The possible use of dalbavancin as a catheter lock solution was previously demonstrated by our study group. However, it was needed to assess whether heparin could affect dalbavancin bioactivity during freezing storage.
MethodsWe tested the bioactivity of a dalbavancin+heparin (DH) vs. dalbavancin (D) against Staphylococcal biofilms comparing DH median value of cfu counts and metabolic activity with that obtained for D before and during storage under freezing up to 6 months.
ResultsDespite there was a slight decrease in the median percentage reduction of metabolic activity at month 3 in Staphylococcus epidermidis between DH and D (97.6 vs. 100, p=0.037), considering the clinical criteria, no significant reduction in any of the variables tested was observed at the end of the experiment between D and DH solutions.
ConclusionThe addition of heparin to a dalbavancin lock solution did not affect its bioactivity against staphylococcal biofilms irrespective of its preservation time under freezing.
Nuestro grupo de estudio previamente demostró el posible uso de la dalbavancina (D) como solución de sellado de catéteres. Sin embargo, era necesario evaluar si la heparina (DH) podía afectar a la bioactividad de la D durante el almacenamiento por congelación.
MétodosSe comprobó la bioactividad de una solución de D+DH vs. D frente a biopelículas de estafilococos comparando el valor medio de recuentos de unidades formadoras de colonias (UFC) y actividad metabólica de la DH con el obtenido para la D antes y durante su conservación bajo congelación hasta seis meses.
ResultadosA pesar de que se observó una ligera disminución en la mediana del porcentaje de reducción de la actividad metabólica en el mes tres en Staphylococcus epidermidis entre DH y D (97,6 vs. 100, p=0,037), teniendo en cuenta los criterios clínicos, no se observó una reducción significativa en ninguna de las variables analizadas al final del experimento entre las soluciones D y DH.
ConclusionesLa adición de DH a una solución de sellado de D no afectó a su bioactividad frente a biopelículas estafilocócicas, independientemente de su tiempo de conservación bajo congelación.
Antibiotic lock solutions are indicated in addition to systemic therapy for catheter retention when catheter withdrawal cannot be achieved.1 However, as current guidelines for the management of catheter-related bloodstream infections (C-RBSI) are somewhat outdated, they do not provide for the use of new lipoglycopeptides, such as dalbavancin.2 We previously assessed the efficacy of a dalbavancin-heparin catheter lock solution against biofilms of Staphylococcus spp., demonstrating that its bioactivity was at least equal to that of vancomycin and that a 6-month freezing did not negatively affect it.3,4 Moreover, we observed no reduction in the efficacy of heparin in combination with dalbavancin before and during a 6-month freezing period.4
However, we further needed to assessed whether heparin could affect dalbavancin bioactivity during this freezing process of a stored catheter-lock solution.
MethodsWe applied an in vitro model to evaluate bioactivity of a heparin (60IU)-based dalbavancin (1mg/ml) lock solution vs. a dalbavancin (1mg/ml) lock solution against biofilm of Staphylococcus aureus ATCC43300 (MRSA) and Staphylococcus epidermidis ATCC35984 (MRSE) before and during a 6-month freezing (ATCC, American Type Culture Collection).
The study was performed in the laboratory of a tertiary teaching hospital in Madrid, Spain. We used an in vitro static microplate model as previously described.5
We used Xydalba® powder for concentrate solution for infusion 500mg (Almac Pharma Services Ltd., Seagoe Industrial Estate, Craigavon County Armagh BT63 5UA Reino Unido. Allergan Pharmaceuticals International Ltd., Clonshaugh Business & Technology Park, Dublin 17, D17 E400, Irlanda. Almac Pharma Services, Irlanda. Limited Finnabair Industrial Estate, Dundalk, Co. Louth, A91 P9KD, Irlanda). Xydalba® was reconstituted by slowly adding 25ml of sterile water for injection and then diluted with 50mg/ml (5%) glucose solution. An aseptic technique was used for the reconstitution and dilution of Xydalba®. From the reconstituted vial of Xydalba® (20mg/ml), we prepared a DH solution by transferring 1–18.6ml of glucose saline 5%+0.4ml heparin sodium (60IU), heparin sodium, 5000IU/5ml; Hospira Prod. Farm. y Hosp, S.L., Spain; and a D solution by transferring 1–19ml of glucose saline 5%. We prepared 3-ml aliquots of each solution and stored them at −70°C for 6 months.
Frozen lock solutions of D and DH were thawed at room temperature (30min) before performing the experiments. Biofilms were tested against lock solutions at baseline before freezing (month 0), and during months 3 and 6 of freezing. Data were expressed as median (IQR) percentage reduction of log cfu/ml counts and metabolic activity.
For quantification of bacterial load, wells were vigorously scraped in 100μl of PBS, and the triplicates of each treatment and controls were mixed separately in a pool. Four 1:100 serial dilutions were performed, and 100μl of each dilution was plated on blood agar plates and incubated at 37°C for 24h. Colonies of the plates were counted, and the percentage reduction in cfu/well was calculated. Colony counts were expressed as the median (IQR) number of cfu/ml and on a logarithmic scale.
For quantification of metabolic activity, 100μl of XTT/menadione (0.5mg/ml/1.72mg/ml) was mixed in a proportion of 10ml/1μl and inoculated in each well protected from light. The plate was then incubated at 37°C for 3h. Absorbance was measured at 492nm in a spectrophotometer (Biochrom EZ Read 400), and the percentage reduction in metabolic activity was calculated.
Quantitative variables are expressed as the median and interquartile range (IQR).
For the comparison between DH and D solution results, we defined clinical significance when DH values were within 25% of D values. We arbitrarily choose that 25% cut-off considering it a reasonable value below which there would be no too clinical impact, based on possible oscillations during experimental replications.
We also assessed the statistical significance by comparing median (IQR) percentage reduction values in log cfu/ml counts and metabolic activity of DH solution with those values obtained with D solution at each study periods (months 0, 3, and 6) using the Kruskal–Wallis test.
Statistical significance was set at p<0.05 for all the tests. The statistical analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, New York, USA).
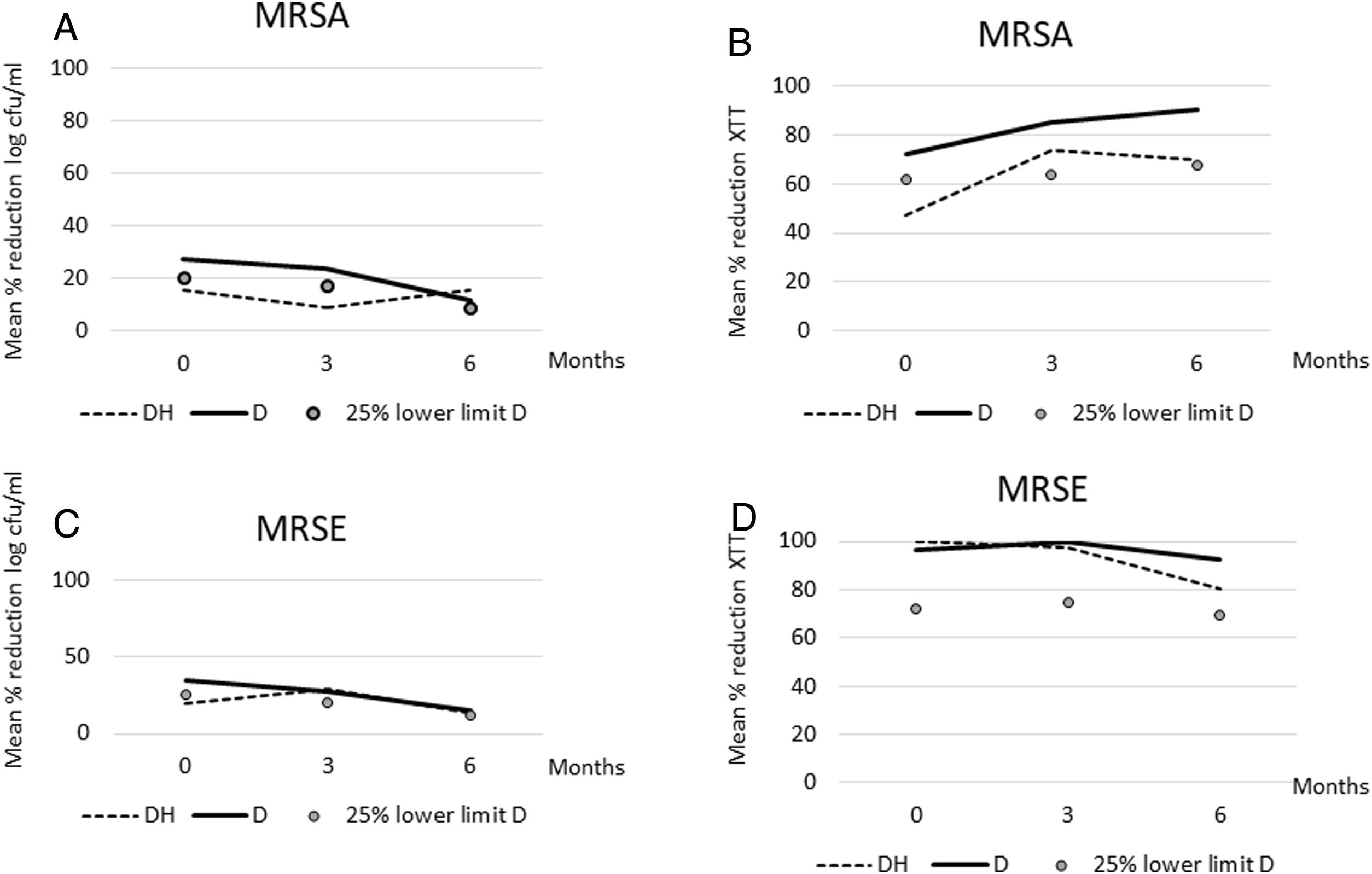
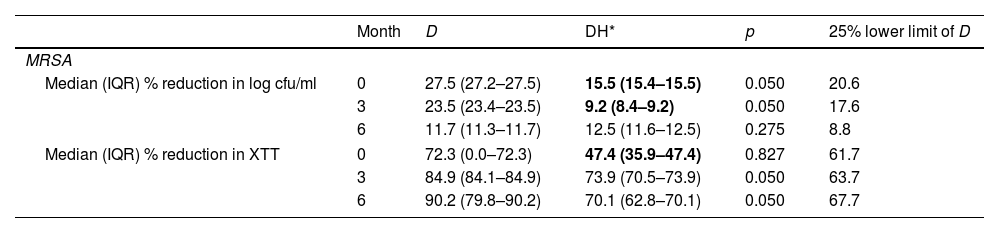
ResultsOverall, considering the clinical criteria, no significant reduction in any of the variables tested was observed at the end of the experiment (month 6) between D and DH solutions against Staphylococci biofilms (Table 1, Fig. 1). DH median values were only below 25% lower limit of D values at basal experiments (month 0) for percentage reduction in log cfu counts (15.5%<20.6%) and metabolic activity (47.4%<67.1%), and at month 3 for percentage reduction in log cfu counts (9.2%<17.6%) in MRSA. In MRSE, clinical significance was only observed at basal experiments (month 0) for percentage reduction in log cfu counts (20.1%<25.7%).
Median (IQR) percentage reduction in log cfu/ml and metabolic activity for dalbavancin alone and dalbavancin-heparin against MRSA and MRSE biofilms during freezing.
| Month | D | DH* | p | 25% lower limit of D | |
|---|---|---|---|---|---|
| MRSA | |||||
| Median (IQR) % reduction in log cfu/ml | 0 | 27.5 (27.2–27.5) | 15.5 (15.4–15.5) | 0.050 | 20.6 |
| 3 | 23.5 (23.4–23.5) | 9.2 (8.4–9.2) | 0.050 | 17.6 | |
| 6 | 11.7 (11.3–11.7) | 12.5 (11.6–12.5) | 0.275 | 8.8 | |
| Median (IQR) % reduction in XTT | 0 | 72.3 (0.0–72.3) | 47.4 (35.9–47.4) | 0.827 | 61.7 |
| 3 | 84.9 (84.1–84.9) | 73.9 (70.5–73.9) | 0.050 | 63.7 | |
| 6 | 90.2 (79.8–90.2) | 70.1 (62.8–70.1) | 0.050 | 67.7 | |
| Month | D | DH | p | ||
|---|---|---|---|---|---|
| MRSE | |||||
| Median (IQR) % reduction in log cfu/ml | 0 | 34.3 (33.9–34.3) | 20.1 (19.9–20.1) | 0.050 | 25.7 |
| 3 | 27.6 (27.2–27.6) | 29.0 (28.9–29.0) | 0.050 | 20.7 | |
| 6 | 15.5 (15.4–15.5) | 13.0 (12.8–13.0) | 0.050 | 11.6 | |
| Median (IQR) % reduction in XTT | 0 | 96.3 (80.0–96.3) | 100 (100–100) | 0.121 | 72.2 |
| 3 | 100 (100–100) | 97.6 (96.0–97.6) | 0.037 | 75.0 | |
| 6 | 92.3 (62.8–92.3) | 80.8 (73.9–80.8) | 0.513 | 69.2 | |
SD, standard deviation; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; D, dalbavancin; DH, dalbavancin-heparin; cfu, colony-forming units.
(A) Median percentage reduction in log cfu/ml for dalbavancin-heparin and dalbavancin solutions against MRSA during freezing time. (B) Median percentage reduction in metabolic activity for dalbavancin–heparin and dalbavancin solutions against MRSA during freezing time. (C) Median percentage reduction in log cfu/ml for dalbavancin-heparin and dalbavancin solutions against MRSE during freezing time. (D) Median percentage reduction in metabolic activity for dalbavancin-heparin and dalbavancin solutions against MRSE during freezing time. DH, dalbavancin-heparin; cfu, colony-forming units; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis. The markers correspond to the lower limit of 25% of the D value.
Considering the statistical criteria, no differences were statistically significant in any of the experiments, except in the median percentage reduction of metabolic activity at month 3 in MRSE between DH and D (97.6 vs. 100, p=0.037) (Table 1).
DiscussionWe demonstrated that heparin (60UI) does not reduce dalbavancin bioactivity against biofilms of MRSA and MRSE ATCC strains even during a 6-month freezing storage time.
C-RBSI can be achieved by combining systemic and catheter lock therapies in some circumstances in which catheter must be retained.6–10 The current international Guidelines for the Management of Catheter-related infections recommend only vancomycin or cefazolin for the treatment of Gram-positive C-RBSI episodes (BII).1 The Clinical Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC) and the Spanish Society of Intensive Care Medicine and Coronary Units additionally included teicoplanin and daptomycin (BII).11 However, little is known about dalbavancin to be used for catheter savage attempts.12
Our group previously demonstrated that the efficacy of a heparin-based dalbavancin catheter lock solution against MRSA and MRSE strains was non inferior to vancomycin and that both heparin and dalbavancin remained effective and stable for 7 days in an antimicrobial lock solution at 37°C, simulating a catheter dwell time of 7 days.3,13–15 Moreover, as it was required to optimize the dalbavancin intravenous vial with a cost-effective approach, we recently demonstrated that a heparin-based dalbavancin catheter lock solution can be stored at −70°C without seriously affecting its bioactivity against MRSA and MRSE biofilms.4 However, it was needed to assess whether, in addition, heparin may affect dalbavancin bioactivity when they are combined in a catheter lock solution both before and after the freezing storage period.
Based on our results, we consider that no relevant differences occurred between D and DH solutions against both MRSA and MRSE biofilms regarding percentage reduction of neither log cfu counts nor metabolic activity. In the occasional cases where both statistical and clinical differences were observed between D and DH solutions, they never occurred at the end time of the study (month 6), which is when they would be considered relevant. So, we found no evidence that DH solution bioactivity was reduced over the shelf life with respect to D solution.
The main limitation of the study is that we only tested our experiments on a single ATCC strain of each specie. In addition, bioactivity of D and DH was indirectly calculated by assessing percentage reduction of log cfu counts and metabolic activity of 24-h formed biofilms.
ConclusionHeparin does not negatively affect dalbavancin bioactivity against staphylococcal biofilms when they are combined in a catheter lock solution frozen up to 6 months. Our supports should be validated in a large sample size and in other species, such as Enterococci.
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Availability of data and materialData sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Funding sourceM. Guembe is supported by the Miguel Servet Program (ISCIII-MICINN, MS18/00008) of the Health Research Fund (FIS) of the Carlos III Health Institute (ISCIII), Madrid, Spain. M. Díaz-Navarro is supported by ISCIII (FI22/00022). The study was partially financed by grants from the ISCIII (PI21/00344), the Fundación Mutua Madrileña (FMM21/01), IiSGM (2022-PI-II-COOPTR-01), and the European Regional Development Fund (FEDER) “A way of making Europe”.
Conflicts of interestsThe authors declare that they have no conflicts of interest.