clinical data of migrants with onchocerciasis.
| Demographic data | Migrants (n=400) |
|---|---|
| Age | |
| Mean±SD (range), years | 37.5±16.7 (16–87) |
| Sex, n (%) | |
| Female | 220 (55.0) |
| Origin, n (%) | |
| Sub-Saharan Africa | 400 (100) |
| Country, n (%) | |
| Equatorial Guinea | 389 (97.2) |
| Cameroon | 5 (1.2) |
| Angola | 4 (1.0) |
| Ghana | 1 (0.2) |
| Mali | 1 (0.2) |
| Time to first assessment in consultation±SD (range), months | 12.6±21.4 (1–177) |
| Clinical data | Migrants (n=400) |
| Asymptomatic | 81 (20.3) |
| Cutaneous manifestations | 319 (79.7) |
| Pruritus | 250 (62.5) |
| Onchocercomata | 116 (29.0) |
| Onchodermatitis (acute & chronic) | 191 (47.8) |
| Number of onchodermatitis per patient | |
| 0 | 209 (52.3) |
| 1 | 133 (33.3) |
| 2 | 43 (10.8) |
| >3 | 15 (3.8) |
| Number of onchocercomata per patient | |
| 0 | 284 (71.0) |
| 1 | 63 (15.8) |
| 2 | 27 (6.8) |
| >3 | 26 (6.6) |
| Direct diagnosis, n (%) | |
| Microfilariae in skin | 313 (78.2) |
| Microfilariae in eye | 0 (0) |
| Microfilariae in blooda | 2 (0.2) |
| Onchocercomata | 110 (27.5) |
| Mazzotti test, n (%) | 41 (10.2) |
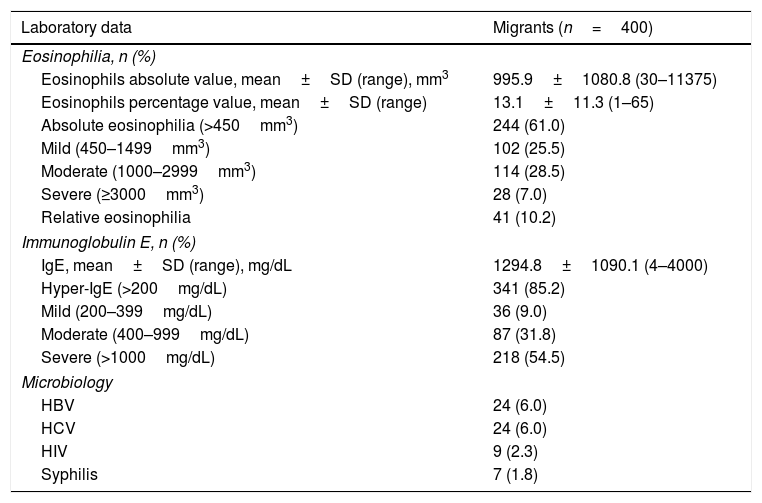
Main laboratory data of patients.
| Laboratory data | Migrants (n=400) |
|---|---|
| Eosinophilia, n (%) | |
| Eosinophils absolute value, mean±SD (range), mm3 | 995.9±1080.8 (30–11375) |
| Eosinophils percentage value, mean±SD (range) | 13.1±11.3 (1–65) |
| Absolute eosinophilia (>450mm3) | 244 (61.0) |
| Mild (450–1499mm3) | 102 (25.5) |
| Moderate (1000–2999mm3) | 114 (28.5) |
| Severe (≥3000mm3) | 28 (7.0) |
| Relative eosinophilia | 41 (10.2) |
| Immunoglobulin E, n (%) | |
| IgE, mean±SD (range), mg/dL | 1294.8±1090.1 (4–4000) |
| Hyper-IgE (>200mg/dL) | 341 (85.2) |
| Mild (200–399mg/dL) | 36 (9.0) |
| Moderate (400–999mg/dL) | 87 (31.8) |
| Severe (>1000mg/dL) | 218 (54.5) |
| Microbiology | |
| HBV | 24 (6.0) |
| HCV | 24 (6.0) |
| HIV | 9 (2.3) |
| Syphilis | 7 (1.8) |
Regarding the 116 (29%) patients having onchocercomata (208 onchocercomata in total), 54.3% (63 patients) had a single onchocercomata. Mean (±SD) onchocercomata/patient was 1.8±1.1 (range, 1–6), the most frequently occurring location being the pelvic girdle (79, 68.1%), followed by the lower extremities (33, 28.4%).
Regarding the 191patients with onchodermatitis, 133 of them had patients (69.6%) had single onchodermatitis. Mean (±SD) onchodermatitis/patient was 1.4±0.6 (range, 1–4), giving (in descending order of frequency) 71 (17.7%) patients having lichenified onchodermatitis-sowda, 40 (10%) depigmentation, 11 (2.7%) parchment skin atrophy, 6 (1.5%) acute papular onchodermatitis, 3 (0.7%) lymphedema 3 (0.7%) orange skin and 1 (0.2%) patient hanging groin.
Of the 400 migrants analysed, 244 (61%) had absolute eosinophilia (>450mm3), 102 (25.5%) mild eosinophilia (>0.45–0.99×109eosinophils/L), 114 (28.5%) moderate eosinophilia (>1.0–2.99×109eosinophils/L) and 28 (7.0%) severe eosinophilia (>3.00×109 eosinophils/L); 41 patients (10.2%) had relative eosinophilia. Regarding the 244 migrants having high eosinophil levels, 54 (22.1%) were asymptomatic and 190 (77.9%) were symptomatic; 152 (62.3%) migrants had pruritus, 68 (27.9%) had onchocercomata and 119 (48.8%) onchodermatitis. There were no significant differences between eosinophilia and clinical manifestations (p>0.050).
Regarding serum immunoglobulin-E levels, 45 (11.7%) migrants had normal levels and 341 (88.3%) hyper-IgE; 36 (9.0%) migrants had mild hyper-IgE levels (>200–399U/mL), 87 (31.8%) moderate hyper-IgE levels (>399–999U/mL) and 218 (54.5%) severe hyper-IgE levels (>1000U/mL). Concerning the 341 migrants having high IgE levels, 72 (21.1%) were asymptomatic, 211 (61.9%) had pruritus, 101 (29.8%) had onchocercomata and 167 (49.0%) had onchodermatitis. No significant differences were found between hyper-IgE and clinical manifestations (p>0.050).
The relationship between demographic and clinical variables, eosinophil count and IgE levels was further analysed; half of the 81 asymptomatic patients (40 migrants, 49.4%) had both eosinophilia and hyper-IgE (OR=7.5, 1.3–65.5 95%CI; p=0.037).
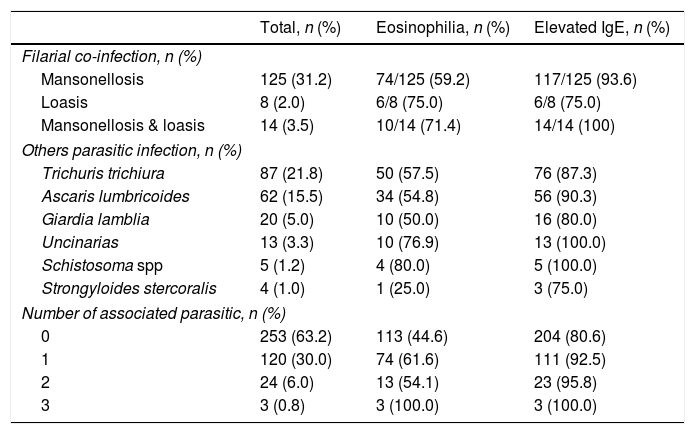
Thirty-two (8.0%) patients had cutaneous mycosis co-infection. Table 4 describes the main types of parasitic co-infection; 137 (34.3%) migrants had Mansonella perstans, 18 (4.5%) Mansonella streptocerca and 22 (5.5%) Loa loa. There was a statistically significant difference between eosinophilia and co-infection (p=0.030) but no significant difference between hyper-IgE and co-infection (p=0.060).
Parasitic co-infection in patients with onchocerciasis.
| Total, n (%) | Eosinophilia, n (%) | Elevated IgE, n (%) | |
|---|---|---|---|
| Filarial co-infection, n (%) | |||
| Mansonellosis | 125 (31.2) | 74/125 (59.2) | 117/125 (93.6) |
| Loasis | 8 (2.0) | 6/8 (75.0) | 6/8 (75.0) |
| Mansonellosis & loasis | 14 (3.5) | 10/14 (71.4) | 14/14 (100) |
| Others parasitic infection, n (%) | |||
| Trichuris trichiura | 87 (21.8) | 50 (57.5) | 76 (87.3) |
| Ascaris lumbricoides | 62 (15.5) | 34 (54.8) | 56 (90.3) |
| Giardia lamblia | 20 (5.0) | 10 (50.0) | 16 (80.0) |
| Uncinarias | 13 (3.3) | 10 (76.9) | 13 (100.0) |
| Schistosoma spp | 5 (1.2) | 4 (80.0) | 5 (100.0) |
| Strongyloides stercoralis | 4 (1.0) | 1 (25.0) | 3 (75.0) |
| Number of associated parasitic, n (%) | |||
| 0 | 253 (63.2) | 113 (44.6) | 204 (80.6) |
| 1 | 120 (30.0) | 74 (61.6) | 111 (92.5) |
| 2 | 24 (6.0) | 13 (54.1) | 23 (95.8) |
| 3 | 3 (0.8) | 3 (100.0) | 3 (100.0) |
Ivermectin had been used as first line anti-filarial drug in 345 cases. Adverse reactions had been described in 11 patients (3.2%): pruritus (6), oedema (3), fever (1) and encephalitis (1). Onchocercomata were removed from all patients. Post-treatment control was clinical in all cases. Mean ivermectin treatment cycle was 1.4±1.3 (Table 5); 231 (57.8%) patients had only received one course, leading to a good response. A preliminary microfilariae skin study had only been made for 10% (40) of the cohort, the result being positive in 31 of them (7.8%); the data for the rest of the cohort had been ignored or they had not been studied.
DiscussionO. volvulus infection causes chronic skin and eye injuries in endemic areas (often progressing to blindness), leading to serious socioeconomic consequences. Ivermectin (an effective microfilaricide) was introduced in 198214 and has been used for mass treatment since 1987 with good results.15 Currently, onchocerciasis treatment is based on administration of ivermectin and doxycycline, which has activity against Wolbachia showing sustained sterility of female worms.16
No broad or well-characterised series regarding onchocerciasis in immigrants are available. The present study describes the data related to 400 cases of imported onchocerciasis over a 17-year period. This disease is being controlled, given onchocerciasis programmes’ have been successfully in recent years and imported cases are likely to decline significantly.17,18 Therefore, this study has added historical value.
Equatorial Guinea was the country of origin for most patients in the target cohort. The island of Bioko is a hyper-endemic area for onchocerciasis. From the clinical point of view, there was a significant amount of asymptomatic cases (20.38%). The most prevalent cutaneous manifestation was pruritus, above 60%; it is often associated with onchodermatitis. The most frequently occurring location for the onchocercomata was on the pelvis. This data are similar to other series.19–21
Regarding laboratory data, 61% of the cohort had eosinophilia. This could be explained because leucopenia could be frequently associated in dark-skinned people. The patients having hyper-IgE exceeded 80%. Cohort data also revealed a high association for onchocerciasis with other filariasis, this being associated with higher eosinophilia.
The greatest onchocerciasis diagnosis cost-effectiveness has been given by skin snipping. Taylor et al., have highlighted the cost-effectiveness of performing six skin snippes (two on the shoulders, hips and calves) for diagnosing onchocerciasis.22 Cohort data showed that the gluteal regions gave more positive results. However, the scapular region was the most profitable location. Microfilariae can be detected in the peripheral blood of patients having high parasitemia (although not frequently) (one case in our series: 0.0025%). The number of patients having onchocerciasis was lower (around 30%) compared to other series reaching more than 80%.23–25 No microfilariae were detected in the ocular chamber.26
A topical Mazzotti test had not been performed on our patients because it had been considered equivalent to taking a cutaneous snippe at the patch site.27,28 The profitability of the oral Mazzotti test was similar to that described in other series.29 Since the cohort had come from a loiasis-endemic area before the Mazzotti test had been performed, the existence of L. loa microfilariae was discarded. We consider that its usefulness is essential in case difficulties arise in diagnosing loiasis and to avoid the general reactions attributed to oral diethylcarbamazine. Serological and molecular test have not been performed in this retrospective study. However, these tools could be used to improve the detection of cases of difficult diagnosis of onchocerciasis.
Adverse reactions only occurred in 2% of ivermectin treatment cycles and only one case was severe in a patient co-infected with Loa loa. Treatment response was satisfactory and similar to that reported in other studies.29,30 The number of cycles required was difficult to assess, given the impossibility of making a correct clinical follow-up in line with a profile of being an immigrant patient and this being an imported disease.
The main limitations are firstly a retrospective study and secondly the biased origin of patients, particularly from Equatorial Guinea. However, this work represents the largest series of imported onchocerciasis described in the literature to date.
Although a decrease in onchocerciasis-related cases is predicted during the next few years,31 a clinical suspicion of onchocerciasis should be maintained regarding immigrants from endemic areas having skin lesions compatible with the disease or in asymptomatic patients having eosinophilia or unexplained high IgE levels. Cutaneous snippes in the scapular region were the first diagnostic choice and treatment with ivermectin was seen to be safe. A single course of treatment resulted in more than 50% of the patients responding satisfactorily.
Conflict of interestsThe authors declare that they have no conflicts of interest.
Thanks to Virginia Velasco-Tirado for her help in this work.