Urinary tract infections (UTIs) are one of the most common infections in solid organ transplant (SOT) recipients.
MethodsExperienced SOT researchers and clinicians have developed and implemented this consensus document in support of the optimal management of these patients. A systematic review was conducted, and evidence levels based on the available literature are given for each recommendation. This article was written in accordance with international recommendations on consensus statements and the recommendations of the Appraisal of Guidelines for Research and Evaluation II (AGREE II).
ResultsRecommendations are provided on the management of asymptomatic bacteriuria, and prophylaxis and treatment of UTI in SOT recipients. The diagnostic-therapeutic management of recurrent UTI and the role of infection in kidney graft rejection or dysfunction are reviewed. Finally, recommendations on antimicrobials and immunosuppressant interactions are also included.
ConclusionsThe latest scientific information on UTI in SOT is incorporated in this consensus document.
Las infecciones del tracto urinario (ITU) son muy frecuentes en los receptores de un trasplante de órgano sólido (TOS).
MétodosInvestigadores y clínicos con experiencia en el TOS han desarrollado este documento de consenso para el mejor abordaje de estos pacientes. Hemos realizado una revisión sistemática y se ha especificado el nivel de evidencia para cada recomendación basado en la literatura disponible. Este artículo se ha redactado de acuerdo con las recomendaciones internacionales sobre documentos de consenso y las recomendaciones del Instrumento para Evaluación de Guías de Práctica Clínica II (AGREE II).
ResultadosSe realizan recomendaciones sobre el abordaje de la bacteriuria asintomática y sobre la profilaxis y tratamiento de las ITU en receptores de TOS. Se han revisado el abordaje diagnóstico-terapéutico de las ITU recurrentes y el papel de la ITU en el rechazo o disfunción del injerto renal. Finalmente, se incluyen recomendaciones sobre las interacciones entre antimicrobianos e inmunosupresores.
ConclusionesSe incorpora a este documento la información científica más actualizada sobre la ITU en el contexto del TOS.
The use of solid organ transplantation (SOT) has been established as an accepted therapy for end-stage disease of the kidneys, liver, heart, and lungs for nearly 30 years. Intestinal and pancreas transplantation are also generally available but are provided on a more limited basis.
Infections remain a major cause of morbidity and mortality in transplant recipients. Urinary tract infections (UTIs) are one of the most common infections in SOT, with a high prevalence, reaching 75% in some series involving kidney recipients.1–6 Experienced SOT researchers and clinicians have developed and implemented this consensus document in support of the optimal management of these patients.
The target population of this document are adults receiving SOT. The intended guideline audience is physicians involved in the care of SOT recipients (including primary care physicians). Here we report a consensus with the objective of assessing the overall available evidence and to propose recommendations on the following key issues:
- 1.
Definitions
- 2.
Epidemiology and risk factors for UTI in SOT recipients.
- 3.
Should SOT recipients receive primary prophylaxis for UTI?
- 4.
What should be the management of asymptomatic bacteriuria in SOT recipients?
- 5.
What is the best empirical treatment of UTI in SOT recipients?
- 6.
What is the best definitive treatment of UTI in SOT recipients?
- 7.
How long should SOT recipients receive antibiotics for a UTI?
- 8.
What should be the management of UTI caused by Candida spp. in SOT recipients?
- 9.
What should be the diagnostic-therapeutic management of recurrent UTI in SOT recipients?
- 10.
What role does UTI play in kidney graft rejection or dysfunction?
- 11.
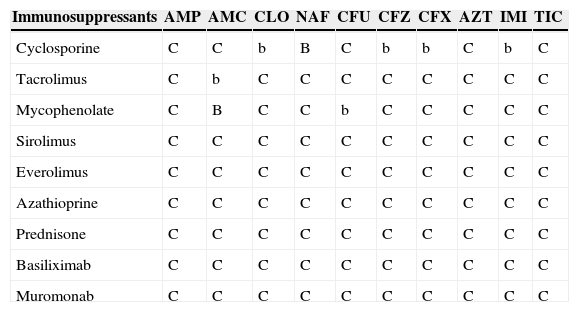
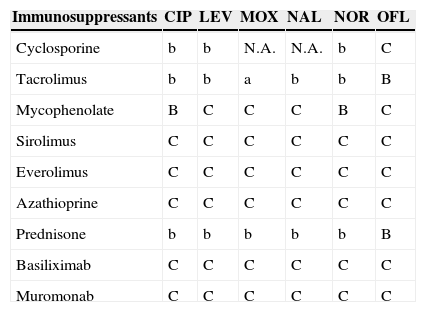
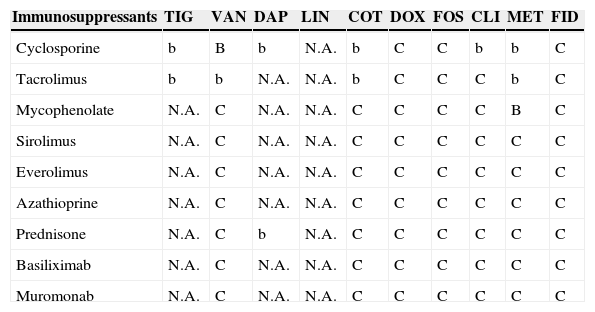
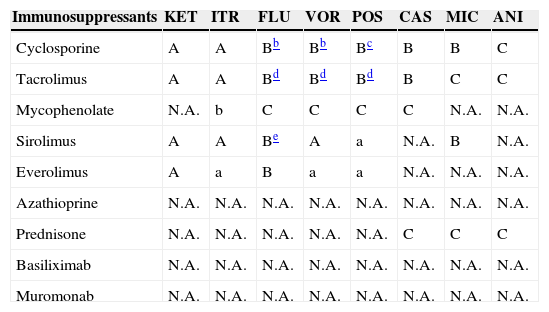
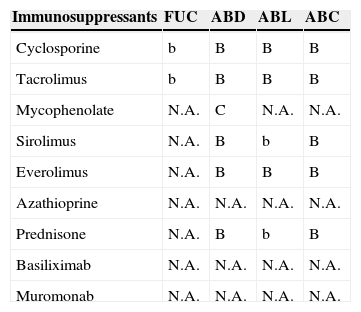
Antimicrobial and immunosuppressant interactions.
We conducted a systematic review to assess the management of UTI in SOT recipients. Data for this document were identified through a search of PubMed and references from relevant articles using the search terms “transplant” and “urinary tract infection”. The search criteria included articles in English that involved human participants. We selected and revised a total of 3043 articles from 1968 to June 2014.
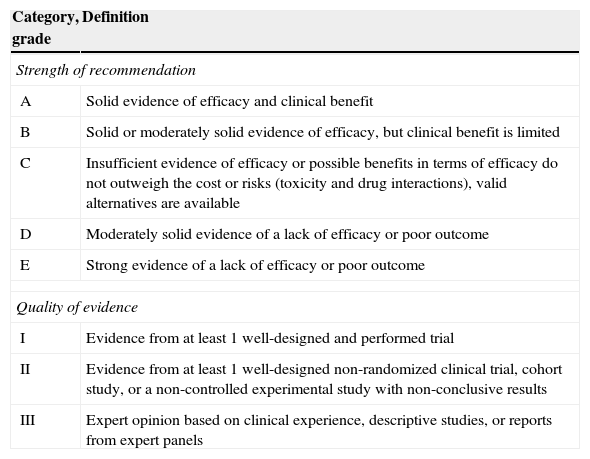
The evidence level based on the available literature is given for each recommendation to assess the strength of the evidence for risk and benefits of the procedure. This article was written in accordance with international recommendations on consensus statements (Table 1)7 and the recommendations of the Appraisal of Guidelines for Research and Evaluation II (AGREE II).8 The authors met twice to discuss the consensus and establish formal recommendations. The coordinators and authors agree on the content and conclusions. The consensus statement was sent to the 96 members of GESITRA for external revision of the manuscript. The board of directors of GESITRA will designate the coordinators to update the statements within 5 years.
Classification of the recommendations of this consensus document based on the strength and quality of the evidence analyzed.
| Category, grade | Definition |
|---|---|
| Strength of recommendation | |
| A | Solid evidence of efficacy and clinical benefit |
| B | Solid or moderately solid evidence of efficacy, but clinical benefit is limited |
| C | Insufficient evidence of efficacy or possible benefits in terms of efficacy do not outweigh the cost or risks (toxicity and drug interactions), valid alternatives are available |
| D | Moderately solid evidence of a lack of efficacy or poor outcome |
| E | Strong evidence of a lack of efficacy or poor outcome |
| Quality of evidence | |
| I | Evidence from at least 1 well-designed and performed trial |
| II | Evidence from at least 1 well-designed non-randomized clinical trial, cohort study, or a non-controlled experimental study with non-conclusive results |
| III | Expert opinion based on clinical experience, descriptive studies, or reports from expert panels |
Bacteriuria is defined according to the criteria proposed by the Infectious Diseases Society of America guidelines.9 For asymptomatic women, bacteriuria is defined as 2 consecutive voided urine specimens with isolation of the same bacterial strain in quantitative counts ≥105colony-forming units (cfu)/ml. A single, clean-catch voided urine specimen with 1 bacterial species isolated in a quantitative count ≥105cfu/ml identifies bacteriuria in men. A single catheterized urine specimen with 1 bacterial species isolated in a quantitative count ≥102cfu/ml identifies bacteriuria in women or men. Asymptomatic bacteriuria (AB) is defined by the presence of bacteriuria in the absence of any symptoms of lower or upper UTI.
CystitisCystitis is defined by the presence of bacteriuria and clinical manifestations such as dysuria, frequency, or urinary urgency in the absence of pyelonephritis criteria.
PyelonephritisPyelonephritis is defined by the simultaneous presence of a urine bacteria count ≥105cfu/ml and/or bacteremia and fever with one or more of the following four categories: costovertebral angle pain (if native kidney involved), renal allograft tenderness (if transplanted kidney involved), chills, criteria for cystitis (bacteriuria and clinical manifestations such as dysuria, frequency, or urinary urgency).10
ReinfectionReinfection is defined by a new episode of infection with the isolation of bacterium other than the one that caused the previous infection or the same bacteria with a different antibiotic sensitivity pattern.11
RelapseRelapse is defined as the isolation of the same microorganism that caused the preceding infection, with the same antibiotic sensitivity pattern, in a urine culture obtained ≥2 weeks after finishing the previous treatment.12
Recurrent infectionRecurrent infection is commonly defined as three or more episodes of symptomatic UTIs over a 12-month period or two episodes in the previous six months.13
Complicated urinary tract infectionA complicated UTI is defined as an infection that is associated with structural or functional abnormalities of the genitourinary tract, or the presence of an underlying disease that increases the risk of acquiring an infection or of failing therapy.14
ProstatitisProstatitis is characterized by discomfort referred to the lower urogenital and perineal and/or ejaculatory discomfort or sexual dysfunction. Acute bacterial prostatitis is presented as fever and chills accompanied by urinary symptoms such as dysuria, frequency, and perineal pain. Chronic bacterial prostatitis has a more prolonged course, usually of at least 3 months. This is usually related to or the result of recurrent urinary infection, or may be a complication of acute prostatitis that is not properly cured, urethritis, or epididymitis. The disease can occur continuously or episodically. The symptoms are milder than in acute prostatitis and sometimes imperceptible. The most common symptoms are perineal or pelvic pain, low back pain, testicular pain, and discomfort when urinating or ejaculating.15,16
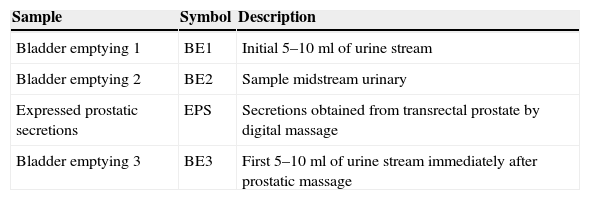
The classification of patients with prostatitis depends on the bacteriological study of lower urinary tract considering sequential urine cultures14 (Table 2).
Sequential urine cultures for anatomical location within the lower urinary tract.a
| Sample | Symbol | Description |
|---|---|---|
| Bladder emptying 1 | BE1 | Initial 5–10ml of urine stream |
| Bladder emptying 2 | BE2 | Sample midstream urinary |
| Expressed prostatic secretions | EPS | Secretions obtained from transrectal prostate by digital massage |
| Bladder emptying 3 | BE3 | First 5–10ml of urine stream immediately after prostatic massage |
Definitive diagnosis of bacterial prostatitis requires that the number of colonies in the BE3 sample exceeds those in the BE1 sample, preferably by more than 10 times. However, the prostate of many patients with chronic prostatitis contains only small amounts of bacteria. In these patients, a prostatic secretions culture is particularly useful. Microscopic examination of the EPS is useful to identify leukocytes and “oval fat bodies” – large lipid-laden macrophages characteristic of prostatic inflammatory response.
Stamey T. Pathogenesis and Treatment of Urinary Tract Infections. Baltimore: Williams & Wilkins; 1980.
The overall incidence of UTI in SOT recipients is 0.23/1000 transplantation days, but varies among the different types of transplant. In kidney transplant recipients (KTR), UTI is responsible for 42–75% of infections1–3,17,18 with an incidence rate of 0.45/1000 transplantation days. Other organs have lower incidence rates: kidney-pancreas 0.22/1000 transplantation days, heart 0.07 episodes/1000 transplantation days, liver 0.06/1000 transplantation days, and lung 0.02 episodes/1000 transplantation days.4 Nevertheless, the prevalence varies in the published studies probably due to different perioperative antibiotic prophylaxis strategies, surgical techniques, and immunosuppression regimens.
Candida UTI is more frequent in KTR than in other SOT groups.19 The frequency of candiduria in KTR cohorts ranges from 3.4 to 11%.20–22
Chronology and risk factors for UTIAlthough UTI can occur any time after transplantation, most cases occur during the first six months after the transplant.2,23 In the RESITRA cohort study, only 15% of the UTI episodes were diagnosed later than 6 months after transplantation. One-third of UTI episodes in renal transplant recipients and two-thirds of UTI episodes in liver transplant recipients occurred in the first month after transplantation.4 Renal transplant bacterial UTI is distributed in a more uniform pattern and occurs in both the perioperative and late postoperative period, while bacterial UTI in non-KTR is usually an early complication that may be associated with urinary catheterization, stays in the ICU, or malnutrition; all of which are associated with postoperative patient care. In a recent study,20 candiduria was detected at a median of 18 months after kidney transplantation and at a median of 54 days after kidney transplantation in another study.21
Female gender is an independent risk factor of UTI in kidney and other types of SOT with an OR of 1.74 (95% CI 1.42–2.13) and 1.7 (95% CI 1.43–2.49), respectively4 (Table 3). Abbott et al. found no differences in the incidence of UTI by gender in the first 6 months after transplantation. However, three years after kidney transplantation, women had a nearly 50% higher incidence of UTI than men.24 The association between female gender and UTI has been previously confirmed not only in SOT recipients, but also in the general population.25–27 Female gender determines a 12.5 times increase (95% CI 6.7–23.0) in candiduria in renal transplant recipients.14
Risk factors for urinary tract infections in solid organ transplantation.
| Female gender |
| Age |
| Mycophenolate mofetil |
| Antithymocyte globulin |
| Need for immediate post-transplant dialysis |
| Ureteral stent placement>30 days |
| Diabetes mellitus |
| Deceased-donor kidneys |
| Number of episodes of acute rejection |
| Reflux kidney disease prior to transplantation |
| Length of hospitalization |
| Length of urinary catheterization |
| Number of episodes of acute rejection |
| Post-transplant urinary obstructions |
| Increase in immunosuppression |
The incidence of UTI increases with age in both kidney and non-kidney SOT4,16 as described in non-immunosuppressed patients.21,28–31 This association may be due to the particular immunological characteristics of these patients and the more complicated postoperative management in elderly patients.
The net state of immunosuppression determines the risk of UTI, but some immunosuppressors, such as mycophenolate mofetil and induction therapy with antithymocite globulin, have been associated with a higher risk of UTI in some studies but not in others.19,28,29 The RESITRA cohort study was unable to associate risk with immunosuppression or acute rejection.4
The need for immediate post-transplant dialysis has been identified as a risk factor in kidney and kidney-pancreas transplants, perhaps because it is a surrogate factor in high-risk patients with a complicated postoperative period, acute rejection, need for additional immunosuppression, or prolonged hospitalization.4
The association between ureteral stents and UTI is controversial. While some authors have reported that patients with stents for more than 30 days have a higher rate of UTIs,30 others have not found this relationship.32
Other risk factors for UTI are diabetes mellitus, deceased-donor kidneys in renal transplants, number of episodes of acute rejection, length of urinary catheterization, stent placement for more than 30 days instrumentation, reflux kidney disease prior to transplantation, length of hospitalization prior to UTI, and post-transplant urinary obstructions.3,4,25,28,30,32 Prophylaxis with cotrimoxazole has been found to diminish the incidence of bacterial UTI in renal transplant patients in some studies3,33 but not in others.4 This might be due to the widespread use of this antibiotic to prevent Pneumocystis jiroveci pneumonia, or to the low doses administered with this aim that might not have an effect on bacterial UTI. Risk factors associated with late UTI are renal insufficiency, prednisone dose higher than 20mg/day, multiple rejections, and CMV infection.23 Other factors associated with candiduria in KTR are ICU care, prior antibiotic use, indwelling urethral catheter, neurogenic bladder, and malnutrition.14,21 No information on other transplantation groups is available.
EtiologyEscherichia coli is the most common cause of UTI in all types of organ transplant recipients and is responsible for 85.7% of bacteremic UTI.3,4,33–36 Other common etiologies are Pseudomonas aeruginosa, Klebsiella spp., Enterococcus spp., Candida spp., and other gram-negative bacteria. In non-kidney recipients, E. coli caused 70.3% of UTI episodes in the GESITRA cohort.4Corynebacterium urealyticum UTI has been associated with obstructive uropathy, symptoms lasting more than one month, and encrusting pyelitis in KTR. Long-term incubation and special media are needed for its isolation.37
Multidrug-resistant (MDR) pathogens are an important issue in UTI in SOT given its association with adverse outcome.38 In the GESITRA cohort study, 26% of the E. coli strain isolates produced extended-spectrum beta-lactamase (ESBL), and resistance to quinolones was achieved in 38–45% of E. coli, 25–31% of Klebsiella spp., and 21% of P. aeruginosa isolates.4 Among Gram-negative bacilli, 60–85% of the strains are resistant to cotrimoxazole.4,39 Antimicrobial resistance has been associated with the prophylactic use of antimicrobials and treatment of AB.39–41
OutcomeIn general, UTI in SOT does not involve attributable mortality, as the majority of cases are cystitis that responds to antimicrobial therapy.4,5 To date, there is no consensus regarding the impact of UTI in graft survival. Some studies have identified pyelonephritis with graft loss and death at 5 years,3,29,42 while others have not.4,28,43
Should SOT recipients receive primary prophylaxis for UTI?Recommendations- 1.
Trimethoprim/sulfamethoxazole (TMP/SMX, cotrimoxazole 160–800mg) antibiotic prophylaxis is recommended during the first 3–6 months post-transplant because it significantly decreases AB and symptomatic UTI, and bacteremia in renal transplant recipients (A-I).
- 2.
Antibiotic prophylaxis is not specifically recommended for UTI in non-kidney SOT recipients.
Prevention of both AB and UTI post-transplant improved with the introduction of routine perioperative antibiotic prophylaxis, minimization of use of indwelling urethral catheters, and long-term use of antimicrobial prophylaxis to prevent Pneumocystis pneumonia and other infections.34,44 Studies published more than 15 years ago demonstrated that prophylaxis with TMP/SMX reduced the risk of UTI threefold and did not result in significant colonization by TMP/SMX-resistant gram-negative bacilli.34,45 This led to recommendations to use prophylactic antibiotics (TMP/SMX) for 6 months to 1 year post-transplant.23 Most transplant centers utilize TMP/SMX for prevention of Pneumocystis pneumonia, which may have the additional benefit of preventing UTI in renal transplant recipients.46
Green et al.39 conducted a systematic review and meta-analysis of randomized controlled trials comparing antibiotic prophylaxis for UTI in KTR vs. placebo, no intervention, or different antibiotics, all beginning postoperatively and continuing for at least one month during the first six months after transplantation. Six trials with 545 patients were included in the review. Three trials compared antibiotic treatment vs. placebo, two trials compared TMP/SMX vs. placebo or no intervention, and the third compared a fluoroquinolone (ciprofloxacin) vs. placebo.34,45,47–51 The other trials compared TMP/SMX vs. ciprofloxacin, different doses of TMP/SMX, or two beta-lactam antibiotics (ampicillin vs. cephalexin).48–50 Prophylaxis lowered the risk of developing sepsis with bacteremia by 87% (RR 0.13, 95% CI 0.02–0.7) and the risk of developing symptomatic and AB by 60% (RR 0.41, 95% CI 0.31–0.56; 3 trials); however, all caused mortality and the graft outcome was not different in this meta-analysis. In addition, TMP/SMX 160/800mg daily seemed superior to 80/400mg.
As uropathogenic bacteria have become more TMP/SMX resistant,52–54 prophylaxis with TMP/SMX may be less effective for the prevention of UTI in KTR. For renal transplant recipients unable to receive TMP/SMX, it remains unclear whether other antibiotics targeted at the prevention of urinary tract pathogens should be routinely used. A randomized trial to compare low-dose TMP/SMX with ciprofloxacin for the prevention of UTI in renal transplant recipients showed that ciprofloxacin was at least as effective as TMP/SMX.48
Although some investigators have recommended the indefinite use of TMP/SMX, data demonstrating clinical benefit beyond the first 9 months following kidney transplantation are not available. Evidence suggests that late UTIs tend to be benign without associated bacteremia, metastatic foci, or effect on long-term graft function.23 For this reason, we recommend prophylaxis for 3–6 months in agreement with other guidelines.14
What should be the management of asymptomatic bacteriuria in sot recipients?Recommendations- 1.
Screening for and treatment of AB in KTR is recommended in the early postoperative period and up to one month after transplantation (B-III).
- 2.
There is not enough evidence to recommend continued screening for and treatment of AB in a clinically stable KTR beyond one month after transplantation (C-III). However, there is no consensus on whether AB by MDR bacteria, mainly gram-negative bacilli, should be treated.
- 3.
Screening for and treatment of AB is not currently recommended for other SOT recipients (D-III). In these cases, guidelines for the general population should be applied.
- 4.
Treatment of asymptomatic candiduria is not currently recommended for SOT recipients. Among patients with a urinary catheter, removal of the catheter may be sufficient to eliminate candiduria without specific antifungal therapy (D-III).
- 5.
Urine culture screening of patients awaiting transplantation is not routinely recommended (D-III).
- 6.
Live donors should be screened and treated for bacteriuria before the organ is harvested (A-III).
The clinical importance of AB has been controversial since the widespread use of quantitative urine cultures provided a reliable method for identification. The main question is whether bacteriuria in the absence of symptoms leads to short-term complications such as symptomatic lower UTI or pyelonephritis, or long-term complications such as urolithiasis, genitourinary cancer, renal failure, hypertension, and death. Alternatively, AB may be beneficial: colonization of the genitourinary tract by an avirulent organism could prevent infection by more virulent organisms through competition for nutrients or receptor sites, or by eliciting a cross-protective host immune or inflammatory response.55
Screening of asymptomatic subjects for bacteriuria is appropriate if bacteriuria has adverse outcomes that can be prevented by antimicrobial therapy. Benefit for treatment of AB has only been proved in pregnant women and before transurethral resection of the prostate or other urologic procedures for which mucosal bleeding is anticipated.9 In other situations, the association of AB with symptomatic UTI is likely attributable to host factors that promote both symptomatic and asymptomatic urinary infection, rather than symptomatic infection being attributable to AB. Hence, treatment of AB neither decreases the frequency of symptomatic infection nor prevents further episodes of AB. According to these guidelines, no recommendation can be made in kidney transplant or other SOT recipients. Moreover, the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines for the care of KTR published in 200956 do not make any specific recommendations about this issue.
Although it is well known that AB is frequent among KTR,57 especially during the first year after transplantation, the need to monitor and treat AB is controversial.58 The Immunocompromised Host Society Consensus Conference recommends periodic monitoring of UTIs after transplantation, but it does not specify the frequency of screening nor does it make a clear recommendation for therapy if bacteriuria is identified.59
Some experts recommend antibiotic therapy for AB in the first few months after transplantation. According to Franz and Hörl, “it is obvious that in the early post transplant period even low-count bacteriuria and AB should be treated”.60 Nicolle et al. consider that it may be appropriate to screen for bacteriuria in the early postoperative period and up to 6 months after transplantation,61 while continued screening for and treatment of AB in a clinically stable KTR beyond 6 months after transplantation does not seem beneficial given the low frequency of bacteriuria and the lack of impact of bacteriuria on graft survival. El Amari et al.41 retrospectively analyzed the outcome of 334 episodes of AB in 77 KTR later than one month post transplantation. They classified the episodes into four groups based on culture and urinary sediment. None of the 101 treated episodes progressed toward symptomatic UTI, although only 55% of them were followed by a sterile control culture. Four of the 233 untreated episodes (2%) progressed toward symptomatic UTI, with spontaneous bacterial clearance being observed in 59% of them. They observed no differences between treated and untreated episodes when comparing progression to symptomatic UTI. Green et al.62 studied a retrospective cohort study including 112 patients with AB between one month and 12 months after kidney transplantation in which 22 patients received antibiotic treatment and 90 did not. The primary outcome of hospitalization for symptomatic UTI or a 25% reduction in the glomerular filtrate at 30 days occurred in 18.2% of treated patients vs. 5.6% of untreated patients. A prospective randomized trial performed by Moradi et al. including 88 patients reported that treatment of AB beyond one year after transplantation did not prevent symptomatic UTIs and that plasma creatinine did not increase significantly.63 In a group of KTR, declining graft function was not ameliorated in patients with prophylaxis during AB, thus indicating that other critical factors are more important for determining the fate of the allograft.64
Most authors recommend that AB occurring after the first post-transplant month be carefully followed and that patients should be warned of symptoms and begin antibiotic therapy when clinical manifestations are present.18 Nevertheless, there are no prospective, placebo-controlled, randomized trials of adequate size to confirm such recommendations.
Some observational evidence indicates that AB may cause subclinical damage to the allograft due to inflammation (increased IL-8 levels measured during such episodes reflect an inflammatory process).65 Damage to renal allografts in AB has been associated with characteristics of the adhesion molecules, fimbriae, and O serotype expressed by E. coli strains.66
Some researchers have recommended AB treatment based on the possibility that even asymptomatic UTI could lead to renal allograft scarring in the context of vesicoureteral reflux.67 Fiorante et al. examined the impact of AB on renal transplant outcome by retrospectively analyzing 189 KTR. In their study, 96 (50.8%) of the subjects presented 289 episodes of AB and 93 did not experience any episode during the 36 months of follow-up. According to their protocol, all episodes of AB were systematically screened and treated. Risk factors for AB were female sex, glomerulonephritis as the disease that led to transplantation, and double renal transplant. The incidence of pyelonephritis in these patients was 7.6 episodes per 100 patient-years compared with 1.07 in those without AB (lower in both cases than the incidence described in previous studies). The detection of two or more episodes of AB was statistically associated with pyelonephritis. Nevertheless, renal function was similar to the group of patients who did not present AB during follow-up. Their data suggest that there are no differences in renal allograft prognosis between patients who do not develop AB and those who do develop AB and are systematically treated during the first three years after transplantation.12,43 The retrospective character of this study did not allow us to assess the influence of untreated AB in the allograft prognosis. There are some ongoing trials to try to answer this question.68,69
It has traditionally been considered that asymptomatic candiduria should be treated promptly in all KTR due to the high risk of developing serious complications. However, the most recent clinical practice guidelines for the management of candidiasis published by the Infectious Diseases Society of America in 2009 do not recommend treatment unless the patient belongs to a group at high risk of dissemination. High-risk patients include neutropenic patients, infants with low birth weight, and patients who will undergo urologic manipulations. Among patients with a urinary catheter, removal of the catheter may be sufficient to eliminate candiduria without specific antifungal therapy.70
Although some groups have observed that the presence of positive preoperative urine cultures prior to transplantation is a risk factor for early postoperative UTI (31% vs. 6% in non-UTI group; p<0.0571), urine culture screening of asymptomatic patients awaiting transplantation is not routinely recommended.72,73
Live donors should be screened and treated for bacteriuria before the organ is harvested, with documentation of its resolution prior to donation.61,72,73 Potential kidney donors with UTI should be investigated to rule out upper tract involvement.72
What is the best empirical treatment of UTI in SOT recipients?Recommendations- 1.
The treatment strategy depends on the time elapsed since transplantation and the severity of the illness (B-III).
- 2.
The choice of empirical antimicrobial agents should be based on local epidemiological data and the patient's history of previous resistant organisms (A-II).
- 3.
Antibiotic therapies prescribed in the previous months should be taken into account (B-III).
- 4.
Review if the patient has recurrent episodes of UTI. The incidence of resistant organisms can rise progressively with the number of episodes (C-III).
- 5.
Especially if resistant organisms are found, expanded antimicrobial testing should be requested from the microbiology lab to identify treatment options for completion of therapy (B-III).
- 6.
Consider removal or replacement of urinary tract instruments such as urethral catheters or urologic stents (B-III).
- 7.
Progression of upper urinary tract disease to a renal or perinephric abscess or emphysematous pyelonephritis usually requires a multidisciplinary approach to treatment, including urologist and/or interventional radiology consultation for percutaneous or surgical drainage of abscesses (A-I).
- 8.
Once culture susceptibility results are available, switch to the narrowest spectrum antibiotic available to complete course of therapy (B-III).
- 9.
Adjust the antibiotic dosage according to the patient's renal function (A-I).
- 10.
In the event of severe infection with sepsis, consider the option of reducing/discontinuing the immunosuppression therapy (B-III).
Silva et al. reported 37.8% of bacteremia due to UTI in KTR, which becomes a life-threatening condition in this immunocompromised population.74 Therefore, when a UTI is diagnosed, empirical antibiotic treatment has to be initiated. Inappropriate antibiotic therapy is associated with an increase in mortality, and thus initial treatment has to treat the most probable microorganisms causing the infectious episode.75,76 To guide empirical therapy, it is necessary to take into account host clinical characteristics, including infection severity, local epidemiological data and the patient's history of resistant microorganisms and prior antibiotic therapies prescribed.
As regards the epidemiology, the most frequent microorganisms causing UTI are Gram-negative bacilli, mainly E. coli followed by P. aeruginosa and Enterococcus spp.77 In the RESITRA cohort, E. coli produced ESBL in 26% of the episodes. Furthermore, the incidence rate of quinolone resistance was as high as 38% in E. coli and 21% in P. aeruginosa and nearly 80% of Gram-negative bacteria were resistant to cotrimoxazole.6 In another Spanish study, MDR bacteria accounted for 14% of infection episodes in KTR.38 Considering these results, empirical treatment with ciprofloxacin is not a good option, and local epidemiological data are essential in choosing the adequate empirical treatment.
Although most infections due to MDR strains are acquired during hospitalization, most patients undergoing SOT have previous risk factors that predispose to these infections such as chronic underlying diseases that lead to multiple hospital admissions and continuous contact with health care devices (e.g. hemodialysis, outpatient parenteral antimicrobial therapy). These conditions, as well as pre-transplant colonization by MDR bacteria, represent major risk factors for infections caused by MDR bacteria. Interestingly, one study found an increasing incidence of ESBL Enterobacteriaceae (ESBLE) fecal carriage among patients undergoing liver transplantation, which is currently 10.6%. These authors also observed that ESBLE fecal carriage increased the incidence of ESBLE infections, especially in the early post-transplantation period compared to non-carrier patients.78 In addition, ESBL-related UTI was associated with reoperations and previous episodes of UTI.79,80
Moreover, prior antibiotic therapy was also related to MDR infections.78,81 Importantly, renal transplantation, urinary source, and previous use of cephalosporins, carbapenems, and glycopeptides were significantly associated with ESBL-producing E. coli or Klebsiella spp.82
Many ESBL producers from community patients are resistant not only to cephalosporins and penicillins, but also to fluoroquinolones and trimethoprim. Pivmecillinam is an oral antibiotic with excellent clinical efficacy in the treatment of uncomplicated UTIs. It has been used extensively in Nordic countries with few problems; despite this, however, it is not widely used in other countries.83 Pivmecillinam has excellent activity against gram-negative bacteria and there is also emerging in vitro and in vivo evidence of its activity against “ESBL-producing” organisms.84,85
Table 4 shows our recommendations for the empirical treatment of UTI in SOT recipients.
Empirical treatment of urinary tract infections in solid organ transplant recipients.
| Clinical presentation | Absence of risk factors for multidrug-resistant (MDR) organisms | Presence of risk factors for MDR organismsc |
|---|---|---|
| Cystitisa | Fosfomycin or amoxicillin/clavulanate or second/third generation oral cephalosporinsAlternative therapy: TMP/SMX or ciprofloxacin | FosfomycinIf recurrence is suspected, consider potential infection for MDR organismsAlternative therapies: ertapenem, pivmecillinam if available |
| Acute uncomplicated pyelonephritisb | CeftriaxoneAlternative therapy: amoxicillin/clavulanate or aztreonam (if beta-lactam allergy) | Ertapenem or piperacillin-tazobactamAlternative therapies: aztreonam+vancomycin (if beta-lactam allergy) |
| Severe sepsis/septic shock | Meropenem±vancomycin (if risk factors for enterococcal infection)±amikacin (if risk factors for P. aeruginosa)Alternative therapies: aztreonam+vancomycin±amikacin (if risk factors for P. aeruginosa) | Meropenem+vancomycin/linezolid (if risk factors for enterococcal infection)±amikacin (if risk factors for P. aeruginosa)If potential infection by extensively-drug resistant (XDR) P. aeruginosa or carbapenem-resistant Enterobacteriaceae or MDR A. baumannii, consider expert consultationAlternative therapies: aztreonam/colistin+vancomycin/linezolid (if risk factors for enterococcal infection)±amikacin (if risk factors for P. aeruginosa) |
In all cases, once culture susceptibility results are available, complete therapy with the most narrow-spectrum antibiotic available.
- 1.
To choose an appropriate antibiotic for the treatment of cystitis caused by Enterobacteriaceae, the recommendations for the general population are adapted to organ transplant patients. For hospitalized patients, we recommend using cotrimoxazole or second- or third-generation oral cephalosporin or amoxicillin-clavulanate or fosfomycin trometamol for susceptible strains (B-I). For outpatients, we recommend ciprofloxacin or fosfomycin trometamol (B-I). For the treatment of cystitis caused by ESBL-producing E. coli, we recommend fosfomycin trometamol (B-I). For cystitis caused by carbapenem-resistant Enterobacteriaceae, we recommend using either fosfomycin trometamol or aminoglycosides (gentamicin or amikacin) (B-II). Contrary to most recommendations for the general population, nitrofurantoin is not recommended as a first-line treatment of cystitis due to the potential occurrence of adverse effects in patients with SOT (D-III).
- 2.
For the treatment of hospitalized patients with acute pyelonephritis caused by Enterobacteriaceae, we recommend the use of a beta-lactam, either third-generation cephalosporins or amoxicillin-clavulanate (B-I). After discharge or in outpatients, we recommend the use of fluoroquinolones (B-I). For pyelonephritis caused by ESBL-producing Enterobacteriaceae, we recommend ertapenem (B-I). Monotherapy with a carbapenem is not recommended for patients with invasive infections caused by carbapenemase-producing Enterobacteriaceae but may be considered in cases of mild invasive infections if adequate source control is readily achieved and the isolate is susceptible (C-III). For patients in which combination therapy is indicated, a regimen with a carbapenem plus one or two fully active drugs (including colistin, an aminoglycoside, or fosfomycin) is recommended if the carbapenem minimum inhibitory concentration (MIC) is ≤ 8mg/L; this applies mainly to patients with severe infections caused by KPC-producing K. pneumoniae (B-II). There are not enough data to recommend including a carbapenem in combination regimens if MIC is>8mg/L. If this is the case, carbapenems are probably useless. Particularly if MIC is>16mg/L, we recommend including at least two fully active drugs in the combination regimen according to susceptibility testing results (drugs to be considered: colistin, aminoglycosides, and fosfomycin) (C-III).
- 3.
For the treatment of cystitis caused by P. aeruginosa, we recommend ciprofloxacin for susceptible strains (B-III). For pyelonephritis by P. aeruginosa we recommend the use, when possible, of beta-lactams active against P. aeruginosa in hospitalized patients and quinolones in outpatients (B-III). For the treatment of pyelonephritis by multidrug-resistant P. aeruginosa, we recommend colistin or amikacin with monitoring of renal function when no other options are available (C-III).
- 4.
For ampicillin-susceptible enterococci strains, we recommend oral amoxicillin for the treatment of cystitis (B-III) and intravenous ampicillin for the treatment of pyelonephritis (C-III). For ampicillin-resistant E. faecium, we recommend glycopeptides (C-III). For vancomycin-resistant Enterococcus strains, the treatment should be guided by antibiogram and we recommend the use of quinolones, cotrimoxazole, fosfomycin, nitrofurantoin, and linezolid in order of preference (B-III).
- 5.
For the treatment of infected cysts in patients with renal polycystic disease, we recommend the use of fluoroquinolones or TMP/SMX when possible and percutaneous drainage if necessary (B-III).
- 6.
For the treatment of acute prostatitis we recommend intravenous beta-lactams until apyrexia and consolidation treatment with fluoroquinolones or TMP/SMX when possible (B-I).
To choose the best antimicrobial for treating cystitis in SOT patients we will follow the recommendations for the general population. Several randomized clinical trials have been performed to evaluate the safety and efficacy of beta-lactams, quinolones, TMP/SMX (cotrimoxazole), fosfomycin trometamol and nitrofurantoin for the treatment of cystitis in the general population. Cotrimoxazole has a treatment efficacy of 93% for the treatment of cystitis in the general population.86 The clinical efficacy of fosfomycin trometamol is 91%.87–89 The clinical efficacy of beta-lactams is slightly lower than that obtained with other antimicrobials (89%)90–92 and usually has more side effects than comparators.93 Quinolones achieve 90% of clinical efficacy after 3 days of treatment.94–97
Quinolones are most effective for treating UTIs in susceptible microorganisms but their use may promote the development of multidrug resistance. As the restricted use of quinolones has been associated with a decrease in bacterial resistance, especially for ESBL-producing Enterobacteriaceae98 and P. aeruginosa,99 we recommend restricting the use of quinolones as a treatment option for cystitis in outpatients.
Most recent guidelines and reviews recommend nitrofurantoin (at a dose of 100mg/8h) as the treatment of choice for uncomplicated UTI in the general population93,100 on the basis of several clinical trials that have shown nitrofurantoin to have a treatment efficacy of 93%.86,101–103 However, nitrofurantoin serum levels can increase, leading to toxicity, and decrease urinary concentrations, leading to treatment failure in patients with a low glomerular filtration rate (<60ml/min). Recently, the nitrofurantoin toxicity threshold has been established at glomerular filtration rates of 40mL/min due to the lack of published evidence.104 In addition, some clinical cases of pulmonary hemorrhage attributed to the use of nitrofurantoin have been published in renal transplant patients.105 As many SOT recipients have low glomerular filtration rates due to the use of calcineurin inhibitors, we do not recommend nitrofurantoin as the first-line treatment for cystitis in organ transplant recipients.
For the treatment of pyelonephritis, we will adapt the recommendations from the data in the general population as very few articles have focused on the treatment of pyelonephritis in organ transplant patients. Fluoroquinolones are the antibiotics with the best clinical efficacy in randomized clinical trials, achieving 96% to 78% efficacy when given as empirical therapy.106–108 It must be noted that the efficacy of empirical treatment with fluoroquinolones for acute pyelonephritis has decreased in recent years. Empirical TMP/SMX achieved an overall clinical efficacy of 83%, which reached 92% if the isolated pathogen was susceptible.106
It has been suggested that the occurrence of acute graft pyelonephritis can impair graft function in the long term, but the results published in the literature are controversial.3,28,29,43 To choose an appropriate antibiotic for the treatment of acute pyelonephritis in renal transplant patients, there is no evidence supporting the distinction between acute graft and own kidney pyelonephritis.
For the treatment of acute pyelonephritis in hospitalized patients, we recommend the use of beta-lactams (ceftriaxone or amoxicillin-clavulanate) as there is no experience of TMP/SMX for the treatment of pyelonephritis in organ transplant patients. In addition, as many patients have received previous prophylaxis with this drug, the prevalence of resistance to TMP/SMX in uropathogens could be higher than in the general population. In outpatients or to consolidate treatment after hospital admission, we recommend the use of ciprofloxacin.
ESBL-producing strains of E. coli and Klebsiella spp. are often resistant to quinolones and cotrimoxazole.109,110 For the treatment of cystitis caused by ESBL-producing E. coli, amoxicillin-clavulanate and fosfomycin had a clinical efficacy of 84% and 93%, respectively, when the isolate showed susceptibility to these drugs.111 No available data supports the use of amoxicillin-clavulanate for the treatment of ESBL-producing Klebsiella spp. Carbapenems are active against ESBL-producing Enterobacteriaceae. The use of ertapenem may decrease the use of imipenem and ciprofloxacin and therefore improve the susceptibility of P. aeruginosa.112 In recent years, an increase in the incidence of ertapenem-resistant K. pneumoniae strains due to a deficiency in porins has been detected,113,114 and this may have relevant clinical consequences.115 Although prolonged in vitro exposure to ertapenem has been associated with porin-deficient subpopulations of E. coli,116 this has not yet been associated with clinical consequences.117 We recommend fosfomycin trometamol for the treatment of cystitis caused by ESBL-producing E. coli and ertapenem for pyelonephritis caused by ESBL-producing Enterobacteriaceae.
Carbapenemase-producing Enterobacteriaceae (CPE) are emerging global pathogens. The spread of CPE to transplant recipients has ominous implications.118–120 Centers from CPE-endemic areas report a 3–10% incidence of carbapenem-resistant K. pneumoniae infection in SOT recipients with similar rates after liver, kidney, lung, and heart transplantation.118,119,121–123 Monotherapy with a carbapenem is not recommended for patients with invasive infections caused by CPE but may be considered in cases of mild invasive infections if adequate source control is readily achieved and the isolate is susceptible. It has been suggested that combined antibiotic therapy may be the best alternative for the treatment of critically ill patients with CPE.120,124,125 Daikos et al. found a lower mortality in carbapenem-containing combinations than in carbapenem-sparing combinations (19.3% vs. 30.6%) and also found that cases with a carbapenem MIC≤8mg/L had a lower mortality than those with a MIC>8mg/L (19.3% vs. 35.5%).126 In line with other guidelines125 for patients in which combination therapy is indicated, we recommend a regimen with a carbapenem plus one or two fully active drugs (including colistin, an aminoglycoside, or fosfomycin; tygecycline achieves low bloodstream and urinary tract concentrations and is therefore inadequate for bacteriemias and UTIs) if the carbapenem MIC is ≤8mg/L. This applies mainly to patients with severe infections caused by K. pneumoniae carbapenemase (KPC)-producing K. pneumoniae. There is not enough data about including a carbapenem in combination regimens if MIC is >8mg/L. If this is the case, carbapenems are probably useless, particularly if MIC is >16mg/L. We therefore recommend including at least two fully active drugs in the combination regimen according to susceptibility testing results (drugs to be considered: colistin, aminoglycosides and fosfomycin).
However, in patients with cystitis, monotherapy should be enough to resolve the infection. We recommend using either fosfomycin or aminoglycosides (gentamicin or amikacin) for the treatment of cystitis by CPE.
For the treatment of cystitis caused by P. aeruginosa, we recommend ciprofloxacin for susceptible strains. For pyelonephritis by P. aeruginosa, we recommend the use, when possible, of beta-lactams active against P. aeruginosa in hospitalized patients and quinolones in outpatients. For quinolone-resistant strains, we recommend using ceftazidime, cefepime, piperacillin-tazobactam, aztreonam, and imipenem/meropenem in order of preference. Aminoglycoside use may be problematic following transplantation where renal failure and/or the co-administration of other nephrotoxic agents are common. The use of colistin in SOT patients has been associated with an approximately 33% incidence of renal toxicity, which increases depending on the length of treatment.127 Thus, for the treatment of multidrug-resistant P. aeruginosa, we recommend using either amikacin or colistin when no other options are available.128
A large percentage of E. faecalis remains susceptible to ampicillin. However, most E. faecium strains are resistant to ampicillin due to an increased expression or mutations of PBP5. E. faecalis is rarely resistant to ampicillin due to the production of beta-lactamases. For enterococcal UTIs caused by ampicillin-susceptible strains, we recommend the use of oral amoxicillin for the treatment of cystitis and intravenous ampicillin for the treatment of pyelonephritis. For ampicillin-resistant E. faecium, we recommend glycopeptides. For the treatment of enterococcal UTIs by vancomycin-resistant strains, the treatment should be guided by antibiogram and we recommend the use of quinolones, cotrimoxazole, fosfomycin, nitrofurantoin, and linezolid in order of preference.129 The occurrence of daptomycin resistance during the treatment of vancomycin-resistant E. faecium infection has recently been described.130 For this reason, we recommend using alternative drugs in this setting.
Own kidney cyst infections can represent a complication in patients with renal transplantation due to polycystic renal disease. E. coli causes around 75% of the episodes and around 70% of the cases can be cured with antibiotics, although some cases require percutaneous drainage (especially larger infected cysts).131 There is scarce information about the concentration of systemically administered antibiotics in infected cysts. Fluoroquinolones, TMP/SMX, metronidazole, and clindamycin have good penetration while beta-lactams and aminoglycosides have poor penetration in renal cysts after systemic administration.132–137 Most patients with cyst infection must be admitted to hospital. In patients with polycystic renal disease and cyst infection, we recommend using ciprofloxacin or cotrimoxazole for the treatment of cysts infected by susceptible strains.
Most antibiotics have poor penetration in prostate tissue and fluids for several reasons, the most important one being the presence of nonporous capillaries in the prostate.138 However, in the acute phase of bacterial prostatitis, most antibiotics achieve optimal concentrations in the prostate parenchyma.139 As most SOT patients with acute bacterial prostatitis will require parenteral antibiotic treatment during the first hours, we recommend the use of either intravenous second- or third-generation cephalosporins or intravenous amoxicillin-clavulanate for the treatment of susceptible strains. The preferred consolidation treatment for bacterial prostatitis should be carried out with either fluoroquinolones or TMP/SMX. For the treatment of prostatitis caused by enterococci, we recommend using parenteral ampicillin in the acute phase and fluoroquinolones to complete the treatment. For the treatment of prostatitis caused by P. aeruginosa, we recommend using an intravenous beta-lactam active against P. aeruginosa in the acute phase and switching to ciprofloxacin to consolidate the treatment when possible.
How long should SOT recipients receive antibiotics for a UTI?Recommendations- 1.
Kidney recipients presenting AB within the first month of transplantation should receive an oral antibiotic selected according to the susceptibility of the isolated microorganism for a period of 5–7 days (BIII). In other SOT recipients, guidelines for the general population should be applied (AII).
- 2.
Cystitis in SOT recipients should be treated for 5–7 days with an oral antibiotic. Early post-transplant cystitis in renal transplant recipients may require longer treatment, especially if a ureteral stent is present (BIII). Short courses of therapy (single dose or three days) have not been studied in SOT recipients (CIII).
- 3.
KTR with allograft pyelonephritis should undergo a 14-day course of antibiotics. However, patients with allograft pyelonephritis in the early post-transplant period presenting with sepsis should be treated for at least 14–21 days (BIII). Late uncomplicated allograft pyelonephritis occurring more than six months after kidney transplantation may be treated with antibiotic therapy for 10–14 days (BIII). At least initially, intravenous antibiotic therapy is recommended in kidney recipients with allograft pyelonephritis (AIII).
- 4.
In non-kidney SOT recipients with uncomplicated pyelonephritis, a 10- to 14-day course of antibiotics is recommended (BIII). At least initially, these patients should be treated with intravenous antibiotics (AIII).
- 5.
No data are available on short courses (7 days) of antibiotic therapy for pyelonephritis in SOT recipients. Therefore, short-term treatment is not recommended in SOT (CIII).
- 6.
For SOT recipients with complicated pyelonephritis, an antibiotic course of at least two weeks is recommended and should be extended until abscesses are adequately drained and patient improvement has been achieved (BIII).
- 7.
For SOT recipients with acute bacterial prostatitis, a 2- to 4-week course of antibiotics is recommended. However, antibiotic therapy can be continued for up to four weeks in patients with severe illness, concomitant bacteremia, and undrained abscesses (BIII).
- 8.
In SOT recipients with polycystic kidney disease and infected cysts, treatment of not less than 14 days is recommended and may be extended depending on patient evolution, cyst diameter, and possibility of drainage (BIII).
No simple recommendations can be made concerning the length of antibiotic therapy for UTI in SOT recipients (both renal transplant patients and others).
Most UTIs in kidney recipients occur within the first year of transplantation, mainly within the first few months. In this early post-operative period, infection often presents as pyelonephritis and is associated with rapidly developing bacteremia, sepsis, and relapse.140 As a result, prolonged administration of antimicrobial therapy has classically been recommended for the treatment of early UTIs in kidney recipients. In recent series on renal transplantation, however, UTIs were associated with considerably lower morbidity and mortality rates, probably due to improvements in patients’ care.14,23 Accordingly, a 14-day antibiotic course may be adequate in early UTI after kidney transplantation.
The incidence of UTI decreases after the first year following kidney transplantation. In addition, late UTIs after renal transplantation have been reported to be relatively benign.141 Consequently, UTIs occurring 12 months after kidney transplantation can be safely managed with a conventional therapeutic course.14,23,58
There are no specific recommendations regarding the length of antibiotic therapy in post-transplant UTIs in non-renal transplant recipients.
Furthermore, the length of antibiotic treatment administered may differ according to the type of UTI (asymptomatic bacteriuria, cystitis, pyelonephritis, complicated UTI, prostatitis, relapsing UTI, and cystic infection).
Asymptomatic bacteriuriaIn contrast to the general population, there is no consensus on whether AB should be screened and treated in the transplant patient and, if so, at what time points post-transplant.12,41,56,62 Some authors recommend treatment of AB in the first month after kidney transplantation.60,61 In this case, AB can be treated with an oral antibiotic for five to seven days that is selected according to the susceptibility of the isolated microorganism.
In other cases, the current guidelines for the general population9 should be applied in SOT recipients. For instance, AB should be treated in pregnant women and before transurethral resection of the prostate or other urologic procedures in which mucosal bleeding is anticipated. The duration of antimicrobial therapy in pregnant women with AB should be three to seven days. Prior to urologic surgery in which mucosa bleeding is anticipated, antibiotic therapy should be initiated shortly beforehand. Antimicrobial treatment should not be continued after surgery unless an indwelling catheter remains in place.
CystitisCystitis in SOT should usually be treated empirically with a single oral antibiotic for 5–7 days.14
Short courses of therapy (single dose or three days) accepted as appropriate choices in the general population93 have not been studied in SOT recipients.
Acute pyelonephritisIn KTR, the morbidity and mortality associated with early allograft pyelonephritis in the 1980s led to the recommendation that this infection be treated with a 6-week course of antimicrobials.140 More recently, this condition has been associated with lower morbidity and mortality, and shorter antibiotic courses may be appropriate.14,23,56
Although no conclusive evidence is available on the optimal duration of therapy for uncomplicated kidney allograft pyelonephritis, a 14-day antibiotic course should be adequate.14,56 Nevertheless, patients with allograft pyelonephritis presenting with sepsis should be treated for at least 14–21 days, especially in the early post-transplant period.57 In contrast, considering that UTIs occurring 6–12 months following kidney transplantation are usually more benign, it has been suggested that late infections can be treated with a standard 10- to 14-day antibiotic therapy.
The incidence of infections by MDR bacteria, mainly gram-negative bacilli, is increasing in SOT recipients.4,43,142 It has not been established whether SOT patients with pyelonephritis caused by MDR bacteria should receive prolonged antibiotic therapy.
Salmonella spp. urinary infection occurring in renal transplant recipients is usually associated with bacteremia and distant foci of infection. This infection is difficult to eradicate, and prolonged therapy of up to six weeks has been recommended.143,144
KTR with allograft pyelonephritis should be hospitalized and treated with intravenous antibiotics, at least initially, because of the potential for serious complications.56 Patients can be switched to an oral agent when they present clinical improvement, providing they can tolerate it and the organism is susceptible.
The duration of therapy for uncomplicated acute pyelonephritis in non-KTR has not been specifically studied. Although no conclusive evidence is available on the optimal duration of therapy for pyelonephritis in SOT, a 10- to 14-day antibiotic course can be recommended.14 In these patients, intravenous antibiotic therapy should be considered depending on the severity of pyelonephritis and the patient's immunosuppression situation and should be switched to an oral agent when they present clinical improvement, providing they can tolerate it and the organism is susceptible. As in the general population, it has not been demonstrated that intravenous antibiotic therapy is superior to oral administration with regard to length of hospitalization or cost effectiveness.
Short courses of antibiotic therapy, mainly fluoroquinolones, have been successfully and safely applied in the treatment of uncomplicated acute pyelonephritis in the general population.93,145 However, local drug resistance patterns should be considered in choosing antibiotics as the incidence of fluoroquinolone resistance in Gram-negative bacilli is high in many countries. There are no data to support the use of short-term antibiotics for the treatment of pyelonephritis in transplant patients.
In SOT recipients who present persistent symptoms despite appropriate therapy, genitourinary tract imaging is mandatory to evaluate complicated pyelonephritis.
Complicated pyelonephritisUpper UTI may progress into a nephritic or perinephritic abscess or emphysematous pyelonephritis in SOT recipients. Complicated pyelonephritis usually requires a multidisciplinary approach with percutaneous or surgical drainage of abscesses. Duration of treatment in complicated pyelonephritis should be at least two weeks and should be extended until abscesses are adequately drained and patient improvement has been achieved.14,23,56–58
Acute bacterial prostatitisNo specific recommendations are available regarding the duration of antibiotic treatment in SOT recipients with acute bacterial prostatitis. Recommendations for the general population139 may be adequate. For SOT recipients with prostatitis, as in the general population, a 2-week antibiotic course is recommended. However, antibiotic therapy can be continued for up to 4 weeks in SOT recipients with severe illness or in patients with concomitant bacteremia.
If symptoms persist despite several days of appropriate antibiotic therapy, the diagnosis of a prostatic abscess must be considered. The presence of prostatic abscess requires abscess drainage, if possible, and a prolonged antibiotic course (6 weeks).
Cystic infection in autosomal dominant polycystic kidney diseaseCystic infection represents a serious complication in patients with autosomal dominant polycystic kidney disease (ADPKD). The incidence of cystic infection has been estimated at 0.01 episodes/patients/year. In KTR with ADPKD, the incidence of cystic infection does not appear to increase.146
Prolonged courses of antibiotic therapy should be administered in SOT recipients with infected cysts. At least 14 days of treatment is recommended although this period may be extended depending on patient evolution, cyst diameter, and the possibility of drainage. In a large series of patients with ADPKD and cystic infection, antibiotic treatment was introduced for a mean duration of 5 weeks (range 2–12 weeks). In large (diameter>5cm) infected cysts, mainly hepatic cysts, the combination of early percutaneous drainage and antimicrobial therapy proved to be more efficient than antibiotics alone. Fluoroquinolones have an increased diffusion in infected cysts; in contrast, beta-lactams usually present poor penetration. Consequently, fluoroquinolones are preferred for treatment of infected cysts if the causative microorganism is susceptible.146
Nevertheless, nephrectomy or partial hepatectomy may be required for persistent or recurrent cystic infections; primarily in candidates awaiting kidney transplantation.147
What should be the management of UTI caused by Candida spp. in SOT recipients?The information on Candida spp. UTI in SOT is very scarce and mostly includes KTR. No clinical trials on the management of Candida UTI in SOT patients have been performed, so recommendations derive from descriptive studies or clinical experience in other populations (level III).
What should be the initial diagnostic approach to a SOT recipient with candiduria?Recommendations- 1.
SOT recipients with candiduria should be classified according to the presence of risk factors for disseminated candidiasis, indications for obtaining a urine culture (surveillance or infection suspicion), and according to their clinical situation (asymptomatic, with urinary tract symptoms or with general manifestations of sepsis) (A-III).
- 2.
Predisposing risk factors should be eliminated or controlled (antibiotic use, malnutrition, hyperglycemia) and urinary catheters should be removed or at least changed if possible. The presence of candiduria should be verified with a second, clean-voided urine culture (A-II).
- 3.
Disseminated candidiasis should be considered in all hospitalized SOT with candiduria. If clinical manifestations are compatible, blood cultures, a second urine culture after removal or replacement of the urinary catheter, fundoscopy, cultures from any other significant site (vascular accesses, peritoneal fluid, etc.), and a kidney imaging study should be obtained (AII).
- 4.
Patients with persistent candiduria and no indwelling bladder catheter should undergo imaging of the kidneys and collecting system to exclude renal abscess, fungus balls, or other urologic abnormalities (A-II).
- 5.
SOT recipients in whom Candida contamination of the preservation fluid is demonstrated or suspected (donors with ruptured abdominal viscus at the time of multiorgan recovery) should undergo urgent diagnostic evaluation including Doppler ultrasound, blood and urine cultures, and cultures from any other significant site (B-III).
Candida spp. may produce local or metastatic infections of the urinary tract and candiduria may thus reflect very different clinical situations, ranging from colonization of an indwelling bladder catheter to fulminant disseminated candidiasis. Ascending infection predominates in patients with urinary catheters. The infection is usually limited to the bladder, but may progress to the ureter and renal pelvis and rarely produce fungal balls that can obstruct the urinary tract. Candiduria may be the first clue of disseminated hematogenous infection in a septic patient, since the kidneys are frequently involved in systemic candidiasis. Blood cultures may be negative in half of patients with disseminated candidiasis. A search for surrogate markers, such as Candida albicans Germ Tube Antibody test (CAGTA), Platelia-Candida, B-d Glucan, or PCR, is under investigation.
Clinical history should include type of transplantation (kidney vs. other types), time after transplantation (early vs. late candiduria), and presence of indwelling bladder catheter or other prosthetic material in the urinary tract (pig-tail catheters, etc.). Functional or structural abnormalities in the urinary tract, place of acquisition of the candiduria, indication of the urine culture, and detection of local or systemic clinical manifestations should also be recorded. Clinical manifestations of Candida UTI are indistinguishable from those of bacterial infections. In a recent series, 83 episodes were described in 34 KTR. Predisposing factors included urinary catheter in 41% and antimicrobial use in 60% of the episodes. Fever was detected in 8% of the episodes (usually caused by a concomitant bacterial infection).148 Physical examination and analytical evaluation of renal function are also needed.
The presence of candiduria should be verified with a second, clean-voided urine culture or after removal or replacement of urinary catheters. Gram stain of the urine may reveal gram positive, budding yeasts. Culture is usually positive in 24h, although some Candida glabrata strains may take longer to grow. Second cultures will be negative in half of the cases.149–151 Colony counting is not useful to separate colonization from infection.152 Finding casts in urine may be suggestive of renal tissue invasion.
Invasive candidiasis is now uncommon in most SOT recipients142,153 although it remains a problem in pancreas and intestine recipients. However, candiduria should be initially considered as a potential marker of disseminated candidiasis in unstable patients.149,154 Blood cultures, a second urine culture after removal or replacement of the urinary catheter, fundoscopy, and cultures from any other significant site (vascular accesses, peritoneal fluid, etc.) should be obtained. If confirmed, these patients should be managed according to standard guidelines and will not be dealt with in depth in this consensus.70,149,155,156
Imaging of the kidneys and collecting system is recommended in all SOT with persistent candiduria, even if asymptomatic, to exclude renal abscess, fungus balls, or other urologic abnormalities. Emphysematous pyelonephritis and pneumaturia have been described. Fungus balls consist of large mycelial clumps that may obstruct the renal pelvis, ureters, and bladder. They occur mostly in neonates and diabetics, but have also been described in KTR.157–162 Oliguria, strangiuria (difficult and painful urination), the passage of particulate matter, and/or pneumaturia suggest the presence of a fungus ball.70,152
Finally, contamination of the preservation fluid with Candida is described in ∼4% of organ procurement procedures and has resulted in a high risk of severe complications in KTR.163 In the recipients, symptoms appeared at a median of 27 days after transplantation (range, 3–154 days). Although the most severe complication is Candida arteritis, infected urinomas, fungus ball, peri-renal hematoma, and abscesses have also been documented. Candidemia early after kidney transplantation obliges excluding renal aneurysm (2/18), but renal arteritis cannot be ruled out in the absence of positive blood cultures. These patients should undergo Doppler ultrasound on day 0 and 7 (consider CT or MRI if negative and high clinical suspicion), cultures from blood, urine, drainage fluids, and from other clinically relevant sites, and receive early antifungal therapy.164Candida species isolates should be stored for genetic analysis and the infection reported to the coordination team.
Donor candiduria does not contraindicate acceptance of the organ, but recipients should be managed as if the preservation fluid were contaminated.164 The American Transplantation Society (ATS) does not recommend accepting donors with untreated candidemia, but treated patients with documented mycological response are very unlikely to transmit the infection.
Which patients should receive antifungal drugs?Recommendations- 1.
Asymptomatic candiduria in SOT patients that are not neutropenic or undergoing a urologic procedure should not be treated with antifungal therapy (D-II).
- 2.
Candiduria in an unstable SOT should be initially considered as a potential marker of disseminated candidiasis. Prompt effective antifungal therapy has to be provided until an alternative diagnosis is obtained (A-III).
- 3.
Candida cystitis or pyelonephritis should be treated with systemic antifungals for 2–4 weeks (B-III).
- 4.
Fungus balls or casts in the pelvis or urinary bladder need surgery and systemic and/or local antifungal therapy (A-III).
- 5.
KTR with contamination of the preservation fluid or with a donor with digestive tract rupture should receive early effective antifungal therapy (B-II).
Asymptomatic patients with no risk factors for disseminated candidiasis should not receive antifungal therapy21,148 unless the patient is undergoing a urologic procedure or is neutropenic.70 Concerns about potential allograft damage due to asymptomatic candiduria in KTR have not been confirmed.23 In the non-transplant population, funguria resolved spontaneously in 76% of patients and administration of fluconazole had no long-term clinical impact.151 In a recent series, only 43% of KTR with candiduria were treated with antifungals; recurrence was observed in 48% and was more common in treated patients (55% vs. 39%). All patients that required admission also had a bacterial UTI.148
Patients with risk factors for candiduria and disseminated candidiasis should be considered for antifungal preemptive therapy or prophylaxis if asymptomatic.149,154 An example could be patients early after transplantation still in the ICU with indwelling bladder catheter and drainages. In this situation, the criteria for providing antifungal therapy to kidney, heart, and liver transplant recipients include re-intervention, renal failure requiring dialysis, CMV disease, and in the case of liver transplantation, prolonged complicated transplant procedure with high transfusion needs, fulminant liver failure, choledochojejunostomy, and peri-transplant colonization. The American Transplantation Society (ATS) recommends that preservation fluid cultures be taken into consideration when establishing the indication for prophylaxis164; however, this information is not always available. These high-risk, colonized patients should receive antifungal prophylaxis with drugs active against Candida spp. and Aspergillus.165 Antifungal prophylaxis is usually provided to all pancreatic, intestinal, and lung transplant recipients.155
Patients with Candida cystitis or pyelonephritis must be treated with systemic antifungals for 2–4 weeks. Exceptionally, bladder instillation of amphotericin B (AMB) has been used to treat cystitis.149,166
Patients with Candida fungus balls must receive therapy with antifungal agents that may sometimes result in spontaneous disruption and passage of the mass of hyphal filaments and debris.167,168 Antifungal treatment should be continued until urine culture becomes negative, and renal ultrasonography shows the disappearance of fungus balls and recovery of normal renal parenchymal echogenicity.168 However, an invasive procedure is usually needed to relieve obstruction and remove the bulk of the mass. If the required urologic procedure (e.g., percutaneous nephrostomy) provides access to the renal pelvis, urethers, or bladder, local irrigation with intermittent or continuous AMB or fluconazole can be considered.158,167,169 Other methods to facilitate the breakdown and passage of fungus balls have included intermittent irrigation with saline, insertion of thrombectomy devices through a percutaneous nephrostomy, percutaneous endoscopic disruption and drainage, antegrade guidewire fragmentation, and percutaneous irrigation with streptokinase. Urology consultation is promptly recommended.
The outcome of KTR with donor-transmitted Candida infection or contamination of the preservation fluid is frequently complicated. In a recent series, 3/18 patients died and 9 lost the allograft.163 Systemic antifungal therapy should be provided early and if a renal aneurysm is identified, embolization or open surgery with extra-anatomic reconstruction, with or without nephrectomy, is usually needed.
Which drug should be prescribed and for how long?Recommendations- 1.
Fluconazole is the agent of choice for most patients with Candida UTI due to the high concentration achieved in urine (>100μg/ml, which is 10-fold the simultaneous plasma level) (A-II).
- 2.
Other antifungal agents should only be considered for patients in unstable clinical condition, allergic to fluconazole, or in whom therapy has clearly failed despite maximum fluconazole doses and optimal management of urologic abnormalities or other predisposing conditions (B-III).
- 3.
A single dose of parenteral AMB deoxycholate, with or without oral 5-flucytosine, reach high concentrations in urine, and may be used to treat Candida cystitis in patients not responding to or not treatable with fluconazole. Candida pyelonephritis can also be treated with AMB. However, potential kidney toxicity limits its use in the transplant population (B-I).
- 4.
Liposomal AMB, with or without 5-flucytosine, may be used to treat Candida pyelonephritis in patients not responding to or not treatable with fluconazole. However, due to the low concentration reached in urine, a relapse may occur if the collecting system is infected (C-III).
- 5.
AMB deoxycholate bladder irrigation may be used in patients with symptomatic cystitis that cannot be treated with other drugs (C-II).
- 6.
Echinocandins are the preferred initial agents for systemic candidiasis in unstable patients, in patients who have been exposed to azoles in the previous 3 months, and in patients with renal insufficiency requiring external replacement therapy (A-I).
- 7.
Echinocandins achieve low concentrations in the urinary tract but may be used in patients not responding to or not treatable with fluconazole. If the collecting system is infected, relapse may occur (C-III).
- 8.
All symptomatic UTIs due to Candida species in KTR should be considered complicated and treated for at least 14 days (B-II).
All UTIs in patients with abnormal urine flow due to functional or structural abnormalities should be classified as complicated; accordingly all UTIs in KTR are by definition complicated and should be treated for at least 7–14 days.14 Single-dose therapy has not been studied in symptomatic SOT recipients with candiduria and is currently not recommended.
Fluconazole is the agent of choice for Candida UTI due to its safety profile and to the high concentration achieved in urine (>100μg/ml, which is 10-fold the simultaneous plasma level). Fluconazole is highly water soluble and is excreted as an active drug into the urine. Expected concentrations in urine exceed the MIC of most Candida species, including dose-dependent and even resistant (MIC>64μg/ml) isolates.149 Renal parenchymal levels of fluconazole are threefold higher than plasmatic ones. In the presence of renal insufficiency, the dose should be adjusted for treating systemic infections, but this is not so clear in patients with symptomatic candiduria. Fisher recommends administering at least 400mg/d regardless of the renal function,149 attending to the necessity of maintaining high levels in urine and the tolerability of high-dose fluconazole in other infections such as cryptococcosis. Under these circumstances, drug interactions and hepatic function should be monitored. The drug is almost completely cleared by peritoneal dialysis and by external renal replacement therapies.
Other azoles are not useful for treating Candida cystitis since they are minimally excreted in urine as active agents (itraconazole and posaconazole<1%, voriconazole<5%).149
5-Flucytosine (5-FC) also reaches high concentrations in urine (>30μg/ml) and most C. glabrata isolates and 75% of C. albicans are susceptible. However, 5-FC may cause bone marrow toxicity and should not be given alone due to the emergence of resistance. A dose of 25mg/kg every 6h is recommended.
AMB deoxycholate achieves significantly higher urine concentration than lipidic forms of AMB (L-AMB) or candins. Some small studies showed that a single intravenous dose of AMB eradicated 72% of candidurias, suggesting a delayed urinary excretion of AMB. This approach could eventually be used in Candida cystitis or other forms of refractory Candida UTIs.149,170,171 However, AMB deoxycholate is not available in some centers and the risk of kidney toxicity is high in the SOT population. The lipid formulations of AMB achieve a much lower tissue penetration in the renal parenchyma and are not recommended for treating renal candidiasis.149 However, some successful cases, occasionally with simultaneous local instillation of AMB deoxycholate or flucytosine, have been reported in non-SOT populations.172–174 L-AMB levels were measured in a renal cyst infected with Candida krusei and proved to be lower than the microorganism MIC.175
Echinocandins achieve low concentrations in the urinary tract. Therefore, eradication of Candida in the cortex and interstitium of the kidney is more likely than in the collecting system. Very scarce positive158,176 and negative172 responses have been reported.
Bladder irrigation with AMB deoxycholate or L-AMB has been used in patients with symptomatic Candida cystitis that could not be treated with oral or IV drugs.149,166,177 Usually 50mg of AMB is diluted in 1L of sterile water. Continuous irrigation of the bladder for 5–7 days resolves>90% of Candida cystitis, although relapses are common.178 However, no SOT recipients were included in these studies and this approach is rarely needed nowadays. Bladder irrigation should only be considered in patients requiring urinary catheter for other reasons that are not treatable or are refractory to other strategies.
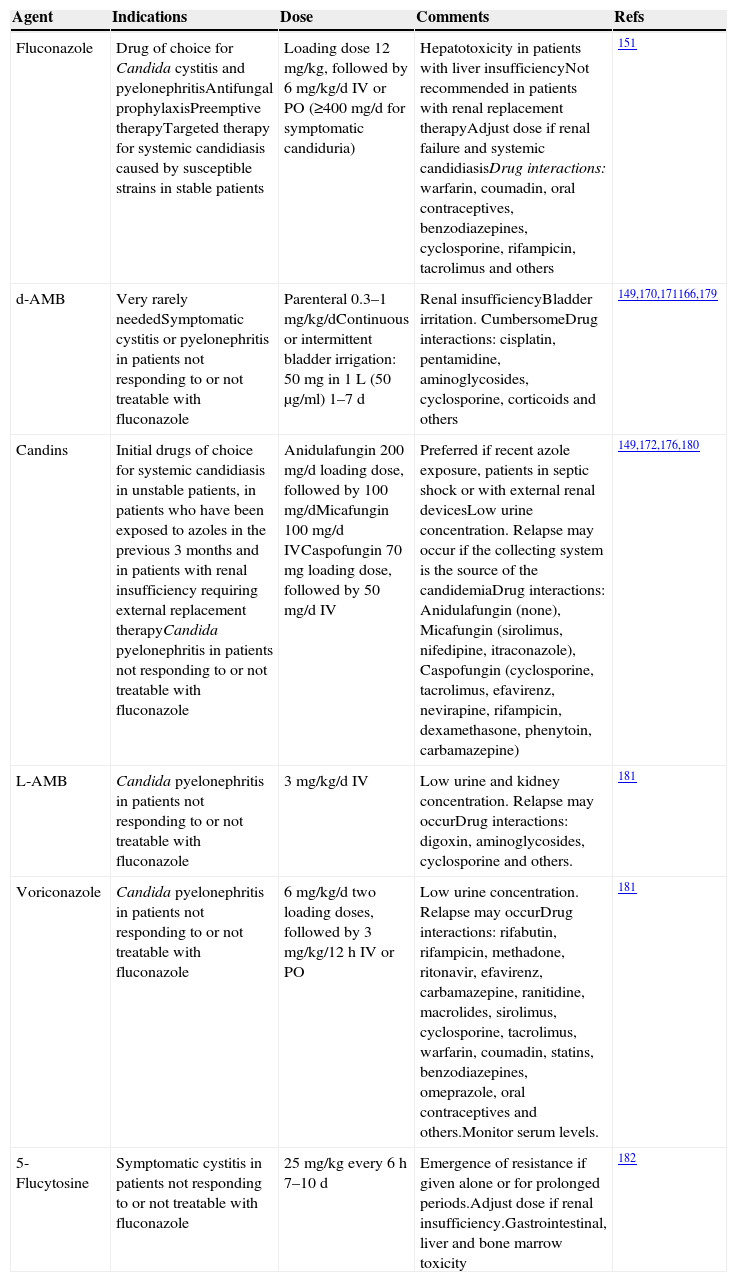
Information about antifungal drugs is completed in Table 5.
Antifungal drugs.
| Agent | Indications | Dose | Comments | Refs |
|---|---|---|---|---|
| Fluconazole | Drug of choice for Candida cystitis and pyelonephritisAntifungal prophylaxisPreemptive therapyTargeted therapy for systemic candidiasis caused by susceptible strains in stable patients | Loading dose 12mg/kg, followed by 6mg/kg/d IV or PO (≥400mg/d for symptomatic candiduria) | Hepatotoxicity in patients with liver insufficiencyNot recommended in patients with renal replacement therapyAdjust dose if renal failure and systemic candidiasisDrug interactions: warfarin, coumadin, oral contraceptives, benzodiazepines, cyclosporine, rifampicin, tacrolimus and others | 151 |
| d-AMB | Very rarely neededSymptomatic cystitis or pyelonephritis in patients not responding to or not treatable with fluconazole | Parenteral 0.3–1mg/kg/dContinuous or intermittent bladder irrigation: 50mg in 1L (50μg/ml) 1–7d | Renal insufficiencyBladder irritation. CumbersomeDrug interactions: cisplatin, pentamidine, aminoglycosides, cyclosporine, corticoids and others | 149,170,171166,179 |
| Candins | Initial drugs of choice for systemic candidiasis in unstable patients, in patients who have been exposed to azoles in the previous 3 months and in patients with renal insufficiency requiring external replacement therapyCandida pyelonephritis in patients not responding to or not treatable with fluconazole | Anidulafungin 200mg/d loading dose, followed by 100mg/dMicafungin 100mg/d IVCaspofungin 70mg loading dose, followed by 50mg/d IV | Preferred if recent azole exposure, patients in septic shock or with external renal devicesLow urine concentration. Relapse may occur if the collecting system is the source of the candidemiaDrug interactions: Anidulafungin (none), Micafungin (sirolimus, nifedipine, itraconazole), Caspofungin (cyclosporine, tacrolimus, efavirenz, nevirapine, rifampicin, dexamethasone, phenytoin, carbamazepine) | 149,172,176,180 |
| L-AMB | Candida pyelonephritis in patients not responding to or not treatable with fluconazole | 3mg/kg/d IV | Low urine and kidney concentration. Relapse may occurDrug interactions: digoxin, aminoglycosides, cyclosporine and others. | 181 |
| Voriconazole | Candida pyelonephritis in patients not responding to or not treatable with fluconazole | 6mg/kg/d two loading doses, followed by 3mg/kg/12h IV or PO | Low urine concentration. Relapse may occurDrug interactions: rifabutin, rifampicin, methadone, ritonavir, efavirenz, carbamazepine, ranitidine, macrolides, sirolimus, cyclosporine, tacrolimus, warfarin, coumadin, statins, benzodiazepines, omeprazole, oral contraceptives and others.Monitor serum levels. | 181 |
| 5-Flucytosine | Symptomatic cystitis in patients not responding to or not treatable with fluconazole | 25mg/kg every 6h 7–10 d | Emergence of resistance if given alone or for prolonged periods.Adjust dose if renal insufficiency.Gastrointestinal, liver and bone marrow toxicity | 182 |
d-AMB: amphotericin B deoxycholate.
L-AMB: liposomal amphotericin B.
- 1.
The diagnostic approach in transplant patients with recurrent UTI must be meticulous in order to rule out the existence of anatomical or functional changes (A-III).
- 2.
If possible, treatment aimed at the sensitivity of the isolated microorganisms must be used in patients with recurrent UTI. TMP/SMX is a good option (B-III). Quinolones must be avoided as empirical therapy (D-II).
- 3.
Duration of antibiotic treatment for recurrent UTIs in transplant patients is not well-defined. At least a 6-week treatment period may be recommendable (B-III), although other authors suggest prolonging treatment more than three months. Indefinite treatment may be evaluated in diabetic patients, patients with a history of UTIs before or soon after transplantation and those receiving high-dose immunosuppressive treatment (equivalent to secondary prophylaxis) (B-II).
- 4.
Anatomical changes related with recurrent UTI must be corrected if possible (A-II).
- 5.
The use of non-antibiotic therapies, such as cranberry extract, L-methionine, topical estrogens, or topical application of Lactobacillus, could be provided to transplant patients with recurrent UTI (C-II).
Recurrent UTI is commonly defined as the presence of three or more episodes of symptomatic UTIs over a 12-month period or two episodes in the previous six months.13 Recurrent UTIs in kidney transplant patients are significant because these infections in themselves have been recognized as a risk factor for the development of subsequent UTIs.3 It has also been associated with other complications such as dysfunction of the kidney graft.183 The appearance of recurrent UTIs in kidney transplant patients has been associated with vesicoureteral reflux,183,184 infections due to MDR bacteria,79–81,185 the lack of use of antibiotic prophylaxis,186or the presence of renal calculi.185
- a.
When should recurrent UTIs be investigated in kidney transplant patients?
For patients with recurrent UTIs who are otherwise healthy with no risk factors or criteria for complications, restricting the investigation of the underlying causes to cases in which recurrence is due to the persistence of the same microorganism has been proposed.187,188 However, a detailed study must be carried out in all transplant patients, regardless of the isolated microorganism.23,57,189,190
- b.
What examinations should be performed in a kidney transplant patient with recurrent UTIs?
In the general population with recurrent UTIs, adequate antibiotic spectrum and duration of the administered treatment must be confirmed.81 Complementary tests must also be requested to rule out the existence of anatomical or functional changes such as vesicoureteral reflux, stenosis of the ureterovesical junction, or neurogenic bladder, which can favor or perpetuate the infection,14,23,57,184 or to find reservoirs where the microorganism has remained hidden.81 These examinations are especially recommended for KTR with recurrent UTIs, since these changes are more common in these patients184 and the repercussions that UTIs may have on mortality and on the graft can be considerable. For this reason, the diagnostic approach must be meticulous57,189:
- •
The patient's medication must be reviewed, as there may be a relation between recurrent UTIs and excessive immunosuppression or there may be resistance to standard prophylaxis.
- •
It is equally important to obtain samples for clinical laboratory testing, including complete blood count, renal function, and inflammatory parameters (ESR and CRP), in order to assess the general repercussions of the process.
- •
The corresponding microbiological cultures should be requested to determine the causative microorganism and its sensitivity. It may also be necessary to request specific tests for the detection of Mycobacterium tuberculosis or polyomavirus BK, when they are suspected.
- •
Imaging studies of the genitourinary tract should be considered (ultrasound examination and CTs) in order to rule out structural changes such as kidney stones or complicated cysts, both in the patient's own kidney and in the graft. It may be useful to combine these studies with a PET scan to rule out, for example, an infected cyst in a patient with polycystic kidneys.191
- •
Anatomical and functional changes, such as vesicoureteral reflux, bladder dysfunction, or obstruction, may be confirmed by cystoscopy, cystogram, uroflowmetry, and other urodynamic techniques. Before these are performed, however, the risk of complications must be considered, as many of these tests are invasive. One of the complications is the infection itself because of the risk of inoculation of microorganisms.
- •
- c.
Should secondary prophylaxis be administered in transplant patients with recurrent urinary infections? How long should it be given?
No general antibiotic guidelines have been established for secondary prophylaxis in transplant patients with recurrent UTI. It can be extrapolated from the literature that treatment aimed at the sensitivity of the isolated microorganisms must be used, ensuring that the dose of the selected antibiotic is as well adjusted as possible with few interactions and side effects such as, for example, TMP/SMX.23,57,189 The use of quinolones in allergic patients has been proposed,189 but empirical treatment must probably be advised against due to the high resistance rate of this group of antibiotics.
Authors addressing the question of duration of antibiotic treatment for recurrent UTIs in transplant patients agree that it is appropriate to administer antibiotics for a longer period than if it were a first episode. However, there are no well-defined recommendations due to the lack of clinical trials. Indeed, some studies propose a 6-week treatment period,23,189 while others suggest prolonging it for at least three months57 or even indefinitely, which, from a practical point of view, is equivalent to initiating secondary prophylaxis. The latter approach may be particularly recommendable in cases in which there are other associated risk factors, as may be the case in diabetic patients, patients with a history of UTIs before or soon after transplantation, or those receiving high-dose immunosuppressive therapy.34 Anatomical changes must be corrected if possible as this approach has been associated with recurrent UTI resolution.14,183
In any case, the decision to initiate secondary prophylaxis is not an easy one. Among the inconveniences to be taken in account, the most obvious is that the use of long-term antibiotic regimens may select for resistant microorganisms, interfere in levels of immunosuppression, and favor fungal overgrowth due to the effect of wide-spectrum antibiotics on the normal flora.57,189
The increase in secondary resistances due to long-term exposure to antibiotics is a problem which is becoming a major healthcare issue, especially in transplant patients. Some studies have described that more than 70% of gram-negative microorganisms are TMP/SMX resistant.36 Valera et al.5 reported that a quarter of isolated E. coli (the pathogen responsible for 71% of gram-negative infections) strains are wide-spectrum beta-lactamase producers, and more than 75% of patients with prior TMP/SMX exposure were resistant to this antibiotic.1 Indeed, there are studies showing how the use of TMP/SMX in the two weeks before the UTI or history of prolonged prior exposure is related with resistance to this antibiotic in successive UTIs.192,193 In an attempt to control this complication, the use of high-dose antibiotics for the shortest time possible has been proposed for these UTIs.194
For patients with kidney transplants, the use of various non-antibiotic therapies already used in recurrent UTIs in non-transplant patients188 may be of particular interest.195 Firstly, the administration of cranberry extract appears to reduce this type of infection, particularly in women.196 However, not all studies are conclusive.197 Its mechanism is not well defined, but it is speculated that cranberry extract interferes with the adhesion of uropathogenic bacteria, primarily E. coli, to the uroepithelial cells.57l-Methionine has also demonstrated usefulness.196 Topical estrogens have also been used with success in post-menopausal patients. These agents appear to reinforce the vesical trigone in these patients who show lowered estrogen levels.57,198 Finally, it also appears that the topical application of Lactobacillus can reduce the incidence of UTIs by reducing vaginal pH.57,188,189 However, more robust studies on the use of these treatments in recurrent UTIs are needed, both in the general population and in transplant patients. This is an area which may be very promising because if these approaches are effective, they may constitute treatments which can be used chronically, considerably reducing the side effects of antibiotics.
What role does UTI play in kidney graft rejection or dysfunction?Recommendations- 1.
Kidney transplant patients are particularly vulnerable to infections, and this is one of the reasons for which primary prophylaxis has been established (A-I) and early aggressive treatment of symptomatic UTI is recommended (A-II).
- 2.
Although UTI has been associated with induction of acute rejection in kidney transplant patients (A-II), there is controversy about the final impact on the graft in terms of chronic rejection or dysfunction (B-II).
- 3.
Late-onset UTIs, which were traditionally associated with a good prognosis, have also been recently related with a risk of rejection or dysfunction of the kidney graft (B-II).
- 4.
The association between AB and graft loss is unclear.
The association between UTI and graft loss in kidney transplant patients is a question that is currently not well-defined. Grafts are known to have a high risk of infection, due to both the patient's immunosuppressed status and to the particular vulnerability of the grafted organ after surgical manipulation.3,199 Indeed, this is one of the reasons for which prophylaxis with TMP/SMX during the first 3–6 months after transplant has been established49 and why early aggressive treatment of symptomatic UTI is recommended.200
Although UTIs are known to be more frequently associated with induction of acute rejection in kidney transplant patients,42,66,199 the final impact of the infection on the graft is unclear. The controversy arises from the lack of consensus in the results of different studies.29,183,201 Some papers have found a relation between UTIs and graft survival or dysfunction, while others have not.29,183,184,201–205 Even studies that have found a relation between UTIs and adverse events in kidney transplant patients are not fully in agreement. Some have reported associations with dysfunction only, with mortality only or both.33,201 There is no consensus regarding late-onset UTIs (those occurring more than 6 months post-transplantation), which have been traditionally associated with a good prognosis, since recent data have also related these infections with a risk of graft loss or death.23,201
Some studies also show an association between AB and graft loss, but other authors do not find the same results.41,65
In an attempt to explain the discrepancies in the published results, it has been speculated that although there may be a greater risk of dysfunction after post-transplant UTI episodes, this may be due to the fact that the causes of UTI are also responsible for the dysfunction of the transplanted organ.74 Such discrepancies may also be due to the different accuracy of the diagnostic techniques used in the measurement of renal impairment.206
Microbiological etiology has also been proposed to explain these differences. The severity of E. coli infections has been found to be associated with the expression of virulence factors by E. coli (O:H serotypes, P fimbriae, and adhesion expression). These factors affect both the individual's defense response and the capacity of the microorganism to invade the urothelium.66 Moreover, virulence factors may trigger some degree of inflammation, which is not always clinical, but may be damaging.65 Accordingly, graft survival and patient response to UTI may depend on the expression of virulence factors by the causative microorganism.
To sum up, the definitive effects of UTIs on kidney transplant patients are unknown. The potentially ominous consequences of UTIs make both primary prophylaxis in the first few months post-transplant and treatment of symptomatic UTIs highly recommendable. More studies are needed to evaluate the treatment of asymptomatic UTIs.
Antimicrobials and immunosuppressant interactionsRecommendations- 1.
The treatment of UTIs in SOT recipients is more complex due to interactions between antimicrobials and immunosuppressants.
- 2.
The interactions may jeopardize the transplanted organ and also increase the specific adverse effects of each drug.
- 3.
Key measures to avoid the consequences of these interactions are to know and to prevent them by monitoring the plasma levels of these drugs, monitoring graft function and characteristic adverse effects, and avoiding contraindicated combinations (AII).
Interactions between antimicrobial and immunosuppressants make treatment more complex in SOT recipients than in the general population. The co-administration of immunosuppressants and antibiotics can modify the pharmacokinetic and pharmacodynamic characteristics of both groups of drugs, causing serious consequences. Among them, reduced plasma levels of immunosuppressive and antimicrobial agents may result in the loss of the transplanted organ and failure to cure the infection, respectively. By contrast, the elevated levels as a result of the interaction may increase the toxicity of both drug groups. Finally, combination treatment may increase the intensity of an adverse effect common to both groups of drugs by synergy.
The most widely used antibiotics in the treatment of UTI include aminoglycosides, beta-lactams, quinolones, glycopeptides, and fosfomycin. The most commonly used antifungals are fluconazole, voriconazole, amphotericin, and echinocandins.
Interactions between these antimicrobials and immunosuppressants are reviewed next in order to recommend more appropriate clinical decisions depending on their intensity: dose adjustment, monitoring plasma levels and/or the graft function, or that it is not necessary to make any changes because interactions do not exist or are not relevant. The tables also show whether interaction has occurred in the clinical setting or is a prediction of potential interaction due to the pharmacokinetic characteristics of the drug. This review is based on a recent article published previously in this journal.207
Antibiotics. The most relevant interactions are described in the text. Information is completed in Tables 6–9.
Interactions between aminoglycosides and immunosuppressants.a
| Immunosuppressants | AMK | GEN | ETM | EPT | KAN | TOB |
|---|---|---|---|---|---|---|
| Cyclosporine | B | B | B | B | B | B |
| Tacrolimus | B | b | b | b | B | b |
| Mycophenolate | C | C | C | C | C | b |
| Sirolimus | b | b | b | b | B | b |
| Everolimus | b | b | b | b | B | b |
| Azathioprine | C | C | C | C | C | C |
| Prednisone | C | C | C | C | C | C |
| Basiliximab | C | C | C | C | C | C |
| Muromonab | C | C | C | C | C | C |
AMK: amikacin; GEN: gentamicin; ETM: streptomycin; EPT: spectinomycin; KAN: kanamycin; TOB: tobramycin.
A/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Interactions between beta-lactams and immunosuppressants.a
| Immunosuppressants | AMP | AMC | CLO | NAF | CFU | CFZ | CFX | AZT | IMI | TIC |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyclosporine | C | C | b | B | C | b | b | C | b | C |
| Tacrolimus | C | b | C | C | C | C | C | C | C | C |
| Mycophenolate | C | B | C | C | b | C | C | C | C | C |
| Sirolimus | C | C | C | C | C | C | C | C | C | C |
| Everolimus | C | C | C | C | C | C | C | C | C | C |
| Azathioprine | C | C | C | C | C | C | C | C | C | C |
| Prednisone | C | C | C | C | C | C | C | C | C | C |
| Basiliximab | C | C | C | C | C | C | C | C | C | C |
| Muromonab | C | C | C | C | C | C | C | C | C | C |
AMP: ampicillin; AMC: amoxicillin-clavulanate; CLO: cloxacillin; NAF: nafcillin; CFU: cefuroxime; CFZ: ceftazidime; CFX: ceftriaxone; AZT: aztreonam; IMI: imipenem; TIC: ticarcillin.
A/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Interactions between quinolones and immunosuppressants.a
| Immunosuppressants | CIP | LEV | MOX | NAL | NOR | OFL |
|---|---|---|---|---|---|---|
| Cyclosporine | b | b | N.A. | N.A. | b | C |
| Tacrolimus | b | b | a | b | b | B |
| Mycophenolate | B | C | C | C | B | C |
| Sirolimus | C | C | C | C | C | C |
| Everolimus | C | C | C | C | C | C |
| Azathioprine | C | C | C | C | C | C |
| Prednisone | b | b | b | b | b | B |
| Basiliximab | C | C | C | C | C | C |
| Muromonab | C | C | C | C | C | C |
CIP: ciprofloxacin; LEV: levofloxacin; MOX: moxifloxacin; NAL: nalidixic acid; NOR: norfloxacin; OFL: ofloxacin.
A/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Interactions between other antibiotics and immunosuppressants.a
| Immunosuppressants | TIG | VAN | DAP | LIN | COT | DOX | FOS | CLI | MET | FID |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyclosporine | b | B | b | N.A. | b | C | C | b | b | C |
| Tacrolimus | b | b | N.A. | N.A. | b | C | C | C | b | C |
| Mycophenolate | N.A. | C | N.A. | N.A. | C | C | C | C | B | C |
| Sirolimus | N.A. | C | N.A. | N.A. | C | C | C | C | C | C |
| Everolimus | N.A. | C | N.A. | N.A. | C | C | C | C | C | C |
| Azathioprine | N.A. | C | N.A. | N.A. | C | C | C | C | C | C |
| Prednisone | N.A. | C | b | N.A. | C | C | C | C | C | C |
| Basiliximab | N.A. | C | N.A. | N.A. | C | C | C | C | C | C |
| Muromonab | N.A. | C | N.A. | N.A. | C | C | C | C | C | C |
TIG: tigecycline; VAN: vancomycin; DAP: daptomycin; LIN: linezolid; COT: cotrimoxazole; DOX: doxycycline; FOS: fosfomycin; CLI: clindamycin; MET: metronidazole; FID: fidaxomicin.
A/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Aminoglycosides (Table 6). Co-administration of aminoglycosides with cyclosporine or tacrolimus potentiates the nephrotoxicity of both drugs.208 Therefore, monitoring renal function along with the usual determination of plasma levels is recommended. If renal clearance is decreased, it is recommended to lower the doses or use an alternative non-nephrotoxic antimicrobial as a substitute.209
Beta-lactams (Table 7). In general, this is a safe combination. It is recommended to avoid nafcillin with cyclosporine because of nephrotoxicity.210 Treatment with amoxicillin-clavulanate may affect mycophenolate absorption by altering the intestinal flora.211
Quinolones (Table 8). Quinolones lack serious interactions. Since ciprofloxacin can reduce intestinal absorption of mycophenolate, it is advisable to monitor levels.211
Vancomycin (Table 9). Vancomycin may potentiate the nephrotoxicity of cyclosporine and tacrolimus, so it is recommended to determine plasma levels of both and monitor renal function.212
Antifungals (Tables 10 and 11). Azoles (Table 10) inhibit hepatic metabolism with different degrees of severity. This can cause serious interactions when administered with immunosuppressive drugs because they increase their plasma levels.213 Co-administration of ketoconazole and itraconazole with cyclosporine, tacrolimus, sirolimus, and everolimus is contraindicated (AII). Itraconazole increases mycophenolate levels, so monitoring is recommended.213–215
Interactions between azoles, echinocandins and immunosuppressants.a
| Immunosuppressants | KET | ITR | FLU | VOR | POS | CAS | MIC | ANI |
|---|---|---|---|---|---|---|---|---|
| Cyclosporine | A | A | Bb | Bb | Bc | B | B | C |
| Tacrolimus | A | A | Bd | Bd | Bd | B | C | C |
| Mycophenolate | N.A. | b | C | C | C | C | N.A. | N.A. |
| Sirolimus | A | A | Be | A | a | N.A. | B | N.A. |
| Everolimus | A | a | B | a | a | N.A. | N.A. | N.A. |
| Azathioprine | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Prednisone | N.A. | N.A. | N.A. | N.A. | N.A. | C | C | C |
| Basiliximab | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Muromonab | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
KET: ketoconazole; ITR: itraconazole; FLU: fluconazole; VOR: voriconazole; POS: posaconazole; CAS: caspofungin; MIC: micafungin; ANI: anidulafungin.
A/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Interactions between flucytosine, polyenes and immunosuppressants.a
| Immunosuppressants | FUC | ABD | ABL | ABC |
|---|---|---|---|---|
| Cyclosporine | b | B | B | B |
| Tacrolimus | b | B | B | B |
| Mycophenolate | N.A. | C | N.A. | N.A. |
| Sirolimus | N.A. | B | b | B |
| Everolimus | N.A. | B | B | B |
| Azathioprine | N.A. | N.A. | N.A. | N.A. |
| Prednisone | N.A. | B | b | B |
| Basiliximab | N.A. | N.A. | N.A. | N.A. |
| Muromonab | N.A. | N.A. | N.A. | N.A. |
FUC: flucytosine; ABD: amphotericin B deoxycholate; ABL: liposomal amphotericin B; ABC: amphotericin lipid complex.
A/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Fluconazole inhibits hepatic metabolism with less intensity, so interactions are minor and occur mainly when it is administered orally, which increases levels of cyclosporine, tacrolimus, sirolimus, and everolimus. It is therefore recommended to reduce the dose of these immunosuppressants, considering that it takes a week for the effect to occur.213–215
The combination of voriconazole and posaconazole with sirolimus and everolimus is contraindicated (AII). If voriconazole is combined with cyclosporine or tacrolimus it is recommended to reduce the dose of cyclosporine and tacrolimus by 50% and 33%, respectively,213–216 and to monitor tacrolimus levels when tacrolimus is combined with posaconazole.217
Echinocandins (Table 10) have very few interactions with immunosuppressants, particularly anidulafungin.218 Caspofungin reduces tacrolimus levels by 26% and increases cyclosporine AUC by 35%. Micafungin increases sirolimus AUC.212
Polyenes (Table 11) may potentiate the nephrotoxicity of cyclosporine or tacrolimus. Liposomal amphotericin B is significantly less nephrotoxic than amphotericin B deoxycholate.
Conflict of interestJMC has received a conference grant from Astellas, Astra-Zeneca, MSD, Novartis, and Pfizer. All other authors have no conflict of interest to declare.
We thank Jesús Rodríguez-Baño MD, PhD and member of SEIMC for his comments on the manuscript.