A considerable increase of imported Zika virus (ZIKV) infection has been reported in Europe in the last year. This is the result of the large outbreak of the disease in the Americas, along with the increase in the numbers of travellers and immigrants arriving from ZIKV endemic areas.
MethodsA descriptive study was conducted in the Tropical Medicine Unit of Hospital La Paz-Carlos III in Madrid on travellers returning from an endemic area for ZIKV from January to April 2016. Demographic, clinical and microbiological data were analyzed.
ResultsA total of 185 patients were screened for ZIKV (59.9% women, median age of 37.7±10.3 years). Main purpose of the travel was tourism to Colombia, Brazil, and México. Just under three-quarters (73%) were symptomatic, mostly with fever and headache. A total of 13 patients (7% of those screened) were diagnosed with ZIKV infections, of which four of them were pregnant. All of them were symptomatic patients, the majority immigrants, and mainly from Colombia. Diagnostic tests were based on positive neutralization antibodies (8 cases, 61.6%) and a positive RT-PCR in different organic fluids (7 cases, 53.8%) The four infected pregnant women underwent a neurosonography every 3 weeks, and no alterations were detected. RT-PCR in amniotic fluid was performed in three of them, with negative results. One of the children has already been born healthy.
ConclusionsOur cases series represents the largest cohort of imported ZIKV to Spain described until now. Clinicians must increase awareness about the progression of the ZIKV outbreak and the affected areas so that they can include Zika virus infection in their differential diagnosis for travellers from those areas.
En el último año se ha registrado un importante aumento de casos de infección por virus Zika (ZIKV) importados en Europa. Este hecho es un reflejo de la epidemia que actualmente se está produciendo en las Américas, así como del aumento del número de viajeros e inmigrantes que proceden de zonas endémicas.
MétodosEstudio descriptivo de los viajeros retornados de área endémica para ZIKV en la Unidad de Medicina Tropical del Hospital La Paz-Carlos III en Madrid, de enero a abril de 2016. Se recogieron y analizaron datos demográficos, clínicos y microbiológicos.
ResultadosSe cribaron para ZIKV un total de 185 pacientes (59,9% mujeres, mediana de edad de 37,7±10,3 años). El propósito por el que habían realizado el viaje fue por turismo a Colombia, Brasil y México. El 73% de los inicialmente cribados presentaron síntomas, fundamentalmente fiebre y cefalea. Se diagnosticó infección por ZIKV a 13 pacientes (7% de los cribados); 4 de ellos eran gestantes. Todos los casos con infección confirmada estaban sintomáticos, y la mayoría eran inmigrantes colombianos. El diagnóstico se basó en la presencia de anticuerpos neutralizantes positivos (8 casos, 61,6%) y RT-PCR positiva en diferentes fluidos orgánicos (7 casos, 53,8%). A las 4 gestantes infectadas se les realizó neurosonografía fetal seriada cada 3 semanas, no detectándose alteraciones en ninguna de ellas. En 3 casos se realizó RT-PCR en líquido amniótico, que fue negativo. Uno de los niños ha nacido, y está completamente sano.
ConclusionesNuestra serie representa la cohorte más grande de infección por ZIKV importada en España hasta la fecha. Los clínicos deben estar alerta sobre la evolución de la epidemia del ZIKV y las zonas a las que afecta, para poder incluir la infección por ZIKV dentro del diagnóstico diferencial de viajeros que regresan de esas áreas.
Zika virus (ZIKV) infection is the paradigm of a re-emergent viral infectious disease. Since its isolation in rhesus monkey in 1947 in Uganda, human ZIKV infections has been sporadic and located in limited geographical regions of Africa and Asia.1 In 2007 ZIKV was isolated for the first time in the Pacific, on the Micronesian island of Yap.2 In 2013, the large outbreak registered in different islands of French Polynesia was associated to the first imported cases of ZIKV infection in countries where this disease had not been previously reported.3 Later on, other territories, mainly touristic destinations as Thailand4 or Indonesia,5 also were the source of imported cases to Europe.
This vector-borne disease rapidly became a worldwide public health issue due to its causal relationship with microcephaly among newborns in Brazil,6,7 and with Guillain–Barre syndrome in adults.8 On February 2016, the World Health Organization considered the current ZIKV epidemic a public health emergency of international concern.9 Therefore it was expected a high demand of screening in exposed population, both among returned travellers and immigrants natives from endemic and risk areas.
Since the beginning of the current outbreak in 2015 in different territories of Centre and South America and Caribbean, many cases of imported ZIKV to Europe have been reported.10 Spain harbours an important immigrant community native from these specific areas of the Americas. Also, Spain is an important tourist source country to these territories. It is therefore not a surprise that imported ZIKV disease has already been described in Spain.11
The aim of this study is to describe the demographic, clinical and microbiological data of patients initially screened for ZIKV infection and patients diagnosed with ZIKV in the first four months of 2016. All of them were seen at the Tropical Medicine Unit of Hospital La Paz-Carlos III in Madrid.
Patients, material and methodsStudy designThis descriptive retrospective analysis was carried out at the outpatient Tropical Medicine Unit from Hospital Universitario La Paz-Carlos III, located in Madrid. This is a referral Unit that attends a media of 4500 returned patients each year (both travellers and immigrants) and a mean of 10,000 subjects who are going to travel and ask for pre-travel medical advice and vaccinations.
For this study, data were registered in a specific database for travellers seen from 1st January to 30th April 2016.
Variables registeredDemographic: age, gender, country of destination, nationality, type of case [tourist, visiting friends and relatives (VFR), business, cooperation, others], duration of travel (stays longer than 365 were not considered for the calculation of means), time elapsed from arrival to consultation, reason for seeking medical care, risk group (pregnant, sexual partner of pregnant women, women or men with gestational desire, others). Clinical: presence of symptoms, type of symptoms (fever, rash, arthralgia, retroocular pain, headache, others).12
Microbiologic test performed consisted on serology for Dengue (Elisa technique, Panbio®) and ZIKV (IFA technique, Euroimmun®) in all cases, and CHIKV (IFA technique, Euroimmun®) only in selected patients. Neutralization antibody test (NT) for ZIKV was performed if needed in the laboratory of reference located in Centro Nacional de Microbiología in Majadahonda, Madrid. Reverse transcription polymerase chain reaction (RT-PCR) for screening was also performed in different biological fluids (urine, blood, semen or amniotic fluid) (Zika RealStar, Altona®). Confirmation with a second PCR was carried out by using a modification of Balm et al.13
Neuroultrasonography was performed in all pregnant women who where screening for ZIKV, regardless they were finally infected or not. In infected pregnants, the echography was repeated every 3 weeks until delivery.
Diagnostic criteriaDiagnostic criteria were those established in the national protocols for surveillance of ZIKV infection.1,14 Briefly, a patient where the detection of RNA of ZIKV by means of a confirmed positive PCR (two positive PCRs designed with different genomic targets and similar sensitivity or in different aliquots of the same sample) was obtained, was considered as a confirmed case. The confirmation of positive cases by IFA requires positive results in microneutralization tests.
Statistical analysisDemographic data were analyzed using descriptive statistics (means and standard deviation for all continues variables; frequencies and percentages are presented for scale or nominal data). Travel and case histories were analyzed for epidemiological, clinical o diagnostic features of infection.
ResultsOf the 184 patients initially screened for ZIKV during the study period, 13 (7%) were diagnosed of ZIKV.
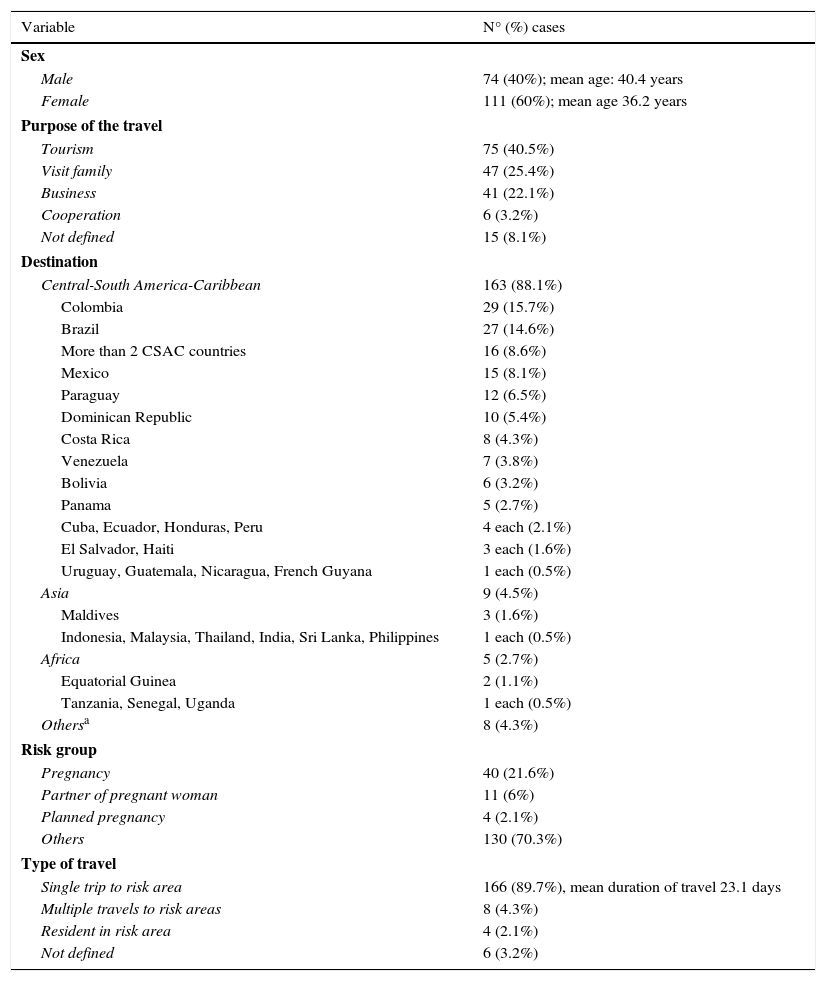
Of the 184 patients screened, 111 (59.9%) were women. Mean±SD age was 37.7±10.3 years. Central and South America was by far the largest geographical region contributing screened cases, with nearly 90%. Most of them travelled to Colombia (29%), Brazil (27%) and México (15%). Up to 16 patients travelled to more than two different ZIKV risk areas. Most patients were on holiday (75 cases, 40.5%), or returning home to VFR (47 cases, 25.4%). The median of time spent in a ZIKV risk area was 15 days (range 1–365). Most relevant epidemiological data are summarized in Table 1.
Epidemiological data of screened patients (N=185).
| Variable | N° (%) cases |
|---|---|
| Sex | |
| Male | 74 (40%); mean age: 40.4 years |
| Female | 111 (60%); mean age 36.2 years |
| Purpose of the travel | |
| Tourism | 75 (40.5%) |
| Visit family | 47 (25.4%) |
| Business | 41 (22.1%) |
| Cooperation | 6 (3.2%) |
| Not defined | 15 (8.1%) |
| Destination | |
| Central-South America-Caribbean | 163 (88.1%) |
| Colombia | 29 (15.7%) |
| Brazil | 27 (14.6%) |
| More than 2 CSAC countries | 16 (8.6%) |
| Mexico | 15 (8.1%) |
| Paraguay | 12 (6.5%) |
| Dominican Republic | 10 (5.4%) |
| Costa Rica | 8 (4.3%) |
| Venezuela | 7 (3.8%) |
| Bolivia | 6 (3.2%) |
| Panama | 5 (2.7%) |
| Cuba, Ecuador, Honduras, Peru | 4 each (2.1%) |
| El Salvador, Haiti | 3 each (1.6%) |
| Uruguay, Guatemala, Nicaragua, French Guyana | 1 each (0.5%) |
| Asia | 9 (4.5%) |
| Maldives | 3 (1.6%) |
| Indonesia, Malaysia, Thailand, India, Sri Lanka, Philippines | 1 each (0.5%) |
| Africa | 5 (2.7%) |
| Equatorial Guinea | 2 (1.1%) |
| Tanzania, Senegal, Uganda | 1 each (0.5%) |
| Othersa | 8 (4.3%) |
| Risk group | |
| Pregnancy | 40 (21.6%) |
| Partner of pregnant woman | 11 (6%) |
| Planned pregnancy | 4 (2.1%) |
| Others | 130 (70.3%) |
| Type of travel | |
| Single trip to risk area | 166 (89.7%), mean duration of travel 23.1 days |
| Multiple travels to risk areas | 8 (4.3%) |
| Resident in risk area | 4 (2.1%) |
| Not defined | 6 (3.2%) |
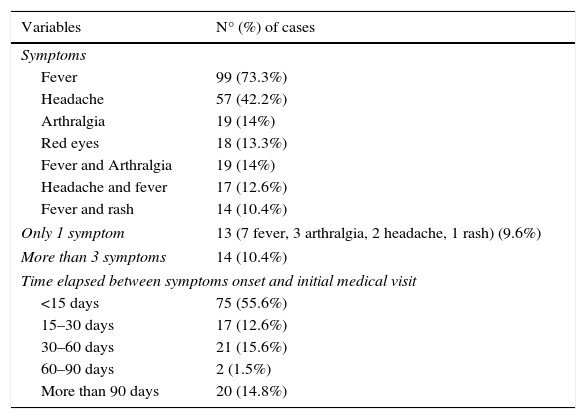
The majority of screened patients were symptomatic at the time of consultation (135 cases, 73% of total). Symptoms consisted of fever in 99 cases (73.3%) or headache in 57 cases (42.2%). Most patients (122; 90.4%) have more than one symptom reported, mostly fever associated to headache. Time elapsed between arrival and consultation was a median of 39 days (range 1–197 days). In 47 patients (34.8%) symptoms started during travel. Median of time elapsed between arrival and seeking medical advice was 5 days (range 1–142). Most symptomatic patients asked for medical advice less than 15 days before arriving (81 patients, 86.6%). Clinical data related to symptomatic patients are described in Table 2. Final diagnosis related to arboviriasis when ZIKV was ruled out was DENV infection in 17 cases (9.2%) and CHIKV infection in 2 cases (1.1%).
Symptoms of screened patients (N=135).
| Variables | N° (%) of cases |
|---|---|
| Symptoms | |
| Fever | 99 (73.3%) |
| Headache | 57 (42.2%) |
| Arthralgia | 19 (14%) |
| Red eyes | 18 (13.3%) |
| Fever and Arthralgia | 19 (14%) |
| Headache and fever | 17 (12.6%) |
| Fever and rash | 14 (10.4%) |
| Only 1 symptom | 13 (7 fever, 3 arthralgia, 2 headache, 1 rash) (9.6%) |
| More than 3 symptoms | 14 (10.4%) |
| Time elapsed between symptoms onset and initial medical visit | |
| <15 days | 75 (55.6%) |
| 15–30 days | 17 (12.6%) |
| 30–60 days | 21 (15.6%) |
| 60–90 days | 2 (1.5%) |
| More than 90 days | 20 (14.8%) |
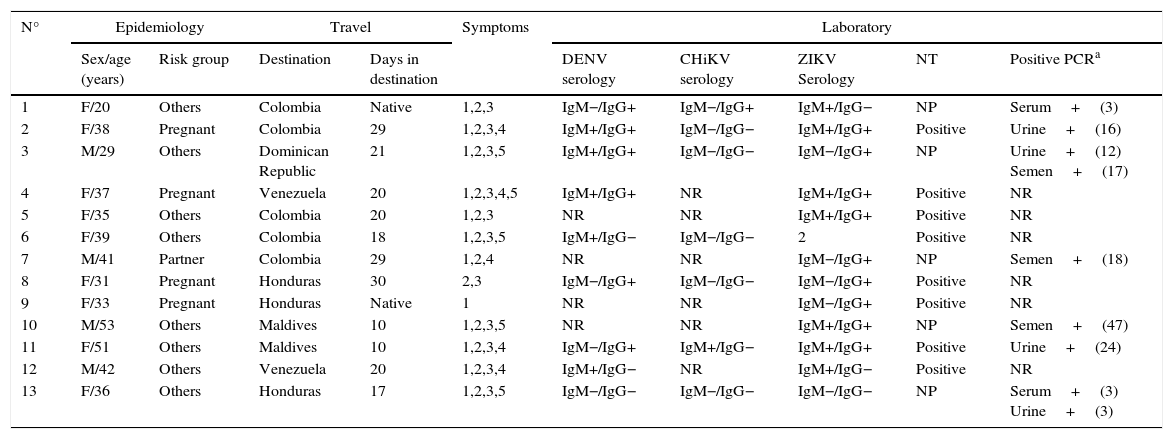
Regarding the 13 patients diagnosed of ZIKV, in 9 cases (69.2%) they were classified as VFR, and the country of acquisition of infection was Colombia (5 cases, 38.5%), followed by Honduras (3 cases, 27%). Four of them (30.8%) were pregnant women, 1 male (7.7%) was the sexual partner of a pregnant women and the rest (61.5%) didn’t belong to any risk group.
Diagnostic test that lead to the diagnosis were a positive serology with a confirmative neutralization antibody assay in 8 cases (61.6%) and a positive RT-PCR in organic fluid in 7 cases (53.8%). In two patients both microbiological techniques were positive.
Most relevant characteristics of patients diagnosed with Zika virus infection are summarized in Table 3.
Characteristic of patients diagnosed with Zika virus infection.
| N° | Epidemiology | Travel | Symptoms | Laboratory | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex/age (years) | Risk group | Destination | Days in destination | DENV serology | CHiKV serology | ZIKV Serology | NT | Positive PCRa | ||
| 1 | F/20 | Others | Colombia | Native | 1,2,3 | IgM−/IgG+ | IgM−/IgG+ | IgM+/IgG− | NP | Serum+(3) |
| 2 | F/38 | Pregnant | Colombia | 29 | 1,2,3,4 | IgM+/IgG+ | IgM−/IgG− | IgM+/IgG+ | Positive | Urine+(16) |
| 3 | M/29 | Others | Dominican Republic | 21 | 1,2,3,5 | IgM+/IgG+ | IgM−/IgG− | IgM−/IgG+ | NP | Urine+(12) Semen+(17) |
| 4 | F/37 | Pregnant | Venezuela | 20 | 1,2,3,4,5 | IgM+/IgG+ | NR | IgM+/IgG+ | Positive | NR |
| 5 | F/35 | Others | Colombia | 20 | 1,2,3 | NR | NR | IgM+/IgG+ | Positive | NR |
| 6 | F/39 | Others | Colombia | 18 | 1,2,3,5 | IgM+/IgG− | IgM−/IgG− | 2 | Positive | NR |
| 7 | M/41 | Partner | Colombia | 29 | 1,2,4 | NR | NR | IgM−/IgG+ | NP | Semen+(18) |
| 8 | F/31 | Pregnant | Honduras | 30 | 2,3 | IgM−/IgG+ | IgM−/IgG− | IgM−/IgG+ | Positive | NR |
| 9 | F/33 | Pregnant | Honduras | Native | 1 | NR | NR | IgM−/IgG+ | Positive | NR |
| 10 | M/53 | Others | Maldives | 10 | 1,2,3,5 | NR | NR | IgM+/IgG+ | NP | Semen+(47) |
| 11 | F/51 | Others | Maldives | 10 | 1,2,3,4 | IgM−/IgG+ | IgM+/IgG− | IgM+/IgG+ | Positive | Urine+(24) |
| 12 | M/42 | Others | Venezuela | 20 | 1,2,3,4 | IgM+/IgG− | NR | IgM+/IgG− | Positive | NR |
| 13 | F/36 | Others | Honduras | 17 | 1,2,3,5 | IgM−/IgG− | IgM−/IgG− | IgM−/IgG− | NP | Serum+(3) Urine+(3) |
F: female; M: male; NR: not requested; NP: Nor performed; NT: neutralization antibody test.
Symptoms: 1: temperature>38°C; 2: maculopapular rash; 3: arthralgia; 4: red eyes; 5: headache.
All pregnant women diagnosed of ZIKV underwent a neurosonography every three weeks until delivery, all of them within normal findings. They were also offered to perform a RT-PCR in amniotic fluid: one expecting mother rejected, the rest had a negative result. At the moment of redaction of this paper, one mother delivered a healthy normal newborn, who has negative RT-PCR in umbilical blood cord for ZIKV, as well as in the placenta.
Positive IgM for DENV was found in 22 patients (11.9%) and for ChIKV in 10 (5.4%), although in five and in one cases respectively ZIKV was concomitantly confirmed by both RT-PCR in any organic fluid and/or neutralization antibody assay. No RT-PCR for DENV nor ChIKV was performed.
A more detailed analysis of DENV cases showed that most of them were from Paraguay and classified as VFR (10 patients; 45.4% both). All of them presented with fever (100% of cases). No differences between DENV and ZIKV diagnosed patients were found in other epidemiological or clinical data.
Regarding the 99 febrile patients attended, final diagnosis apart from ZIKV (12 cases; 12.1%) were self-limited fever without a focus (36 cases; 36.4%), diverse infections affecting urinary tract, prostate, lungs or digestive tract (18; 18.2%), acute DENV (17; 17.2%), upper tract respiratory infections (12; 12.1%) and acute CHiKV (4; 4%).
DiscussionZIKV disease is spreading rapidly through Latin America. According to last official reports Brazil, but also Colombia and El Salvador are the most affected countries.2,15 In our series, Colombia and Brazil are also the most represented countries, but not in the expected proportion. This fact reflects the specific patterns of migration and outbound tourism in Spain. In 2014 nearly 800,000 Spaniards travelled to Central and South America, mainly to México and Dominican Republic.3,16 Besides, the immigrant population established in Spain is mainly from Colombia and Dominican Republic.4,17 Brazil is not a principal focus of tourism, neither of immigration. Anyway, this is an important issue for clinicians evaluating returned travellers.
The overwhelming majority of patients screened travelled for tourism. It is difficult to derive risk estimates from these data, because no surveillance is available regarding the number of travellers that return with health concerns. Our results highlight the increased risk of ZIKV infection for VFR. This specific group of patients experience a higher incidence of travel-related infectious diseases due to multiple factors such as lack of awareness of risk or low rates of pre-travel health care evaluation.5,18 In that sense, it is not surprising that most cases of patients diagnosed of ZIKV in our centre were VFR.
Although it was not possible to establish when infection was acquired, a significant proportion of patients had symptoms during the travel or within the first two weeks of returning, which is compatible with the suggested typical incubation period of 2–14 days.6,19
Our data show that of all screened patients the rate of ZIKV infection was 7.9%. This number has to be taken with caution since no other systematic statistics are available. In the absence of reliable surveillance data, different statistical mathematic models estimate up to 190 cases of symptomatic and asymptomatic imported ZIKV form Brazil into Spain in 2016.7,20,21 In the same way our rate of pregnant women infected with ZIKV (30.8%) should be taken cautiously. This rate could also be an overestimation since our Hospital is the reference Centre in the Madrid Region for pregnant women infected with ZIKV. Another reason that could explain this rate is the active search of this infection in pregnant women.
Microbiological confirmation of ZIKV infections is based mostly on detection of viral RNA in clinical samples by using RT-PCR.8,13 IgM against ZIKV can be detected by currently available ELISA or IFA tests, but due to the cross-reactivity of ZIKV antibodies with other flaviviruses (including DENV), positive results should be confirmed by neutralization assays.9,22 The diagnostic utility of urine as a source for detection of ZIKV RNA by real-time RT-PCR is gaining importance, according to last published information, due to the longer duration of the detection of ZIKV RNA in urine and a higher viral load.10,23 ZIKV RNA is present in the blood11,13 only during the first 3–5 days after the onset of symptoms, while it is detected in urine for >10 days in urine12,23 and in semen for several weeks.13,24 In our series, we have performed the confirmatory diagnosis of ZIKV both with PRNT and RT-PCR. Of note, our series emphasizes that RT-PCR can persist positive in urine (up to 28 days) and semen (up to 47 days). The long persistence of infective semen is of particular concern in terms of public health, and specific guidelines for prevention of ZIKV infection through sexual route have been published recently25 and the first case of sexual transmission in Spain has been reported.26
It should be noted that nearly 12% of patients attended were diagnosed of DENV. A high percentage of them were VFR arriving from Paraguay. That differs from the most affected countries by ZIKV and reflects the special epidemic situation of DENV in that area (Paraguay), in which there has been a significant increase in the incidence of this disease in the last months.27
This study had several limitations. The population analyzed represents only those returned travellers who presented to our Unit; as such, our conclusions may not extend to all returned travellers. Travellers with mild or self-limited illnesses may have sought care in different settings apart for ours. Also, our data do not permit an estimation of incidence rates or destination-specific numeric risks for particular diseases.28 Finally, social and medical alarm may have led to over-screening of ZIKV. Only a few centres in Spain provide standardized diagnostic procedures for patients with suspected ZIKV. Thus, the incidence of ZIKV among returned travellers must be certainly by far underestimated.
We have confirmed that travel to VFR confers particularly high risk, which underscores the need to improve pretravel intervention for a population that is unlikely to seek specific pretravel advice. In that way, in order to provide accurate information about ZIKV infection, we set up in early 2016 a specific phone-line to answer questions related to ZIKV. In the first four months, more than 250 calls were received. The majority of questions were related to the risk of acquiring ZIKV or the probability of being infected after travelling to endemic areas. General and specific advice was given if needed. This phone-line also brought back patients to our clinic for subsequent screening.
The global disease burden of ZIKV is staggering. Continuous expansion and the lack of a vaccine illustrate the limitations of current ZIKV control efforts. Data reported here can contribute to understand the epidemiology and characteristics of imported ZIKV.
Clinicians and travel health clinics must increase awareness about the evolution of the Zika virus outbreak and the affected areas so that they can include Zika virus infection in their differential diagnosis for travellers from those areas.
Conflicts of interestThe authors declare no conflicts of interest.
The clinical research team acknowledges the support provided by Red de Investigación Cooperativa de Enfermedades Tropicales (RICET; RD06/0021/0020).
Mar Lago (Unidad de Medicina Tropical y del Viajero), Concepción Ladrón de Guevara (Unidad de Medicina Tropical y del Viajero), Pablo Barreiro (Unidad de Medicina Tropical y del Viajero), Julio García (Servicio de Microbiología y Parasitología), Elena Martín-Boado (Unidad de Medicina materno-fetal, Servicio de Obstetricia y Ginecología), Nuria Martínez-Sánchez (Unidad de Medicina materno-fetal, Servicio de Obstetricia y Ginecología), Roberto Rodríguez (Sección de Ecografía y Medicina Fetal, Servicio de Obstetricia y Ginecología), Beatriz Herrero (Sección de Ecografía y Medicina Fetal, Servicio de Obstetricia y Ginecología), Francisco López (Sección de Ecografía y Medicina Fetal, Servicio de Obstetricia y Ginecología), Jose Luis Bartha (Servicio de Obstetricia y Ginecología), Maria Dolores Elorza (Servicio de Pediatría, Unidad de Neonatología), Marta Cabrera Lafuente (Servicio de Pediatría, Unidad de Neonatología), Milagros García Hortelano (Servicio de Pediatría, Unidad de Enfermedades Infecciosa y Tropicales).