Fecal microbiota transplantation (FMT) is an effective and safe treatment to treat recurrent Clostridioides difficile infection. It is essential to make every effort to perform FMT rigorously and based on scientific knowledge. Selection of the fecal microbiota donor is a key point of the process to ensure recipient safety. It is necessary to have protocols of action that allow clinicians to act with the maximum guarantees and to minimise the risks of the procedure. For this reason, a multidisciplinary working group has been set up in Cataluña with the aim of establishing recommendations for the selection of the fecal microbiota donor.
La transferencia de microbiota fecal (TMF) es un tratamiento eficaz y seguro para tratar la infección recurrente por Clostridioides difficile. Es esencial extremar esfuerzos para que la TMF se realice con rigor y en base a los conocimientos científicos. La selección del donante de microbiota fecal es un punto clave del proceso para garantizar la seguridad del receptor. Es necesario disponer de protocolos de actuación que permitan a los clínicos actuar con las máximas garantías y minimizar los riesgos del procedimiento. Por este motivo, en Cataluña se ha constituido un grupo de trabajo multidisciplinario con el objetivo de establecer unas recomendaciones para la selección del donante de microbiota fecal.
Faecal microbiota transplantation (FMT) has emerged in recent years as the treatment of choice for recurrent Clostridioides difficile (C. difficile) infection, with overall cure rates of 85%–90%.1 The efficacy of FMT has been widely demonstrated in multiple uncontrolled studies and in several clinical trials.2 Consequently, the main clinical practice guidelines and medical associations recommend FMT as a first-line treatment option in recurrent C. difficile infection.3–7
Continuing advances in knowledge of the human gut microbiome have shown that there is an association between abnormal gut microbiota and a broad spectrum of disorders and/or diseases. These data have sparked growing interest in the scientific community in determining the role of FMT in conditions other than recurrent C. difficile infection, such as inflammatory bowel disease, metabolic syndrome, intestinal colonisation by multidrug-resistant micro-organisms, irritable bowel syndrome, etc.
FMT is considered a safe, well-tolerated procedure with virtually no short-term adverse effects if performed correctly. However, the evidence available on long-term safety is limited. It is therefore essential to establish action protocols that allow clinicians to work with maximum guarantees and minimise the risks of the procedure.
The COVID-19 pandemic caused by the SARS-CoV-2 virus is forcing professionals to take additional measures for the selection of Faecal microbiota donors. Several studies have documented the presence of SARS-CoV-2 virus RNA in faeces,8,9 meaning there is a potential risk of Faecal –oral transmission of the virus. This consensus document establishes a series of recommendations to minimise the risk of contagion of COVID-19 through FMT; these recommendations will be subject to refinement as scientific knowledge in this field advances.
With this objective, a multidisciplinary working group has been set up in Catalonia with specialists in gastroenterology, infectious diseases, microbiology and endocrinology in order to establish recommendations that serve to ensure this treatment is performed according to strict standards and, at the same time, offer guidelines on the methodology to follow.
Donor selectionThe selection of the donor must be rigorous to guarantee the safety of the procedure. Donor screening is vital to prevent the transmission of infectious diseases. There is also a theoretical risk of FMT modulating the recipient's susceptibility to developing conditions or diseases related to the intestinal microbiota. To minimise these risks, prior to donation, each potential candidate will complete a personal interview and undergo clinical laboratory tests, including blood, stool and other tests.
Donor information sheetEveryone who enters the donor selection process will be informed about how the process works and about the purpose of their contribution. They will be given an information document that guarantees the confidentiality and protection of their personal data. They will then be asked to sign an informed consent form. A sample donor information sheet appears in Appendix A (see additional materials).
Personal interview and physical examinationThe safety of the recipient is the main concern. Therefore, donors will be turned down if their personal interview and/or physical examination reveal a significant relevant medical history, behaviours associated with an increased risk of contracting communicable diseases or signs suggestive of active disease. All donors must be provided with contact information for the FMT programme managers during the personal interview so they can immediately report any changes in their symptoms or other significant changes that may occur during the selection and donation period. A sample questionnaire to be used in the personal interview is shown in Appendix B (see additional materials).
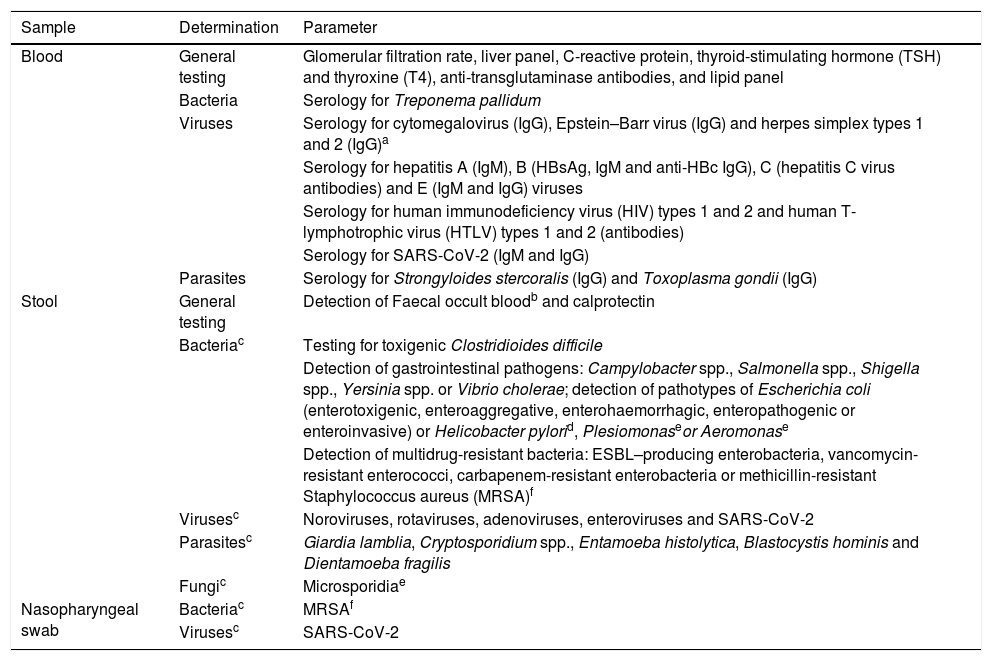
Laboratory testsAfter the personal interview is completed, laboratory screening tests must be performed. Table 1 lists the determinations considered essential. Testing for highly uncommon but potentially pathogenic micro-organisms may be included depending on the recipient's clinical context (e.g. immunosuppression). Tests in donors of other nationalities must be adapted to the epidemiology of their country of origin (e.g. for Trypanosoma cruzi or Schistosoma). The number of entities included on the list may be updated based on the knowledge and experience acquired with FMT.
Screening tests to be performed in all potential stool donors.
| Sample | Determination | Parameter |
|---|---|---|
| Blood | General testing | Glomerular filtration rate, liver panel, C-reactive protein, thyroid-stimulating hormone (TSH) and thyroxine (T4), anti-transglutaminase antibodies, and lipid panel |
| Bacteria | Serology for Treponema pallidum | |
| Viruses | Serology for cytomegalovirus (IgG), Epstein–Barr virus (IgG) and herpes simplex types 1 and 2 (IgG)a | |
| Serology for hepatitis A (IgM), B (HBsAg, IgM and anti-HBc IgG), C (hepatitis C virus antibodies) and E (IgM and IgG) viruses | ||
| Serology for human immunodeficiency virus (HIV) types 1 and 2 and human T-lymphotrophic virus (HTLV) types 1 and 2 (antibodies) | ||
| Serology for SARS-CoV-2 (IgM and IgG) | ||
| Parasites | Serology for Strongyloides stercoralis (IgG) and Toxoplasma gondii (IgG) | |
| Stool | General testing | Detection of Faecal occult bloodb and calprotectin |
| Bacteriac | Testing for toxigenic Clostridioides difficile | |
| Detection of gastrointestinal pathogens: Campylobacter spp., Salmonella spp., Shigella spp., Yersinia spp. or Vibrio cholerae; detection of pathotypes of Escherichia coli (enterotoxigenic, enteroaggregative, enterohaemorrhagic, enteropathogenic or enteroinvasive) or Helicobacter pylorid, Plesiomonaseor Aeromonase | ||
| Detection of multidrug-resistant bacteria: ESBL–producing enterobacteria, vancomycin-resistant enterococci, carbapenem-resistant enterobacteria or methicillin-resistant Staphylococcus aureus (MRSA)f | ||
| Virusesc | Noroviruses, rotaviruses, adenoviruses, enteroviruses and SARS-CoV-2 | |
| Parasitesc | Giardia lamblia, Cryptosporidium spp., Entamoeba histolytica, Blastocystis hominis and Dientamoeba fragilis | |
| Fungic | Microsporidiae | |
| Nasopharyngeal swab | Bacteriac | MRSAf |
| Virusesc | SARS-CoV-2 |
ESBL: extended-spectrum beta-lactamase; MRSA: methicillin-resistant Staphylococcus aureus; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; TSH: thyroid-stimulating hormone.
Donors will be eligible candidates if their answers to the questionnaire specify that they have no risks, their pathogen results are negative and the results of their additional tests indicate no significant disease. It is essential to train donors during the initial interview to report any change in their health status to the medical team during the donation period.
Once this selection process is complete, donors will be able to make all the donations (stools) they wish during a two-week period. Donors who wish to continue donating must undergo further screening with repeat stool tests every two weeks and repeat blood tests and nasopharyngeal swabs every two months as set out in Table 1.
To ensure the safety of the recipients, as an additional measure, it is recommended that the donation be quarantined for two to eight weeks to confirm that the donor shows no significant changes in their health status in the weeks subsequent to their most recent donation.9 This measure is intended to detect infections in a window period or not detected in the initial study.
Donor exclusion criteriaDonors must be turned down if risk factors for the transmission of infectious agents or other characteristics that could affect the health of the recipient are detected.
At present, the ideal composition of the donor's gut microbiota for FMT to be effective is not known, so donors are selected by a principle of exclusion rather than inclusion. It must be taken into account that if they are properly screened, in the end only a minority will be able to act as donors.10
Donor exclusion criteria- •
Under 18 or over 50 years of age.
- •
Having taken antimicrobials (antibiotics, antivirals or antifungals) or probiotics in the six months prior to donation.
- •
Positive result for any pathogen determined in microbiology tests of blood or feces during the screening period (Table 1).
- •
Smoking (>10 cigarettes/day).
- •
Having a fever or gastrointestinal symptoms (diarrhoea, nausea, vomiting, constipation, abdominal pain, etc.).
- •
Significant medical history (neoplasm, communicable diseases, etc.) and, specifically, history of gastrointestinal disorders, including inflammatory bowel disease, coeliac disease, irritable bowel syndrome, chronic constipation, chronic diarrhoea, previous history of C. difficile infection and/or gastrointestinal bleeding.
- •
History of autoimmune diseases (e.g. multiple sclerosis, connective tissue disorders, type 1 diabetes mellitus), atopy-related diseases, asthma, other types of diabetes mellitus, current treatment with immunomodulatory agents, history of chronic pain syndromes (e.g. fibromyalgia, chronic fatigue), neurological or neurodevelopmental disorders, psychiatric disorders, metabolic syndrome (NCEP ATP III criteria), obesity (body mass index >30 kg/m2), or malnutrition (body mass index <18.5 kg/m2).
- •
Family history of colorectal cancer, polyposis syndrome, inflammatory bowel disease, coeliac disease or autoimmune diseases.
- •
Substance abuse.
- •
Taking medication that may be excreted in the faeces, pose a risk to the recipient or cause changes in the intestinal microbiota or dysbiosis (e.g. proton pump inhibitors).
- •
History of behaviours associated with increased risk of contracting communicable diseases:
- -
Risky sexual behaviour: sexual relations in the last six months with anonymous partners, multiple partners, HIV carriers, people who have used intravenous drugs or people who practice or have practiced prostitution.
- -
Having got a tattoo, body piercing and/or acupuncture in the last six months.
- -
Current incarceration or history of incarceration.
- -
Recent travel (in the last six months) to tropical countries, countries with endemic diarrhoeal diseases or high risk of traveller's diarrhoea (Africa, Southeast Asia, Mexico, Central America, South America or the Caribbean).
- -
Recent needle-stick injury.
- -
Having received blood products in the last six months.
- -
Having received live or attenuated vaccines in the last six months.
- -
Individuals who work with animals (to decrease risk of zoonosis transmission).
- •
Having risk factors for colonisation by multidrug-resistant micro-organisms:
- -
Healthcare workers.
- -
People in contact with the healthcare system defined as: recent hospitalisation, recent admission to long-term care centres, regular attendance at day hospitals and/or outpatient surgery.
- •
Major gastrointestinal surgery.
- •
Major non-gastrointestinal surgery in the last four months (e.g. pneumonectomy, cardiac intervention or thoracic surgery, severe fracture [femur, pelvis, etc.] or joint replacement [hip, knee, etc.]).
- •
Having risk factors for Creutzfeldt–Jakob disease (spongiform encephalopathy).
- •
Having SARS-CoV-2 infection that is confirmed (by PCR) or clinically suspected (with fever, fatigue, dry cough, myalgia, dyspnoea and/or headache).
- •
Contact with a patient with confirmed or clinically suspected SARS-CoV-2 infection in the last four weeks.
Several studies have documented the presence of SARS-CoV-2 virus RNA in faeces and found that it can continue to be detected even after respiratory samples yield negative results.8,9 This means there is a potential risk of Faecal –oral SARS-CoV-2 transmission. To minimise the risk of transmitting COVID-19 with FMT, in addition to the specific tests established in Table 1, the following measures are recommended:
- A
A person who has had COVID-19 (microbiologically confirmed or clinically suspected) cannot be assessed as a possible donor until 12 weeks after the resolution of the infection.
- B
A person who has had contact with a case of COVID-19 (microbiologically confirmed or clinically suspected) cannot be assessed as a possible donor until four weeks after the contact.
- C
The presence of symptoms or any positive microbiology results means that the candidate, as well as any samples the candidate may have provided in the four weeks prior to clinical and/or microbiological diagnosis, must be turned down.
- D
An asymptomatic person with positive IgG and negative results for all other tests will be considered a suitable candidate for stool donation.
The authors declare that they have no conflicts of interest.
Xavi Aldeguer. Gastroenterology Department. Hospital Universitari de Girona Doctor Josep Trueta [Doctor Josep Trueta University Hospital of Girona].
Francesc Balaguer. Gastroenterology Department. Hospital Clínic de Barcelona [Clinical Hospital of Barcelona].
Xavier Bessa i Caserras. Gastroenterology Department. Hospital del Mar [Mar Hospital].
Natalia Borruel Sainz. Gastroenterology Department. Hospital Universitari Vall d'Hebron [Vall d'Hebron University Hospital].
Xavier Calvet Calvo. Gastroenterology Department. Hospital Universitari Parc Taulí [Parc Taulí University Hospital].
Antoni Castells. Gastroenterology Department. Hospital Clínic de Barcelona. President of the Societat Catalana de Digestologia [Catalan Society of Gastroenterology].
Guillermo Cuervo. Infectious Disease Department. Hospital Universitari de Bellvitge [Bellvitge University Hospital].
Maria Esteve Comas. Gastroenterology Department. Hospital Universitari MútuaTerrassa [MútuaTerrassa University Hospital].
Francisco Guarner Aguilar. Gastroenterology Department. Hospital Universitari Vall d'Hebron.
José Manuel Fernández-Real. Endocrinology Department. Hospital Universitari de Girona Doctor Josep Trueta.
Juan P. Horcajada. Infectious Disease Department. Hospital del Mar.
Joaquin López-Contreras González. Infectious Disease Department. Hospital de la Santa Creu i Sant Pau [Santa Creu i Sant Pau Hospital].
Marc Llirós Dupré. Institut d'Investigació Biomèdica de Girona [Girona Biomedical Research Institute] (IdIBGi).
Míriam Mañosa i Ciria. Gastroenterology Department. Hospital Universitari Germans Trias i Pujol [Germans Trias i Pujol University Hospital].
Lurdes Matas Andreu. Clinical Microbiology Department. Hospital Universitari Germans Trias i Pujol.
Ferran Navarro Risueño. Clinical Microbiology Department. Hospital de la Santa Creu i Sant Pau.
Roger Paredes. Infectious Disease Department. Hospital Universitari Germans Trias i Pujol.
Virginia Rodríguez-Garrido. Clinical Microbiology Department. Hospital Universitari Vall d'Hebron.
José R. Santos. Infectious Disease Department. Hospital Universitari Germans Trias i Pujol.
Nieves Sopena Galindo. Infectious Disease Department. Hospital Universitari Germans Trias i Pujol.
German Soriano Pastor. Gastroenterology Department. Hospital de la Santa Creu i Sant Pau.
Judith Villar-García. Infectious Disease Department, Hospital del Mar.
Appendix A lists the members of the Catalan group for the study and development of fecal microbiota transfer.
Please cite this article as: Aira A, Arajol C, Casals-Pascual C, González-Suárez B, Martí S, Domínguez MÁ, et al. Recomendaciones para la selección del donante para la transferencia de microbiota fecal. Documento de posicionamiento avalado por la Societat Catalana de Digestologia, la Societat Catalana de Malalties Infeccioses i Microbiologia Clínica y el grupo GEMBIOTA de la Sociedad Española de Enfermedades infecciosas y Microbiología Clínica. Enferm Infecc Microbiol Clin. 2022;40:142–146.