Neisseria gonorrhoeae (NG) is one of the main causes of sexually transmitted infections and it is reaching high resistance levels worldwide. The aim of this study was to describe the antibiotic resistance, incidence and circulating sequence types of NG in the province of Lleida (Spain).
MethodsA total of 487 NG isolates were included in the study (2017–2024). Antibiotic susceptibility testing was performed by gradient diffusion following EUCAST criteria. NG-MAST was performed to 211 isolates in Centro Nacional de Microbiología (Majadahonda, Spain). The study of co-infections was done by real-time PCR (Allplex™ STI, Seegene®).
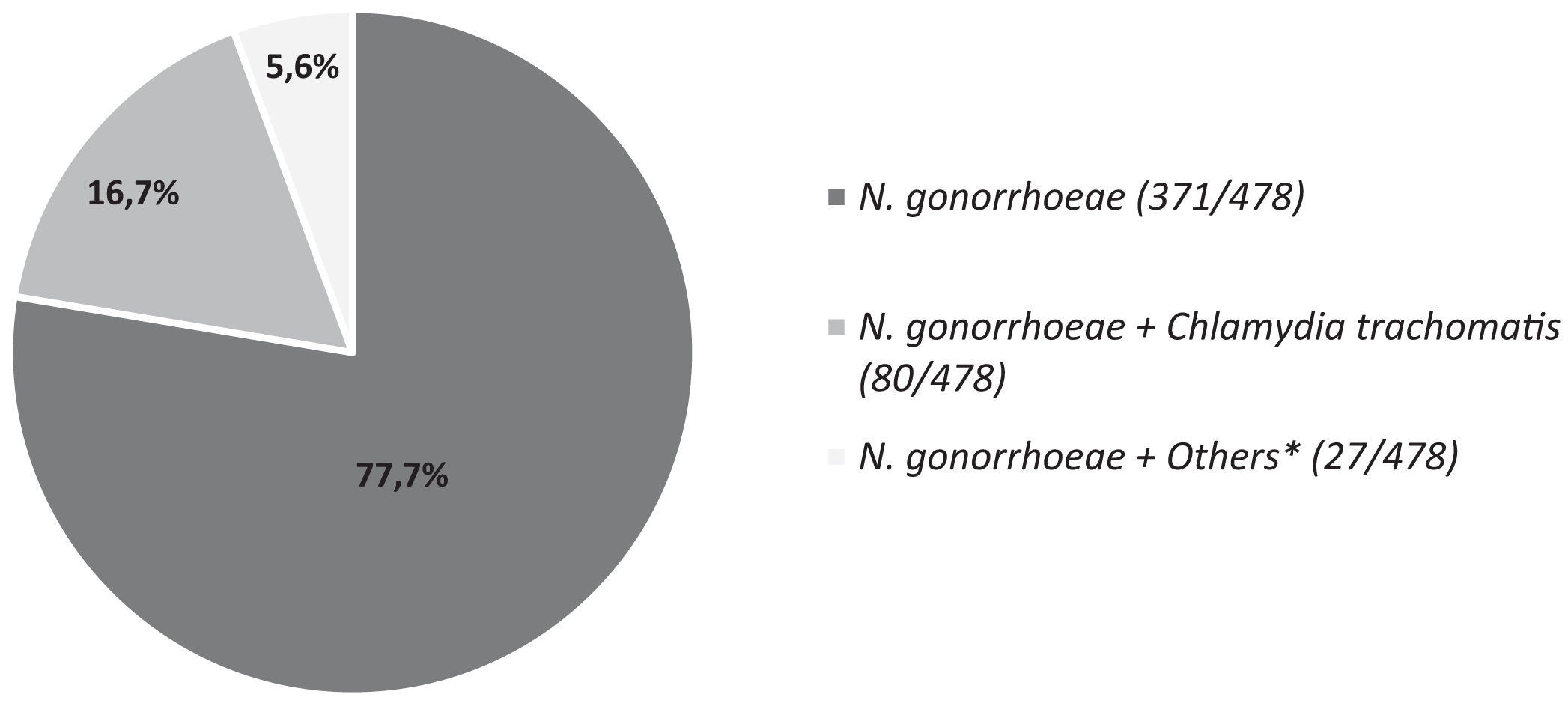
ResultsAll NG isolates remained susceptible to third-generation cephalosporins. The percentages of resistance to tetracycline, ciprofloxacin and penicillin G were 89.1%, 69.2% and 22.6% respectively. A 7.8% of isolates presented a MIC >1mg/L for azithromycin. A decrease in the incidence of gonococcal infections was detected during 2020, followed by a pronounced increase in next years. Ninety-seven different sequence types were detected. ST14994 (14.7%) and ST19792 (6.6%), were the most frequent ST detected in our study. NG appeared as a single STI agent in most cases (77.7%) and Chlamydia trachomatis was the most frequently detected STI agent (74.8%) in samples with co-infections.
ConclusionsNG incidence is increasing in our area. The lack of resistance to third-generation cephalosporins and the low level of azithromycin resistance suggest that the use of these antibiotics is a suitable option. Continuous surveillance is essential to prevent the emergence and spread of resistant NG isolates.
Neisseria gonorrhoeae es uno de los principales causantes de infecciones de transmisión sexual (ITS) y está alcanzando elevados niveles de resistencia a antibióticos. El objetivo de este estudio fue describir la resistencia a antibióticos, la incidencia y los secuenciotipos circulantes de N. gonorrhoeae en la provincia de Lleida.
MétodosSe realizó un estudio prospectivo (2017-2024) en el que se aislaron 487 cepas de N. gonorrhoeae. El antibiograma se realizó mediante tiras de difusión en gradiente siguiendo los criterios EUCAST. Se realizó N. gonorrhoeae-MAST en 211 aislados en el Centro Nacional de Microbiología (Majadahonda). El estudio de coinfecciones se realizó mediante PCR a tiempo real (Allplex™ STI, Seegene®).
ResultadosTodos los aislados permanecieron sensibles a cefalosporinas. Los porcentajes de resistencia a tetraciclina, ciprofloxacino y penicilina G fueron 89,1%, 69,2% y 22,6%, respectivamente. El 7,8% de los aislados presentó una CIM de azitromicina superior a 1mg/l. Se detectó una disminución de la incidencia de infección gonocócica en 2020, seguido de un aumento en los siguientes años. Se describieron 97 secuenciotipos diferentes. ST14994 (14,7%) y ST19792 (6,6%) fueron los más frecuentes. N. gonorrhoeae apareció como único agente de ITS en la mayoría de los casos (77,7%) y Chlamydia trachomatis fue el detectado con mayor frecuencia en las muestras con coinfección (74,8%).
ConclusionesLa incidencia de NG está aumentando en nuestra área. La ausencia de resistencia a cefalosporinas y los bajos niveles de resistencia a azitromicina sugieren que el uso de estos antibióticos es adecuado. Los estudios de vigilancia son esenciales para prevenir la diseminación de N. gonorrhoeae resistentes a los antibióticos.
Gonococcal infection is a sexually transmitted infection (STI) caused by Neisseria gonorrhoeae (NG), also known as gonococcus. It is a nonmotile Gram negative diplococcus that lacks a polysaccharide capsule and exclusively colonizes and infects humans.1 The urogenital epithelium including the cervix, uterus, and fallopian tubes in women, and the urethra in women and men, is the main site of infection; NG can also infect the conjunctiva, pharynx and rectal mucosa. Gonococcal infection is mainly transmitted through direct contact between mucosal surfaces, and it has a short incubation period (2–7 days after exposure to an infected partner). It also can be transmitted perinatally.1,2
Gonococcal infection is commonly asymptomatic or mildly symptomatic. The most frequent symptoms are urethritis in men and cervicitis in women, which can result in complications such as salpingitis or infertility.1–3
NG is currently the second most commonly reported sexually transmitted infection (STI) trailing only Chlamydia trachomatis.4 Regarding data published by the European Centre for Disease and Control, increasing rates of gonococcal infection incidence has been observed in Europe. A total of 70,881 cases were confirmed in 28 European countries in 2022 (incidence of 17.9 cases/100,000 inhabitants). The highest incidences were observed in Ireland (75.3 cases/100,000 inhabitants), Luxemburg (73.6 cases), Denmark (66.9 cases) and Spain (48.3 cases). The incidence reported in Europe in previous years (2021 and 2020) were 11.7 and 9.5 cases/100,000 inhabitants respectively.5 In addition, a notable increase of gonococcal infection since 2001 has been reported in Spain being Catalonia the most affected area.6
Samples collection, transport and processing are of particular importance for NG diagnosis. Early diagnosis along with appropriate treatment, in addition to all preventive and educational measures, are essential for the control of gonococcal infections.1–3
NG presents different mechanisms that make it resistant to different antimicrobials, such as the presence of β-lactamases, modification of the antimicrobial target or activation of efflux mechanisms. These antibiotic resistance mechanisms are the result of spontaneous mutations in DNA or acquisition of plasmids.2 The increasing resistance of NG to different treatment options is of great concern for public health. Since 2012, ceftriaxone plus azithromycin combination therapy has been officially recommended in all European countries. However, increasing resistance to broad-spectrum cephalosporins (ceftriaxone and cefixime) and especially azithromycin has conditioned their use. This fact underlines the importance of a careful selection of antibiotic prescriptions and the need to perform a continuous surveillance of the antibiotic susceptibility of NG isolates.1,8–10
This alarming scenario together with the increase in the number of cases worldwide and the absence of effective vaccines, show the need to implement control plans for gonococcal infection. In addition, molecular typing by N. gonorrhoeae multi-antigen sequence typing (NG-MAST) is essential to study dynamics of different NG populations.7,11–13
Moreover, there are studies that determine the rate of co-infection between different microorganisms responsible for STI. It is known that the co-infection rate is related to the microorganism involved and NG has the highest rate of single infection and the lowest rate of co-infection, followed by C. trachomatis.14,15
The aim of this study was to describe the antibiotic resistance profile of NG isolates from clinical samples and the sequence types circulating in the province of Lleida (Spain). The incidence of gonococcal infection in recent years has been determined. In addition, the co-existence of gonococcal infection along with other microorganisms related to STI was studied.
Material and methodsNG isolatesA prospective study was performed from January 2017 to March 2024 at Hospital Universitari Arnau de Vilanova (Lleida, north-east Spain), a referral tertiary hospital covering an area with approximately 340,000 inhabitants. Patient data was recovered from the Laboratory Information System. Samples obtained from different anatomical locations (urethral, endocervical, vaginal, ocular, pharyngeal, rectal and abscess) were studied. Samples with suspected STI arrived at the laboratory on DeltaSwab Amines Deltalab® swabs. After culture, NG isolates were obtained from Thayer-Martin selective culture medium (BioMerieux), or chocolate agar (BioMerieux), after incubation for 48h at 36±1°C and atmosphere at 5–10% CO2. NG identification was performed by matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF, Bruker Daltonics) obtaining a score >2 in all isolates, indicating high confidence of identification.
Antibiotic susceptibility testing and NG-MASTSix antibiotics (penicillin G, ceftriaxone, cefixime, tetracycline, ciprofloxacin and azithromycin) were included in the antibiotic susceptibility testing (AST). It was done by gradient diffusion using E-test strips (bioMèrieux) on Mueller Hington agar supplemented with 5% horse blood and incubated 24h at 36±1°C and atmosphere at 5–10% CO2. Classification of the antibiotic susceptibility results was performed using the following susceptibility breakpoints according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria16: penicillin ≤1mg/L, ceftriaxone ≤0.125mg/L, cefixime ≤0.125mg/L, tetracycline ≤0.5mg/L, and ciprofloxacin ≤0.06mg/L. The ECOFF cut-off point ≤1mg/L was used to categorize azithromycin.
Hospital Universitari Arnau de Vilanova (Lleida, Spain) began participating in the NG national surveillance program in May 2019 (https://cnm-laboratorios.isciii.es/Customization/News/Publicaci%c3%b3n_archivos/Page439.htm). Conducted at Centro Nacional de Microbiología (Majadahonda, Spain), this project performs multi-antigen sequence typing (NG-MAST) of NG collected from national microbiology laboratories.
Detection of co-infections by multiplex PCR (STI-PCR)Multiplex STI-PCR was performed in all samples with NG isolation. DNA was extracted using EZ1 or QIASymphony equipment (QIAGEN®), and real-time PCR screening was performed on the CFX96 qPCR instrument (Bio-Rad®) using the Allplex™ STI Essential Assay (Seegene®) for the detection of NG and other pathogens causing STI (C. trachomatis, Trichomonas vaginalis, Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma urealyticum, Ureaplasma parvum).
ResultsClinical and epidemiological dataA total of 487 culture-confirmed NG cases (one per patient) were detected during the period of study in the province of Lleida (catchment of 340,000 inhabitants) in north-east Spain. NG was isolated from different clinical samples: urethral (n=437), endocervical/vaginal (n=42), rectal (n=4), eye discharge (n=2), articular abscess (n=1), and pharynx (n=1).
Regarding sex distribution, the 90.6% (441/487) of samples corresponded to males and 9.4% (46/487) to females. The mean age was 31.9 years, with an age range of 15–76 years (median 29 years).
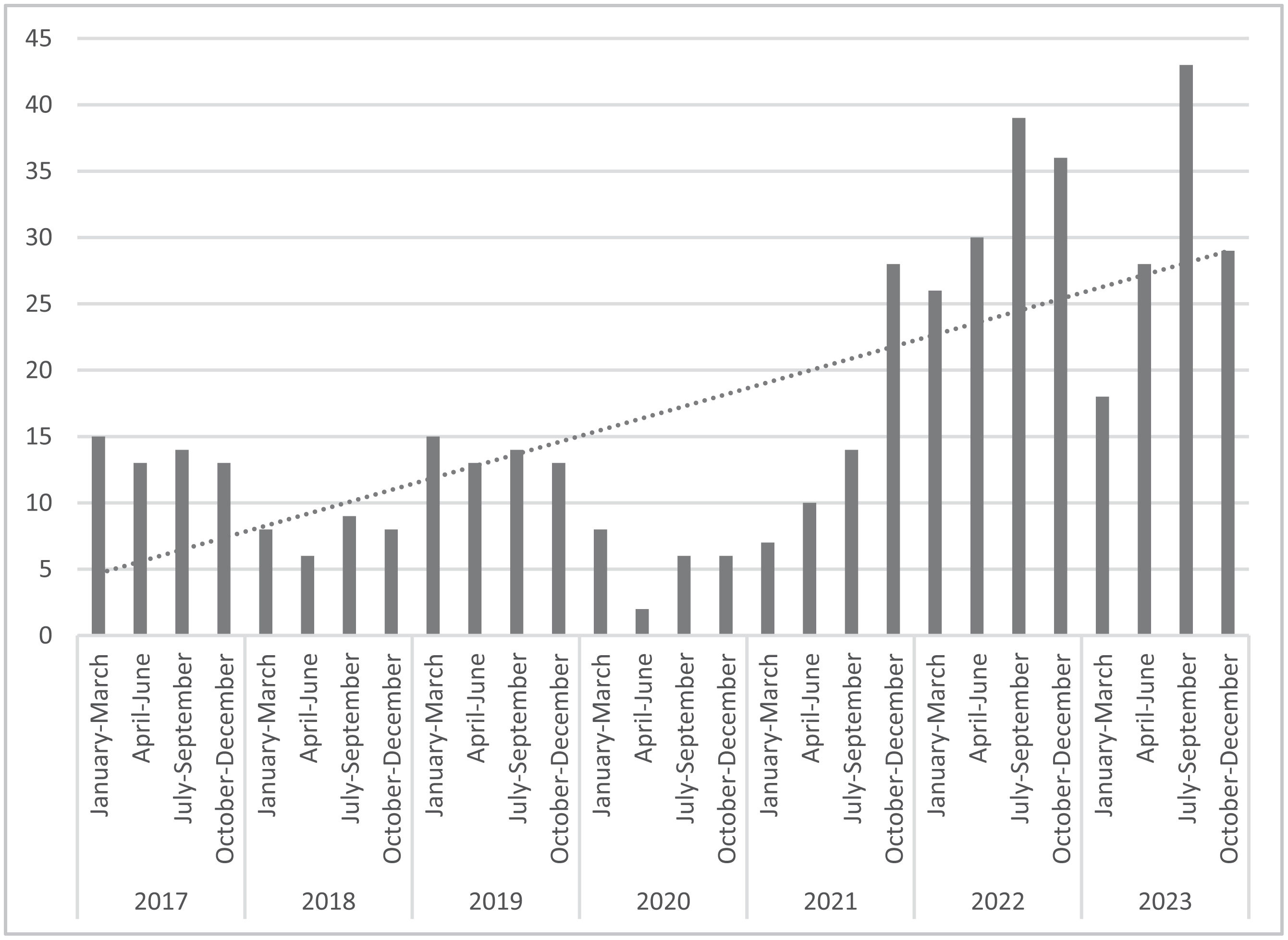
Temporal distribution of NG isolationFig. 1 shows the number of NG isolates by quarters (2017–2024). A large increase in the number of gonococcal infections was observed since 2017. A total of 55 NG were reported in 2019 (incidence 16.2 cases/100,000 inhabitants) followed by a decline in incidence during 2020 with 22 NG isolates (6.5 cases/100,000 inhabitants). The increase detected in 2021 with 59 cases (17.3 cases/100,000 inhabitants) surpassed 2019 incidence. The highest number of gonococcal infections was noticed in 2022 with 131 culture-confirmed cases (incidence 38.5 cases/100,000 inhabitants). A slight decrease in the number of gonococcal infection (n=118) was detected in 2023 but the incidence was higher than 2021 data (34.7 cases/100,000 inhabitants).
Antibiotic susceptibility of NG isolatesAll NG isolates studied were susceptible to third-generation cephalosporins (ceftriaxone and cefixime). The highest percentages of resistance detected were to tetracycline (89.1%; 438/487) and ciprofloxacin (69.2%; 337/487). The percentage of penicillin G resistant isolates was 22.6% (110/487). On the other hand, 7.8% (38/487) of NG isolates had an azithromycin MIC >1mg/L (ECOFF point), so they were classified as carriers of resistance mechanisms (non-wild-type phenotypes).7,8
Co-resistance to two antibiotics was detected in the 71.2% (347/487) of NG isolates. The most frequent co-resistance phenotype was tetracycline and ciprofloxacin (n=231) followed by tetracycline and penicillin G (n=78). Moreover, all azithromycin NG isolates with an azithromycin MIC >1mg/L were resistant to tetracycline (n=38). Sixty-nine isolates (69/487; 14.2%) were concomitantly resistant to three antibiotics (penicillin G, ciprofloxacin and tetracycline). Resistance to four antibiotics (penicillin G, ciprofloxacin, tetracycline and azithromycin MIC >1mg/L) was found in three NG isolates (3/487; 0.62%).
NG characterization by multi-antigen sequence typing (NG-MAST)Molecular typing was performed on 211 NG strains (43.3%, 211/487) and a great variety was detected. Isolates were grouped into ninety-seven different sequence types (STs) consisting of 74 porB and 42 tbpB alleles. The most frequently detected STs were ST14994 (14.7%, 31/211), ST19792 (6.6%, 14/211), ST13489 (4.3%, 9/211) and ST19665 (3.8%, 8/211). Seven NG isolates (3.3%, 7/211) belonged to ST587 and ST6765 and six belonged to ST5441 (2.8%, 6/211). The sequence types ST10421, ST17972 and ST21666 were represented with five isolates each one (2.4%, 5/211), and ST4186 and ST21388 with four (1.9%, 4/211). Three strains (1.4%, 3/211) belonged to ST3935, ST9208, ST10386 and ST21410. Two strains (0.9%, 2/211) belonged to each of the following types: ST5, ST26, ST5405, ST5743, ST17764, ST19002, ST19838, ST19912, ST19914, ST19987, ST20718, ST22292 and ST22642. Only one strain belonged to each of the following STs: ST338, ST340, ST384, ST470, ST612, ST758, ST1042, ST1407, ST1792, ST2997, ST4244, ST4261, ST4530, ST4822, ST5120, ST5547, ST6232, ST9918, ST11522, ST12307, ST12626, ST13892, ST14764, ST15090, ST15597, ST15737, ST15852, ST17495, ST17769, ST18598, ST18660, ST19383, ST19762, ST19764, ST19769, ST19843, ST19845, ST19913, ST19915, ST19956, ST20046, ST20157, ST20286, ST20343, ST20465, ST20532, ST20620, ST20698, ST20701, ST20704, ST20953, ST21020, ST21645, ST21668, ST21676, ST21743, ST21783, ST22001, ST22026, ST22214, ST22215, ST22253, ST22293, ST22684, ST22685, ST22695 and ST22816.
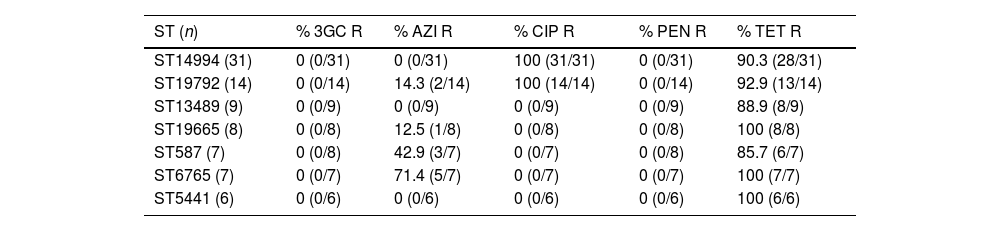
Table 1 shows the relation between resistance phenotypes and the main STs characterized among our NG isolates. It should be noted that all the 45 NG strains that belong to ST14994 and ST19792 were resistant to ciprofloxacin, while all the other isolates were susceptible to this antibiotic. A high percentage of resistance to tetracycline was observed in all NG strains regardless of the ST. The highest rates of azithromycin resistance were detected in ST587 and ST6765 with a 42.9% and 71.4% respectively. On the other hand, the total of these isolates remained susceptible to third-generation cephalosporins (ceftriaxone and cefixime) and penicillin G.
Antibiotic resistance and main sequence types of NG isolates.
| ST (n) | % 3GC R | % AZI R | % CIP R | % PEN R | % TET R |
|---|---|---|---|---|---|
| ST14994 (31) | 0 (0/31) | 0 (0/31) | 100 (31/31) | 0 (0/31) | 90.3 (28/31) |
| ST19792 (14) | 0 (0/14) | 14.3 (2/14) | 100 (14/14) | 0 (0/14) | 92.9 (13/14) |
| ST13489 (9) | 0 (0/9) | 0 (0/9) | 0 (0/9) | 0 (0/9) | 88.9 (8/9) |
| ST19665 (8) | 0 (0/8) | 12.5 (1/8) | 0 (0/8) | 0 (0/8) | 100 (8/8) |
| ST587 (7) | 0 (0/8) | 42.9 (3/7) | 0 (0/7) | 0 (0/8) | 85.7 (6/7) |
| ST6765 (7) | 0 (0/7) | 71.4 (5/7) | 0 (0/7) | 0 (0/7) | 100 (7/7) |
| ST5441 (6) | 0 (0/6) | 0 (0/6) | 0 (0/6) | 0 (0/6) | 100 (6/6) |
3GC: third-generation cephalosporin; AZI: azithromycin; CIP: ciprofloxacin; PEN: penicillin; R: resistance; ST: sequence type; TET: tetracycline.
The multiplex STI-PCR was performed on the 98.2% (478/487) of samples with NG isolation. Fig. 2 shows the percentages of co-infections. NG appeared as a single STI agent in most cases (371/478; 77.7%). Co-infections were noticed in the 22.3% (107/478) of samples and C. trachomatis was the most frequently identified STI agent (80/107; 74.8%). Other microorganisms causing STI as Mycoplasma sp. or Ureaplasma sp. appeared in the 5.6% (27/478) of samples tested.
DiscussionSexually transmitted infections have a deep impact on sexual and reproductive health worldwide. It is estimated that more than one million of these diseases are acquired every day.17
An increasing trend of gonococcal infection has been detected in the Microbiology Unit of the Hospital Universitari Arnau de Vilanova de Lleida (Catalonia, Spain) during the period of study, with a decrease observed in 2020 coinciding with the COVID-19 pandemic. This same trend has been recently described. The incidence of gonococcal infection per 100,000 inhabitants in Spain was 26.95 in 2019, with a decrease to 21.25 in 2020, and a notable increase again to 31.40 in 2021 and 49.00 in 2022. In fact, the highest rate per 100,000 inhabitants of gonococcal infection in Spain was that recorded in Catalonia in 2022 (121.88/100,000 inhabitants).6,18
Similarly, the same trend was observed in the data reported for the whole of Catalonia, with a decrease coinciding with the pandemics and an increase in the overall rate of gonorrhea of 44.1% in 2021 (6674 cases reported) compared to 2020. In this report, Lleida obtained the second lowest rate of gonococcal infection in all of Catalonia.19 According with our study, the incidence detected in the province of Lleida (16.2 in 2019, 6.5 in 2020, 17.3 in 2021, 38.5 in 2022 and 34.7 in 2023) was lower than aforementioned data. In our case, the highest number of gonococcal infection was also noticed in 2022 with an incidence of 38.5 cases/100,000 inhabitants and most patients were men (90.6%) as previously described.3,6
The excessive use of unnecessary antibiotics is the main cause of the worldwide increase in antibiotic resistance. Resistance to previously effective antibiotics in NG is of great concern. This fact has led to reconsider antibiotic prescriptions for gonococcal infections. The therapeutic strategy proposed by the English guideline is to double the dose of ceftriaxone as first-line treatment, reducing the combined therapy ceftriaxone and azithromycin due to the increase in azithromycin resistant NG isolates.20 In the same line, the American guideline suggests increasing the dose of ceftriaxone and reducing therapies that include azithromycin as much as possible.21 On the other hand, the European guideline proposes the combination therapy of ceftriaxone and high doses of azithromycin or ceftriaxone monotherapy in uncomplicated cases if antibiotic susceptibility is unknown.22
The total of NG isolates tested in the present study remained susceptible to third-generation cephalosporines (ceftriaxone and cefixime) according to data obtained from the European gonococcal surveillance program (Euro-GASP, 2020).23 However, Spain was one of the countries with the highest rates of cefixime resistance (≥5.5%) in 2017 and two ceftriaxone-resistant NG strains were detected in 2018.24,25 In addition, Serra-Pladevall et al. reported a percentage of resistance to third-generation cephalosporins of 1% in 2011,26 and a study performed in Valencia (Spain, 2013–2019), detected 3% and 1% of cefixime- and ceftriaxone-resistant NG isolates respectively.27 Despite this fact, a decrease in third-generation cephalosporins resistance was observed in whole Europe from 2016 to 2020.23–25
Macrolides have been widely used for treat respiratory and sexually transmitted infections, which has been associated with increasing rates of resistance to this antibiotic. The increase in azithromycin resistant NG (MIC >1mg/L) is of great concern. Azithromycin resistance in NG is mainly acquired though point mutations in 23S rRNA: A2059G related to high-level resistance and the C2611T related to low and moderate-level resistance to this antibiotic.8,29 A total of 38 out of 487 NG isolates (7.8%) included in this study presented an azithromycin MIC >1mg/L. Our results are similar to those reported in several studies performed in Spain, with resistance rates between 6 and 9.7%.7,28 On the other hand, a high percentage of ciprofloxacin resistant NG has been detected in our study (69.2%; 337/487) compared with other reports with percentages between 53 and 57%.7,23,26
A wide variety of NG sequence types was found in our study (97 different STs). Most of them (70.1%; 68/97) were represented by only one isolate. ST14994 has been the most frequent ST detected in our study (14.7%, 31/211) and was one of the most frequent ST reported in Europe (3.2%) and Spain (6.9%).29 According to a study carried out in Canada, a large increase in the incidence of this ST was also observed in that country, from 2.1% in 2017 to 16.6% in 2018.30 Six isolates belonged to ST5441 (2.8%, 6/211). This ST was also one of the most predominant in Europe (3.7% in 2018). On the other hand, and supporting the results obtained in our study, ST14994 has been associated with a higher rate of ciprofloxacin resistance.30
In our study, single gonococcal infection was more prevalent (77.7%) compared to co-infection (22.3%). Among samples with co-infection, C. trachomatis was the most frequently detected STI agent (74.8%) consistent with previous findings.14
In conclusion, this report suggests that gonococcal infection incidence is increasing in our area. The lack of resistance to third-generation cephalosporins and the low level of azithromycin resistance in our isolates suggest that the use of these antibiotics for gonococcal infection treatment remains as a suitable option. Continuous microbiological surveillance is essential to detect and to prevent the emergence and spread of resistant NG isolates to antibiotics currently used for gonococcal infection treatment.
Conflict of interestsThe authors declare that they have no conflict of interest.