Proper adherence is essential to obtain the desired results of antiretroviral therapy; thus, new interventional strategies for this purpose must be sought.
ObjectiveComparison of 2 interventions, one conducted by a health professional and the other by a peer (patient on antiretroviral therapy), to improve adherence to antiretroviral therapy.
Design and settingIn 2003, a randomized, concurrent, follow-up study was conducted at 3 hospitals.
ParticipantsPatients were recruited consecutively at infectious disease visits scheduled to monitor their disease from May to September 2003. A sealed envelope was used to assign patients to each intervention group. A previous phase was conducted to unify data collection, and the intervention consisted of 4 visits at weeks 0, 8, 16, and 24.
ResultsAmong the 240 patients included, 198 completed follow-up, and in 11 of these, treatment was interrupted at the doctor's decision. At baseline, 46.8% were classified as adherent. Multilevel analysis showed that as the visits progressed, the probability of adhering to treatment increased (OR 1.23; P<.01). Although differences were not significant, the group treated by a peer showed better results than the group treated by a health professional (OR 1.60; P=0.25). A lower probability of antiretroviral adherence was observed in patients receiving a drug combination including a protease inhibitor (OR 0.27; P<0.01) and in those with psychological distress (OR 0.44; P=0.03). Patients with a higher score on the physical quality of life index (OR 1.05; P<0.01) presented a higher probability of adherence.
ConclusionsThe psychoeducational intervention studied is viable and effective for improving antiretroviral adherence. When the intervention is conducted by a peer the results are at least as good as those obtained by a health professional, and this implies cost-saving for the health system.
La adecuada adherencia es esencial para obtener los resultados deseados de la terapia antirretroviral, y por tanto, deben buscarse nuevas estrategias de intervención para este fin.
ObjetivoComparación de 2 de intervenciones para mejorar la adherencia a la terapia antirretroviral: una realizado por un profesional de la salud y el otro por un «igual» (paciente VIH+ en terapia antirretroviral).
DiseñoEn 2003 se llevo a cabo en 3 hospitales, un ensayo clinico aleatorizado.
ParticipantesLos pacientes fueron reclutados consecutivamente en las unidades de infecciosas en sus visitas programadas para controlar su enfermedad desde mayo a septiembre de 2003. La asignación a cada grupo de intervención se realizó mediante un sobre cerrado. Previamente a la intervención se realizó entrenamiento para unificar la recolección de datos, y la intervención consistió en 4 visitas en las semanas 0, 8, 16 y 24.
ResultadosDe los 240 pacientes incluidos, 198 completaron el seguimiento, y en 11 de estos, el tratamiento fue interrumpido por decisión del médico. Al inicio del estudio, el 46,8% fueron clasificados como adherente. Mediante análisis multinivel se mostró que a medida que avanzaban las visitas, la probabilidad de adherirse al tratamiento aumento (OR: 1,23; p<0,01). Si bien las diferencias no fueron significativas, el grupo tratado por un «igual» mostró mejores resultados que el grupo tratado por un profesional de la salud (OR: 1,60, p=0,25). Una menor probabilidad de adherencia a los fármacos antirretrovirales se observó en los pacientes que recibieron una combinación de farmacos que incluía un inhibidor de la proteasa (OR: 0,27; p<0,01) y en aquellos con trastornos psicológicos (OR: 0,44, p=0,03). Los pacientes con una puntuación más alta en el índice de calidad física de vida (OR: 1,05; p<0,01) presentaron una mayor probabilidad de cumplimiento terapeutico.
ConclusionesLa intervención psicoeducativa realizada es viable y eficaz para mejorar la adherencia antirretroviral. Cuando la intervención se lleva a cabo por un «igual», los resultados son al menos tan buenos como los obtenidos por un profesional de la salud, y esto implica ahorro de costes para el sistema de salud.
Approximately 32,000 (20,000–84,000) individuals in North America, and Western and Central Europe died of AIDS in 2007.1 Nonetheless, since the introduction of highly active antiretroviral therapy (HAART), there has been a reduction in opportunistic diseases and a rise in the survival of HIV-infected patients.2
Incomplete adherence and lack of potency of antiretroviral agents are the principal causes of decreased drug efficacy, which is mainly due to the development of viral resistance caused by the impossibility of halting viral replication.3 It should be noted that, unlike other pharmaceuticals, short periods of non-compliance with HAART can make the treatment permanently ineffective and accelerate the advance of the disease.2 Antiretroviral treatment costs 9500 to 10,000€ a year per patient4 and accounts for 21.5% of the total hospital pharmacy expenditure.5 Thus, for both clinical and economic reasons, strategies to achieve full adherence to antiretroviral therapy are worthy of study.3
Several elements that affect adherence to HAART have been identified, including factors related to the physician, patient, and therapy received, among others.6 Because of the importance of antiretroviral adherence from a clinical and public health perspective, it is necessary to search for and develop interventions to improve compliance to therapy.
A systematic review from the Cochrane Library concluded that interventions have a beneficial effect on adherence, although there was no concomitant decrease in viral load. Interventions targeting practical medication management skills and those delivered over 12 weeks or more were associated with better adherence outcomes.2 Since publication of this systematic review, a meta-analysis has reported that participants in the intervention arm were more likely than those in the control arm to achieve 95% adherence (OR: 1.5; 95% CI: 1.16–1.94).7
Knowledge transfer between peers has proven to be very effective in several health-related problems, such as reducing risk behavior in HIV infection and reducing traffic accidents, but its effectiveness for increasing adherence to antiretroviral therapy is unknown.8
The aim of this study is to compare the efficacy of two interventions to improve adherence to antiretrovirals: one conducted by a health professional and the other by a “peer” (HIV patient on antiretroviral therapy) in three Spanish hospitals. Particular attention is paid to plasma viral load and the presence of psychological distress.
MethodsAll patients included in the study were at least 18 years of age, on antiretroviral therapy, and attending scheduled appointments for disease monitoring at infectious disease units at three Spanish hospitals.
A randomized, concurrent, follow-up study was conducted to compare two strategies to improve adherence to antiretrovirals. Patients were recruited consecutively from May to September 2003, and 1 patient was included per day at each hospital. The doctor randomly (stratified by center) assigned patients to the corresponding intervention group, using an opaque, sealed envelope that had been delivered previously. Group A was treated by a health professional (physician or pharmacist with extensive knowledge about HIV) and group B was treated by a “peer”; that is, a patient adherent to treatment, capable of communication and empathy, and with no previous experience as a therapist. Patients in both groups received a psychoeducational intervention to increase their adherence to antiretroviral therapy, as well as regular visits by their doctor. In addition to the baseline visit, patients were seen at weeks 8, 16, and 24. The study ended in March 2004. The intervention visits were scheduled to coincide with routine hospital visits to facilitate attendance by the patients (adherence to the intervention).
The sample size was calculated to detect an absolute difference of 0.15 in efficacy between the two groups, considering a 10% success rate (adherence increase) in group A and 25% in group B. This required a total of 240 patients, 120 in each group. Patients were excluded if they were unlikely to complete the study, if their physical or mental conditions made the study follow-up impossible, or if they were participating in another study.
Study outcomes: 1) Adherence to antiretroviral treatment, 2) Plasma viral load, and 3) Psychological distress.
Variables collected: 1) Sociodemographic variables, including age, sex, educational level, sexual orientation, injection drug use at any point; 2) Clinical variables, including CD4 count, viral load (detectable yes/no), AIDS stage (using the Centers for Disease Control and Prevention [CDC] clinical staging system for HIV infection adapted for Europe), hepatitis C virus positivity, length of treatment, and number of months with HIV-positive status; 3) Therapy-related variables, including the antiretroviral combination used and whether the combination included a protease inhibitor, number of tablets prescribed per day, and difficulty in taking the medication (confrontation); and 4) Psychosocial variables, including social support, psychological distress, and health-related quality of life.
Measurement tools: 1) Adherence to antiretroviral therapy was measured with the SMAQ questionnaire (the only validated questionnaire at the time of the study), which has been validated in Spain and has a sensitivity of 72% and specificity of 91%.9 The questionnaire contains 6 questions and classifies patients as nonadherent if they answer yes to any of the qualitative questions, “two or more forgotten doses in the last week, or two or more days without taking medication in the last three months”; 2) Social support was measured with the Duke-UNC-11, in a version validated and adapted to our setting (Cronbach α >0.80).10 This is a self-administered tool with 11 items that evaluate the patient's perception of functional and qualitative social support; 3) Psychological distress was measured with the 12-item General Health Questionnaire (GHQ-12), which has 76% sensitivity and 80% specificity. Patients with a score ≥3 were considered positive for psychological distress11; and 4) Health-related quality of life was evaluated with the Spanish version of MOS-HIV12, which contains 35 items summarized in a mental health score and a physical health score.
Procedure: After the ethics committee of each hospital had approved the trial, each patient was contacted in consecutive order. The study objectives were explained to the patient, who was then asked to sign the informed consent form. If a patient declined to participate, the next patient attending an appointment was asked to participate. This process was repeated until 80 patients per hospital had been recruited.
All the variables were collected at the first and last visit, and at the second and third visit, variables relating to adherence, CD4 count, viral load and quality of life were collected. CD4 count and viral load were obtained from the patients’ clinical records, using the values that were nearest in time to the therapist's visit. A flow cytometry technique was used for lymphocyte count and NASBA or PCR were used for plasma viremia (undetectable at <50 copies/mL).
Intervention: To unify the intervention style, the 6 participating therapists were trained by one of the authors (I.R.) in cognitive, emotional, and communication aspects (in accordance with the American Heart Association13). The intervention process was standardized by designing guidelines with the steps to be followed at each visit. The first intervention lasted approximately 1h and had several parts: an information sheet formulated by the infectious disease unit, and a one-to-one session to provide a detailed explanation of the drug regimen prescribed. The following points were discussed: increase in confrontation, psychological state, correct self-administration of antiretrovirals, possible side effects, importance of adherence, and development of antiretroviral resistance. In addition, strategies to achieve adherence by overcoming obstacles and forgetfulness through the development of specific skills were discussed. Lastly, risk behavior, such as injection drug use, was discussed. Great importance was placed on solving any cognitive and psychological aspects that were relevant to the patient.
At the follow-up visits, which lasted 30min, the strategies implemented to remember doses were assessed, the importance of adherence to antiretrovirals was repeated, and solutions were offered to any problems that had occurred, emphasizing the skills for correct self-administration of medication at all times. Even though it was a standardized process, the intervention was adapted to the needs of each patient.
Statistics: Statistical analyses were conducted using the S-Plus 6 software package. Significance was established at a P value of <.05. Responses were evaluated by independent, blinded, personnel. First, to assess the magnitude and direction of the potential selection bias, a comparative analysis was made between the groups at the beginning of the study. To this purpose, a description of the sample profile was made and percentages between groups were compared using the chi-square test for categorical variables and Student t test for continuous variables.
McNemar's test was used to analyze changes in adherence, viral load, and psychological distress. An intention-to-treat analysis, applied for values that were lost for the three outcomes at the last visit, used the worst of all situations (non-adherence, detectable viral load, and psychological distress, respectively).14 This is one of the most common methods to impute missing values in clinical trials.
A multilevel analysis, adjusted for variables with differing percentages between groups at baseline and for potential confounders, was performed to determine adherence to antiretroviral therapy. A hierarchical fixed-effects logistic regression model was designed, in which level 1 was repeated measures and level 2 was subjects. The adherence of the individual was entered at each visit. As compared to traditional methods, hierarchical analysis has the advantage of appropriately handling repeated observations for each subject, and does not require the same number of observations (complete data) for each subject (an observation may be missing). A logistic function is used to represent the dichotomous response variable (adherent vs. non-adherent) This results in a reduction in sample size which leads to lesser precision in estimates and a selection risk, since the final sample is a subsample of study participants and may differ from the total sample with regard to the distribution of variables under study.15
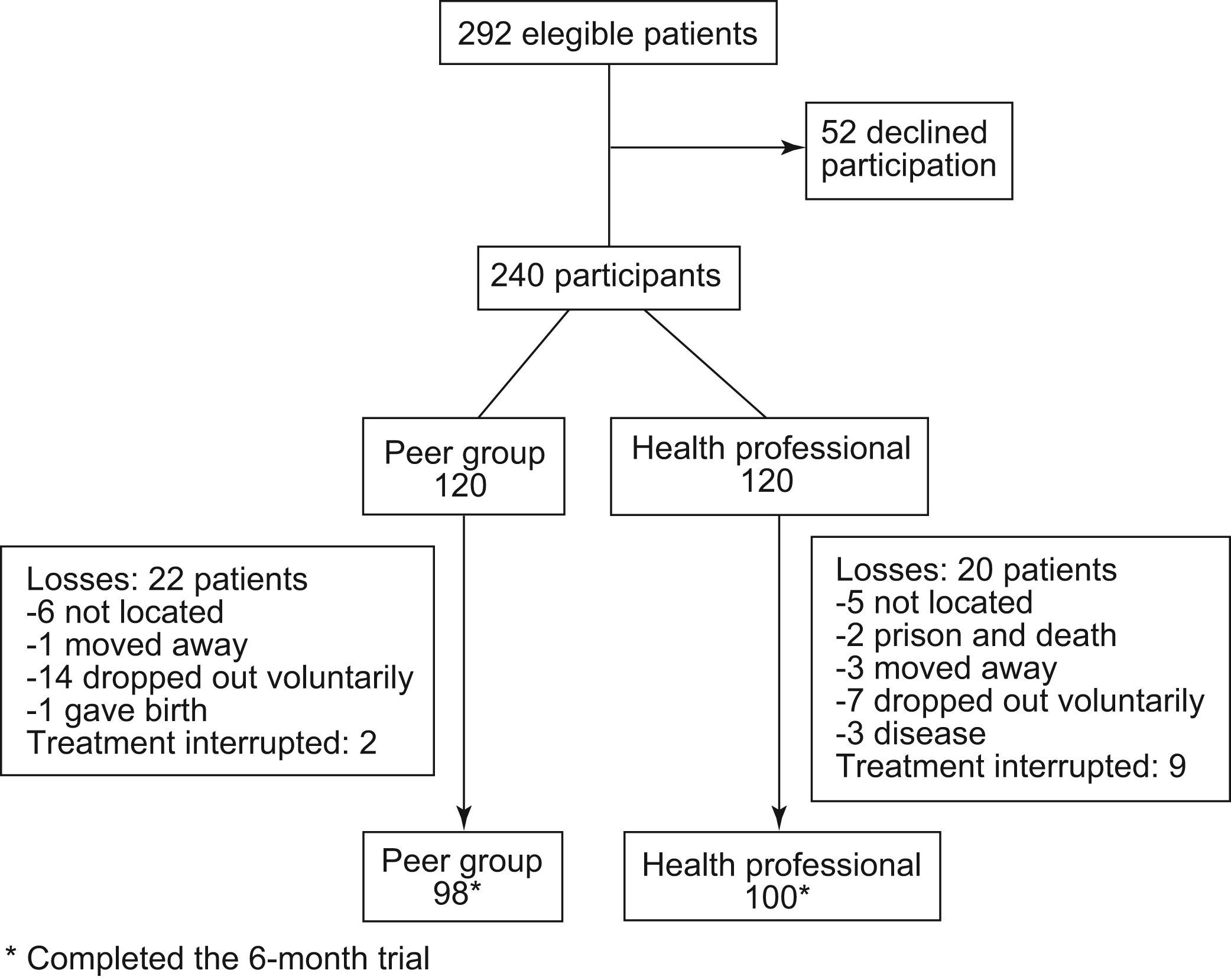
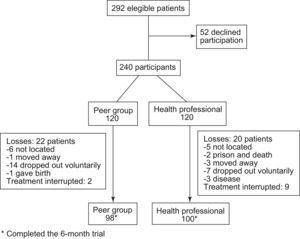
ResultsStudy population. Fifty-two patients declined participation (17.8%). Of the 240 participants, 42 (18.4%) (20 in the health professional group and 22 in the peer group) did not complete follow-up and in 11 (4.6%) (9 health and 2 peer), therapy was interrupted at some point by their doctor. Of the 42 who did not complete follow-up, 21 voluntarily dropped out of the intervention and 11 could not be located at some point. Causes of the remaining dropouts were as follows: 4 moved away, 3 became ill, 1 was sent to prison, 1 died, and one woman had a baby (Fig. 1).
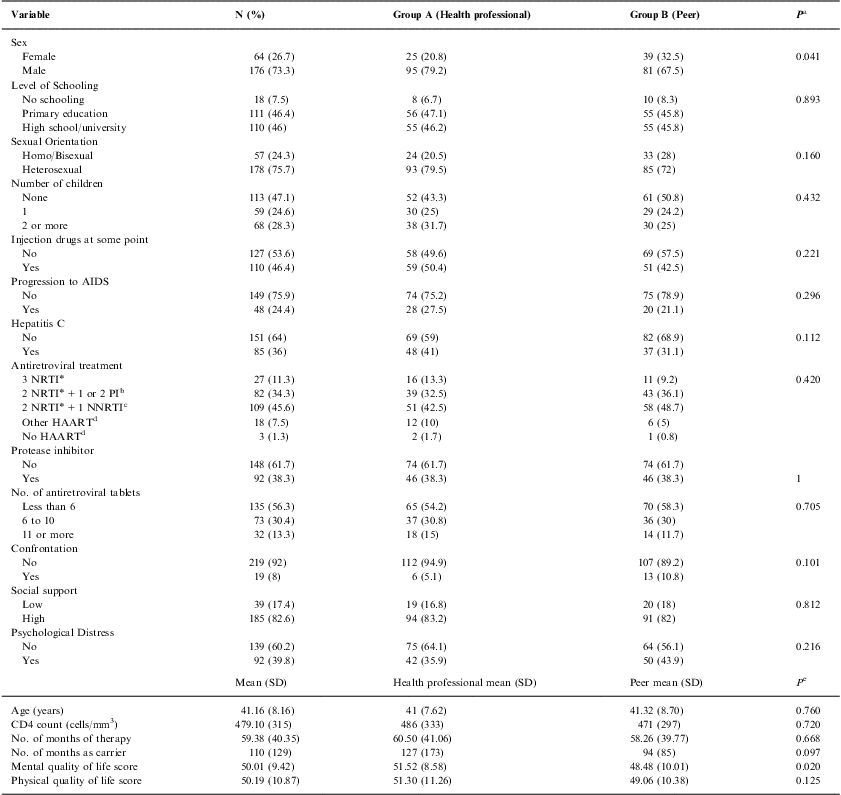
The baseline characteristics of the 240 participants are shown in Table 1. Mean age of the sample was 41.16 years and 26.7% were women. Among the total, 28.3% of the sample had two or more children, 46.4% had injected drugs at some time, and 7.5% lacked a basic education. With regard to psychosocial characteristics, 82.6% had a high level of social support, and 39.8% presented psychological distress. Mean physical and mental quality of life scores were 50.19 and 50.01, respectively. Participants had been receiving antiretroviral therapy for an average of 59.38 months, and had been HIV-positive for 110 months. Mean CD4 count was 479 cells/mm3, 24.4% of patients had developed AIDS, and 36% had hepatitis C virus coinfection.
Patient characteristics at baseline
| Variable | N (%) | Group A (Health professional) | Group B (Peer) | Pa |
| Sex | ||||
| Female | 64 (26.7) | 25 (20.8) | 39 (32.5) | 0.041 |
| Male | 176 (73.3) | 95 (79.2) | 81 (67.5) | |
| Level of Schooling | ||||
| No schooling | 18 (7.5) | 8 (6.7) | 10 (8.3) | 0.893 |
| Primary education | 111 (46.4) | 56 (47.1) | 55 (45.8) | |
| High school/university | 110 (46) | 55 (46.2) | 55 (45.8) | |
| Sexual Orientation | ||||
| Homo/Bisexual | 57 (24.3) | 24 (20.5) | 33 (28) | 0.160 |
| Heterosexual | 178 (75.7) | 93 (79.5) | 85 (72) | |
| Number of children | ||||
| None | 113 (47.1) | 52 (43.3) | 61 (50.8) | 0.432 |
| 1 | 59 (24.6) | 30 (25) | 29 (24.2) | |
| 2 or more | 68 (28.3) | 38 (31.7) | 30 (25) | |
| Injection drugs at some point | ||||
| No | 127 (53.6) | 58 (49.6) | 69 (57.5) | 0.221 |
| Yes | 110 (46.4) | 59 (50.4) | 51 (42.5) | |
| Progression to AIDS | ||||
| No | 149 (75.9) | 74 (75.2) | 75 (78.9) | 0.296 |
| Yes | 48 (24.4) | 28 (27.5) | 20 (21.1) | |
| Hepatitis C | ||||
| No | 151 (64) | 69 (59) | 82 (68.9) | 0.112 |
| Yes | 85 (36) | 48 (41) | 37 (31.1) | |
| Antiretroviral treatment | ||||
| 3 NRTI* | 27 (11.3) | 16 (13.3) | 11 (9.2) | 0.420 |
| 2 NRTI*+1 or 2 PIb | 82 (34.3) | 39 (32.5) | 43 (36.1) | |
| 2 NRTI*+1 NNRTIc | 109 (45.6) | 51 (42.5) | 58 (48.7) | |
| Other HAARTd | 18 (7.5) | 12 (10) | 6 (5) | |
| No HAARTd | 3 (1.3) | 2 (1.7) | 1 (0.8) | |
| Protease inhibitor | ||||
| No | 148 (61.7) | 74 (61.7) | 74 (61.7) | |
| Yes | 92 (38.3) | 46 (38.3) | 46 (38.3) | 1 |
| No. of antiretroviral tablets | ||||
| Less than 6 | 135 (56.3) | 65 (54.2) | 70 (58.3) | 0.705 |
| 6 to 10 | 73 (30.4) | 37 (30.8) | 36 (30) | |
| 11 or more | 32 (13.3) | 18 (15) | 14 (11.7) | |
| Confrontation | ||||
| No | 219 (92) | 112 (94.9) | 107 (89.2) | 0.101 |
| Yes | 19 (8) | 6 (5.1) | 13 (10.8) | |
| Social support | ||||
| Low | 39 (17.4) | 19 (16.8) | 20 (18) | 0.812 |
| High | 185 (82.6) | 94 (83.2) | 91 (82) | |
| Psychological Distress | ||||
| No | 139 (60.2) | 75 (64.1) | 64 (56.1) | 0.216 |
| Yes | 92 (39.8) | 42 (35.9) | 50 (43.9) | |
| Mean (SD) | Health professional mean (SD) | Peer mean (SD) | Pe | |
| Age (years) | 41.16 (8.16) | 41 (7.62) | 41.32 (8.70) | 0.760 |
| CD4 count (cells/mm3) | 479.10 (315) | 486 (333) | 471 (297) | 0.720 |
| No. of months of therapy | 59.38 (40.35) | 60.50 (41.06) | 58.26 (39.77) | 0.668 |
| No. of months as carrier | 110 (129) | 127 (173) | 94 (85) | 0.097 |
| Mental quality of life score | 50.01 (9.42) | 51.52 (8.58) | 48.48 (10.01) | 0.020 |
| Physical quality of life score | 50.19 (10.87) | 51.30 (11.26) | 49.06 (10.38) | 0.125 |
With regard to the drug therapy combination, 1.3% were not receiving HAART, 38.3% had a protease inhibitor as part of their antiretroviral treatment, 56.3% had been prescribed less than 6 antiretroviral tablets per day, and 13.3% were prescribed 11 or more. The study groups were well balanced at baseline, although group B had a poorer mental quality of life than group A (P<.05). Furthermore, there were fewer women in group A than in group B (P<.05) (Table 1).
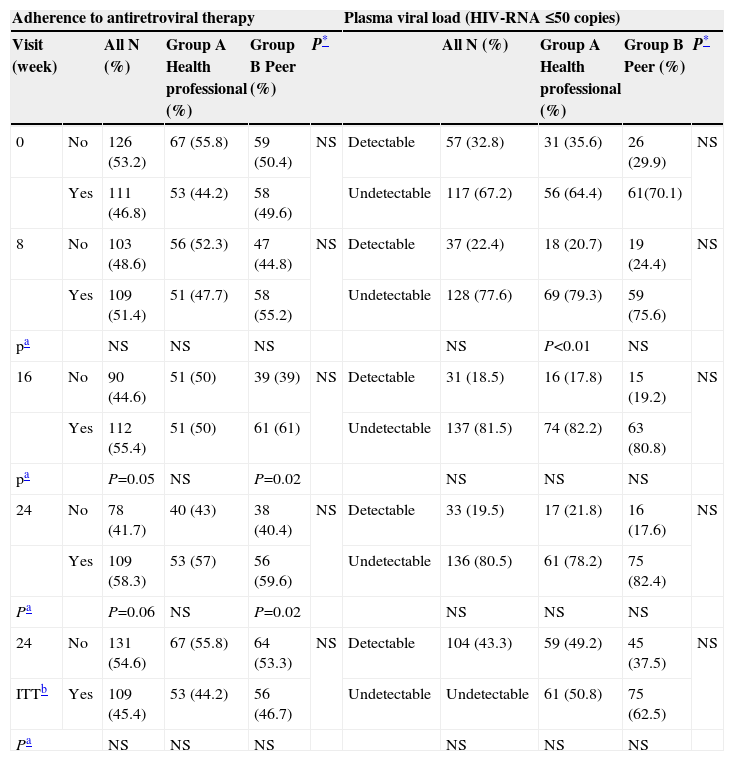
Primary Outcome: Adherence to antiretroviral therapy. At baseline, 46.8% of the sample was adherent to antiretroviral therapy, 49.6% in the peer intervention group and 44.2% in the health professional intervention group. At the last visit, 59.6% of patients in the peer group, 57% in the health professional group, and 58.3% of the total sample were classified as being adherent to their antiretroviral prescription. No differences in the percentage of adherence were observed between the two groups at any of the visits.
Analysis of the change in the percentage of adherence to antiretrovirals in comparison to the first visit showed that adherence had increased 11.4% in the group receiving the peer intervention at week 16 (P=.02) and 10% at week 24 (P=.02). In the overall sample, an adherence increase of 8.6% was observed at week 16 (P=0.05). No significant differences in adherence were found in the health professional group (Table 2).
Analysis of change in adherence and viral load as compared to first visit and between groups
| Adherence to antiretroviral therapy | Plasma viral load (HIV-RNA ≤50 copies) | |||||||||
| Visit (week) | All N (%) | Group A Health professional (%) | Group B Peer (%) | P* | All N (%) | Group A Health professional (%) | Group B Peer (%) | P* | ||
| 0 | No | 126 (53.2) | 67 (55.8) | 59 (50.4) | NS | Detectable | 57 (32.8) | 31 (35.6) | 26 (29.9) | NS |
| Yes | 111 (46.8) | 53 (44.2) | 58 (49.6) | Undetectable | 117 (67.2) | 56 (64.4) | 61(70.1) | |||
| 8 | No | 103 (48.6) | 56 (52.3) | 47 (44.8) | NS | Detectable | 37 (22.4) | 18 (20.7) | 19 (24.4) | NS |
| Yes | 109 (51.4) | 51 (47.7) | 58 (55.2) | Undetectable | 128 (77.6) | 69 (79.3) | 59 (75.6) | |||
| pa | NS | NS | NS | NS | P<0.01 | NS | ||||
| 16 | No | 90 (44.6) | 51 (50) | 39 (39) | NS | Detectable | 31 (18.5) | 16 (17.8) | 15 (19.2) | NS |
| Yes | 112 (55.4) | 51 (50) | 61 (61) | Undetectable | 137 (81.5) | 74 (82.2) | 63 (80.8) | |||
| pa | P=0.05 | NS | P=0.02 | NS | NS | NS | ||||
| 24 | No | 78 (41.7) | 40 (43) | 38 (40.4) | NS | Detectable | 33 (19.5) | 17 (21.8) | 16 (17.6) | NS |
| Yes | 109 (58.3) | 53 (57) | 56 (59.6) | Undetectable | 136 (80.5) | 61 (78.2) | 75 (82.4) | |||
| Pa | P=0.06 | NS | P=0.02 | NS | NS | NS | ||||
| 24 | No | 131 (54.6) | 67 (55.8) | 64 (53.3) | NS | Detectable | 104 (43.3) | 59 (49.2) | 45 (37.5) | NS |
| ITTb | Yes | 109 (45.4) | 53 (44.2) | 56 (46.7) | Undetectable | Undetectable | 61 (50.8) | 75 (62.5) | ||
| Pa | NS | NS | NS | NS | NS | NS | ||||
NS non significant.
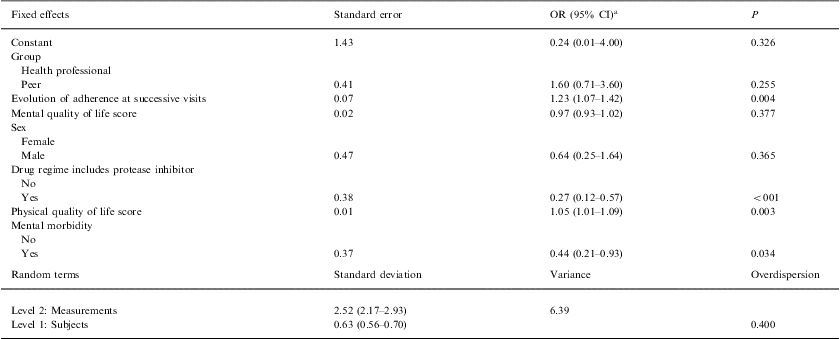
On multilevel analysis, it was found that as the visits progressed, the probability of treatment adherence increased (OR: 1.23; P=.004. Although differences were not significant, the group with the peer intervention obtained better results than those with the health professional intervention (OR: 1.60; P=0.255). There was a lower probability of adherence in patients prescribed a pharmacological combination including a protease inhibitor (OR: 0.27; P<.001) and in those with psychological distress (OR: 0.44; P=0.034). However, patients with the highest scores in physical quality-of-life (OR: 1.05; P=0.003) had a higher probability of adherence (Table 3).
Multilevel analysis of factors related to adherence to antiretroviral therapy
| Fixed effects | Standard error | OR (95% CI)a | P |
| Constant | 1.43 | 0.24 (0.01–4.00) | 0.326 |
| Group | |||
| Health professional | |||
| Peer | 0.41 | 1.60 (0.71–3.60) | 0.255 |
| Evolution of adherence at successive visits | 0.07 | 1.23 (1.07–1.42) | 0.004 |
| Mental quality of life score | 0.02 | 0.97 (0.93–1.02) | 0.377 |
| Sex | |||
| Female | |||
| Male | 0.47 | 0.64 (0.25–1.64) | 0.365 |
| Drug regime includes protease inhibitor | |||
| No | |||
| Yes | 0.38 | 0.27 (0.12–0.57) | <001 |
| Physical quality of life score | 0.01 | 1.05 (1.01–1.09) | 0.003 |
| Mental morbidity | |||
| No | |||
| Yes | 0.37 | 0.44 (0.21–0.93) | 0.034 |
| Random terms | Standard deviation | Variance | Overdispersion |
| Level 2: Measurements | 2.52 (2.17–2.93) | 6.39 | |
| Level 1: Subjects | 0.63 (0.56–0.70) | 0.400 | |
Plasma viral load. The percentage of patients with undetectable viral load at the first visit was 71.1% in the peer intervention group and 64.4% in the health professional intervention group. At the last visit, the values were similar between the two groups, at 82.4% and 78.2%, respectively. The number of patients with a detectable viral load fell 14.9% between the first and second visit (P<.01) in the group of patients treated by a health professional (Table 2).
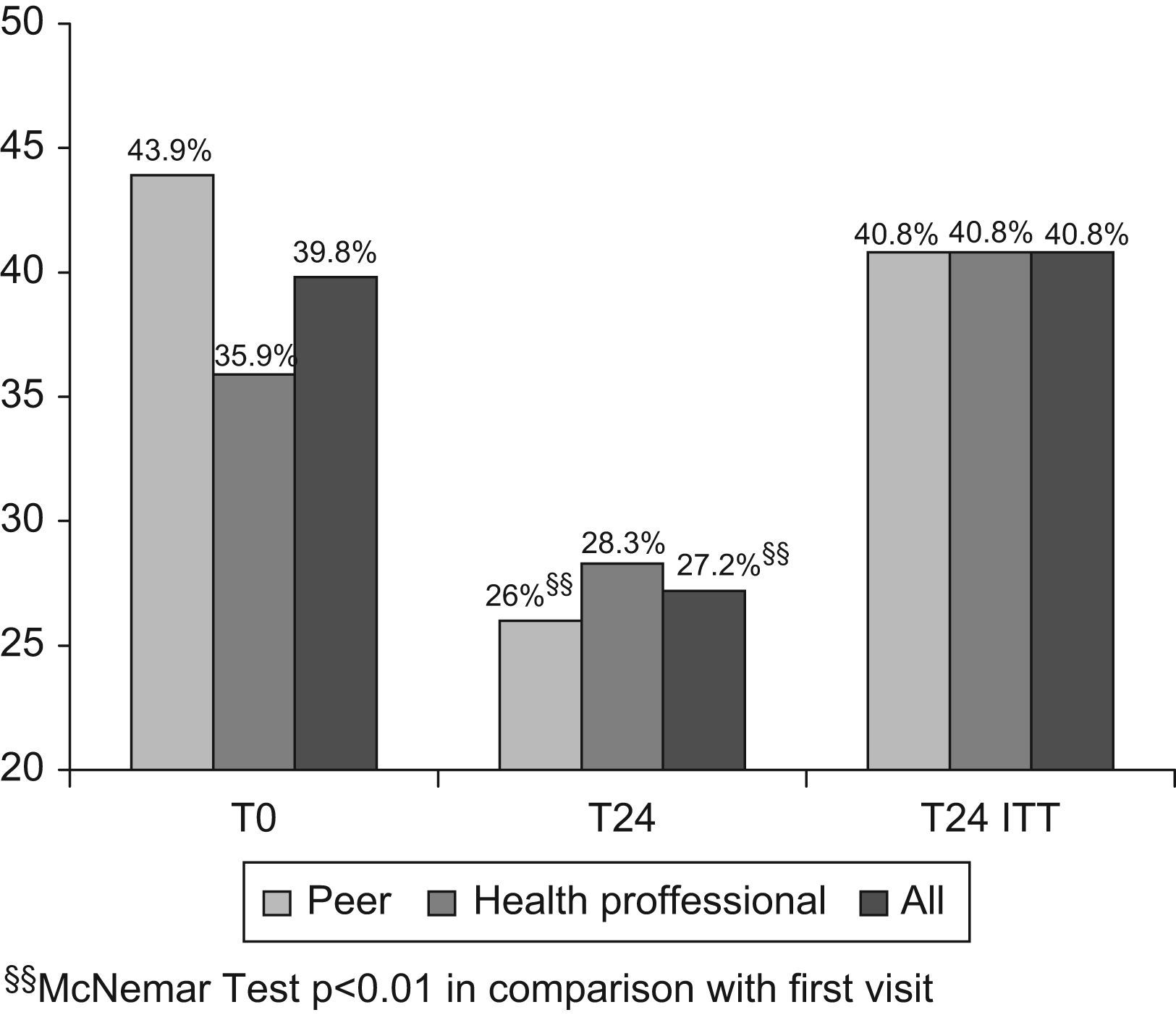
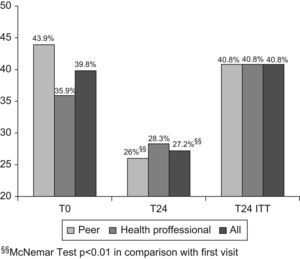
Psychological distress. At the first visit, 43.9% of patients had psychological stress in the peer intervention group and 35.9% in the professional intervention group, and at the last visit, these values were 26% and 28.3%, respectively. Analysis of the changes showed that the number of patients with psychological distress fell in the peer intervention group (P<.01) and in the total sample (P<.01), but there were no significant changes in the group receiving the health professional intervention (Fig. 2).
DiscussionThis study provides further evidence that psychoeducational interventions to improve adherence to antiretroviral therapy are viable, effective, and well accepted by patients, and offer a useful tool to achieve correct pharmacological monitoring. Furthermore, if the intervention is provided by an HIV-positive patient taking antiretroviral therapy, the results obtained are at least as good as in interventions provided by a health professional.
At the end of the intervention, there was an increase in adherence to treatment of 10% to 12.8%. This result is of particular clinical relevance since recent studies have shown that a 10% difference in correct adherence to antiretroviral drugs reduces the risk of death and the AIDS stage by 20%.17 Nonetheless, the differences were not significant in either group. This may be because of the inclusion criteria of the study. A systematic review has recently shown that interventions addressed to patients with detected adherence problems result in a significant increase in adherence (OR=3.07), whereas interventions without this inclusion criterion show a less pronounced effect (OR=1.41).18
This study has some limitations that should be taken into consideration when interpreting the results. Since there was no group that did not receive the intervention, we cannot be certain that the increase in adherence was not the result of other factors, apart from the intervention itself. However, no event occurred during the course of the study to explain the increased adherence. In addition, previous Spanish and international studies have shown that interventions improve adherence.2,16,18–21 A report by Simoni et al20 concluded that these findings can be explained by insufficient exposure to the intervention or other factors. The length of the intervention in this study was similar to the duration used in other reports.16,17,20 Moreover, the main objective of our study was not only to determine whether there was an improvement, but also which intervention was the most effective. Lastly, it has been reported that the mere fact of measuring adherence may result in an improvement, and this is unavoidable.22–24
It seems clear that peer interventions provide benefits, but it is difficult to identify the mechanism underlying this fact. In addition to the rapport and empathy the occurs between peers and patients, we believe that peers may provide new sources of information and alternative explanations when considering and resolving psychosocial aspects and care-related problems of patients with HIV, whereas health professionals may have less experience and information in this regard.
This favorable rapport may be useful in other aspects related to the health of the HIV-infected population. From the economic viewpoint, a study by Broadhead et al. demonstrated that a peer intervention performed to bring injection drug users under the cover of the health system was effective and up to 30-fold less expensive than a conventional intervention.25
The results of this study are consistent with other studies showing that correct adherence to antiretroviral therapy is essential to attain the desirable clinical parameters.26 Although there were no differences in viral load at baseline between groups with differing adherence to therapy (P=.527), adherent patients showed a significant reduction in viral load (P=.038), and this reduction was not significant in the non-adherent participants (P=.700) (data not presented). This is important because viral load is one of the best predictors of progression to AIDS and death.27
Other factors that affect adherence to antiretroviral therapy have been identified in this study. Higher quality-of-life scores were associated with greater adherence. This is consistent with studies reporting that continuous use of treatments improves all the quality-of-life dimensions.28 This provides further evidence of the importance of psychosocial factors in correct adherence to antiretroviral therapy.
The fact that patients who were prescribed protease inhibitors showed lower adherence can be explained by several reasons. First, these drugs can have significant side effects.29 Second, protease inhibitors are metabolized in the liver, and drug interactions are frequent, thus increasing the toxicity. Moreover, in this study, patients who were prescribed protease inhibitors took a larger number of tablets per day. All these factors are related to poor adherence.6,26
The prevalence of psychological distress was found to be greater in the study population than in the general population in Spain.30 This is of particular relevance since, as in the case of other chronic therapies, the presence of mental disorders reduces adherence.26 This association appears to be due to several factors, such as less social support, a reduction in cognitive ability, reduced motivation regarding personal care, and decreased skills in complying with the complex instructions in antiretroviral therapy, which are essential to ensure correct pharmacological follow-up. This result is also important because there is some evidence that psychological distress, independently of adherence, jeopardizes the immune system, increases viral load and accelerates the advance of HIV infection/AIDS.31,32 The reduction in psychological distress observed is relevant for all these reasons, and may have occurred because patients have more information more about their disease, which could facilitate better management of the infection.
In conclusion, in view of the importance of correct adherence to antiretroviral therapy and the high prevalence of psychological distress in HIV-infected patients, this simple intervention provides a true benefit in improving the health of this population and tends to increase the efficacy of antiretroviral therapy.
Study financed by Fondo de Investigación Sanitaria (FIS 020311). The authors are grateful to Professor Ricardo Ocaña Riola and Professor Antonio Daponte Codina for their suggestions and comments on this paper.