Bordetella bronchiseptica (BB) is a strictly aerobic Gram-negative coccobacillus that causes diseases in the respiratory tract of domestic animals.1,2 BB infections in humans are very rare and less than 40 cases have been described.3 Most of them are respiratory-tract infections in patients with HIV, post-transplant immunosuppression, lung cancer or pre-existing lung disease.4–7 We report the clinical course and management of a BB severe cavitary pneumonia in a patient with HIV.
A 38-year-old man was admitted in an Infectious Disease Intensive Care Unit in Lisbon with one week of productive cough, shortness of breath, bilateral chest pain, anorexia and night sweats. HIV was diagnosed ten years before, without AIDS criteria and had been on combined antiretroviral therapy (cART) with good compliance (last CD4 count of 814cells/μl – 32%). He was a smoker with a 20-pack-year smoking history. Used inhaled cocaine and heroin in the past and was up to date in a methadone substitution programme. He had frequent contact with pet dogs, without other significant exposure to animals or travel history.
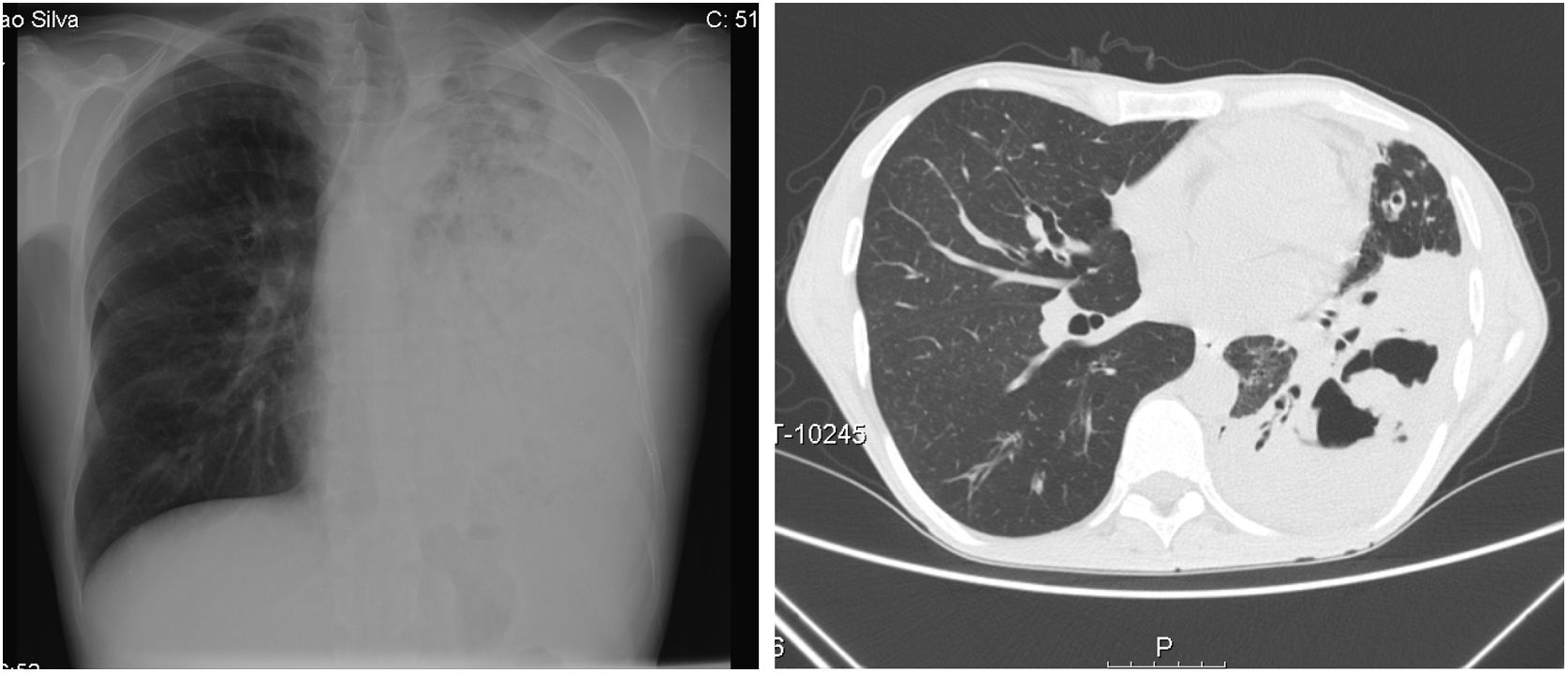
On admission he had a global respiratory failure with moderate acidemia, hypotension with normal lactate and oliguria. Pulmonary auscultation revealed decreased lung sounds of the entire left lung with diffuse dry crackles. Blood tests showed leucocytosis and neutrophilia, C-reactive protein of 29mg/dL, acute kidney failure (creatinine 4.48mg/dL and urea 256mg/dL) and cellular lysis (uric acid 13mg/dL, LDH 6700mg/dL, hypocalcemia, hypophosphatemia and hyperkalemia). The chest radiograph showed diffuse opacity of the left lung and the chest CT scan revealed a left pulmonary consolidation mainly affecting the lower lobe with cavitation areas of considerable size (Fig. 1).
The patient was empirically started on piperacillin/tazobactam. A bronchoalveolar lavage was performed and the culture only grew BB sensitive to carbapenems, aminoglycosides, piperacillin/tazobactam, fluoroquinolones and ceftazidime. All the other cultures and microbiological studies were negative, including for mycobacteria, fungus and fastidious bacteria. It was decided to maintain piperacillin/tazobactam for four weeks.
The cellular lysis was treated with rasburicase and supportive measures. In aim to investigate eventual comorbidity or adjacent malignancy, we performed a bone marrow biopsy that was not suggestive of any lymphoproliferative disease. Further, the full body CT scan didn’t reveal any other changes. Viral load remained undetectable while admitted and re-evaluation of T-CD4 lymphocyte-count revealed an absolute decrease to 434cells/μl (31%).
With the supportive measures, antibiotics and respiratory rehabilitation, the patient progressed favourably, with non-invasive ventilation weaning at 72h. He also had resolution of the renal dysfunction, without any need for replacement technique. He was discharged without supplemental oxygen and with good functional status. On one-year follow-up he stayed compliant with cART and had no signs of infection relapse.
BB is responsible for rare cases of respiratory tract infections in humans, especially among individuals with underlying diseases.8 Cases among immunosuppressed patients have been reported especially in those with HIV. The clinical condition is generally associated with infections in the upper respiratory tract, pneumonia, endocarditis and bacteraemia.9,10 Unfortunately, only case reports and case reports series of BB infections are available.6,8 Wernli et al. described eight cases of BB that caused infection or colonization in human beings over a 15-year period.6 A study by García-de-la-Fuente et al. in 2015 showed that most of the patients from whom BB was isolated presented a compromised immune system as well as an underlying disease, and 82% presented respiratory symptoms.8
Despite of the fact that BB is associated with colonization in the respiratory tract and bronchopulmonary disease, it is difficult to clearly establish the pathogenic effect of this microorganism in human beings. Curiously, in our case the T-CD4 lymphocyte count persisted above 200cells/μl and we didn’t identify any adjacent malignancy, suggesting that the former smoking history and a relative immunosuppression could be the only risk factors.
BB might be included as a differential diagnosis in all immunosuppressed patients presenting with pneumonia and chronic cough, especially those with animal exposure. No guidelines in the management of BB infection are available. Further, treatment failures have been reported despite the use of susceptibility guided antibiotics, so there exists a real need to understand how antibiotic susceptibility data in vitro is related to efficacy in vivo.
Conflict of interestThe authors declare no conflict of interests.
The authors thank Microbiology Laboratory of Hospital de Santa Maria for her invaluable assistance with the identification of Bordetella bronchiseptica.
Master of Medicine, 5th Year Infectious Disease Resident in Serviço de Doenças Infeciosas in Hospital de Santa Maria – Centro Hospitalar e Universitário Lisboa Norte, Portugal.
Master of Medicine, Infectious Disease Specialist in Serviço de Doenças Infeciosas in Hospital de Santa Maria – Centro Hospitalar e Universitário Lisboa Norte, Portugal.
Master of Medicine, Infectious Disease Specialist in PhD programme in Serviço de Doenças Infeciosas in Hospital de Santa Maria – Centro Hospitalar e Universitário Lisboa Norte, Portugal.