Sexually transmitted infections caused by Chlamydia trachomatis, including lymphogranuloma venereum and Mycoplasma genitalium have increased in last decade. This epidemiological scenario presents new challenges in order to improve and strengthen our control and prevention strategies. The routine clinical diagnosis of urethritis and cervicitis must be combined with the active search for the causal agent in men with symptoms of dysuria or proctitis, and in women with pelvic inflammatory disease. We should also include sexually transmitted infections screening in asymptomatic patients with sexual risk behaviours or sexual contact with patients diagnosed with a sexually transmitted infection. The microbiological diagnosis must be based on molecular techniques capable of detecting C. trachomatis (discriminating between L genotypes associated with lymphogranuloma venereum and other genotypes) and M. genitalium (ideally including the identification of macrolide-resistant strains). A faster and specific diagnosis will allow for a targeted treatment with a suitable antibiotic regimen. We also recommend including contact tracing of sexual partners and, occasionally, a cure test. Finally, sexually transmitted infection screening must be widely implemented in those population groups with a high prevalence of sexually transmitted infections.
El incremento en las infecciones de transmisión sexual por Chlamydia trachomatis, incluyendo el linfogranuloma venéreo, y Mycoplasma genitalium registrado en la última década plantea nuevos retos para mejorar su control y reforzar su prevención. El diagnóstico clínico habitual (uretritis/cervicitis) debe completarse con una búsqueda activa de la infección en varones con disuria o proctitis, mujeres con enfermedad inflamatoria pélvica y contactos asintomáticos. El diagnóstico microbiológico debe basarse en técnicas moleculares, capaces de detectar Chlamydia trachomatis (diferenciando el genotipo L para linfogranuloma venéreo) y Mycoplasma genitalium (incluyendo idealmente la detección de cepas resistentes a macrólidos). Un diagnóstico más rápido y específico permitirá un tratamiento dirigido con la pauta antibiótica idónea. El manejo de estas infecciones de transmisión sexual debe incluir un estudio de los contactos sexuales y en ocasiones un test de cura. Finalmente, deben ser valorados los cribados de infección en grupos de población con mayor prevalencia.
Over the past decade, the management of sexually transmitted infections (STIs) caused by Chlamydia trachomatis (C. trachomatis) and Mycoplasma genitalium (M. genitalium) has become increasingly important for various reasons, including: (a) increased incidence; (b) increased diagnostic demand; (c) new clinical manifestations associated with these STIs; (d) improved diagnostic methods; (e) description of antibiotic resistance in M. genitalium; (f) screening for C. trachomatis infection.
Since the beginning of this century, the observed increase in the incidence of STIs has been partly due to the reduced use of preventive measures during sexual intercourse as a result of the success of antiretroviral therapy in HIV infection. This reduced use has resulted in an increase in high-risk sexual behaviour (unprotected sex) among some sectors of the population, such as young people or determined groups of men who have sex with men (MSM). This increase in STIs has also been enhanced by the extensive use of social networks promoting sexual contact and the use of recreational drugs during sex (chemsex). It is therefore essential to reinforce the need for primary prevention (sex education and campaigns promoting the use of condoms) and to assess the implementation of STI screening based on prevalence according to age group, gender and/or sexual behaviour.
An STI may be diagnosed by various different medical specialties (Primary Care, Dermatology, Gynaecology, Urology, Ophthalmology, Paediatrics, A&E as well as Infectious Diseases and Microbiology) based on its clinical manifestations, gender and age. It is necessary to remember the importance of an active search for infection in cases of dysuria in young men with leukocyturia and a negative urine culture, in young women with abdominal pain that is possibly of gynaecological origin, in cases of proctitis in MSM, etc. One general consideration when diagnosing STIs caused by C. trachomatis and M. genitalium is that their symptoms may be indistinguishable from other STIs. The implementation of diagnostic platforms that allow simultaneous detection of other pathogens, such as Neisseria gonorrhoeae (N. gonorrhoeae), Ureaplasma urealyticum and Trichomonas vaginalis, is therefore recommended. It is also advisable to take a blood sample to rule out other STIs (HIV, syphilis, etc.).
In symptomatic cases, the absence of gram-negative diplococci on Gram stains means that a gonococcal STI is very unlikely and therefore empirical therapy can be started with doxycycline or azithromycin, as recommended by the main guidelines.1,2 The description given for M. genitalium of variable and increasing rates of macrolide resistance has questioned the use of azithromycin as empirical treatment in non-gonococcal urethritis, although the clinical response rates to doxycycline described for this microorganism are also low. The development of nucleic acid amplification tests (NAATs) has resulted in major diagnostic improvements in terms of susceptibility and response time compared to previously used culture techniques and immunological techniques. Commercial NAATs come in various formats and allow the detection of target DNA of various microorganisms (C. trachomatis, M. genitalium, N. gonorrhoeae, U. urealyticum, etc.) in a single reaction. NAATs also allow detection of C. trachomatis genotype L in patients with risk factors and adjustment of the antibiotic regimen with doxycycline. They can also be useful for rapid detection of mutations associated with macrolide resistance in M. genitalium, allowing use of a more-appropriate targeted treatment regimen to improve clinical response and reduce the possibility of transmission.
Finally, a fundamental requirement for the management of these STIs is contact control to break the chain of transmission and prevent new infections. All sexual partners from the 2–3 months prior to the onset of symptoms in the index case must be diagnosed and treated. If sexual partners are unlikely to turn up for treatment, “expedited partner therapy” may be preferred, which involves providing medicines to the index patient to give/take to his partner(s).
This article will separately review the varying epidemiology, symptoms, diagnosis, treatment and prevention of STIs caused by C. trachomatis, including lymphogranuloma venereum (LGV), and M. genitalium, highlighting those practical aspects that are most important for improving management and control of STIs in Spain.
Chlamydia trachomatisEpidemiologyC. trachomatis is the most common bacterial cause of STI and is estimated to cause more than 130 million new infections worldwide each year.3 In 2016, 403,807 cases of this infection were reported in Europe, with an overall notification rate of 185cases/100,000 people and the highest incidence among heterosexuals and women, especially among those aged 20–24 years (1745/100,000).4 A stable trend in overall incidence has been observed over recent years, although there are marked differences in notification rates (with the highest rates more than 5000 times higher than the lowest rates) between the different national surveillance systems, and therefore the overall incidence may be underestimated.
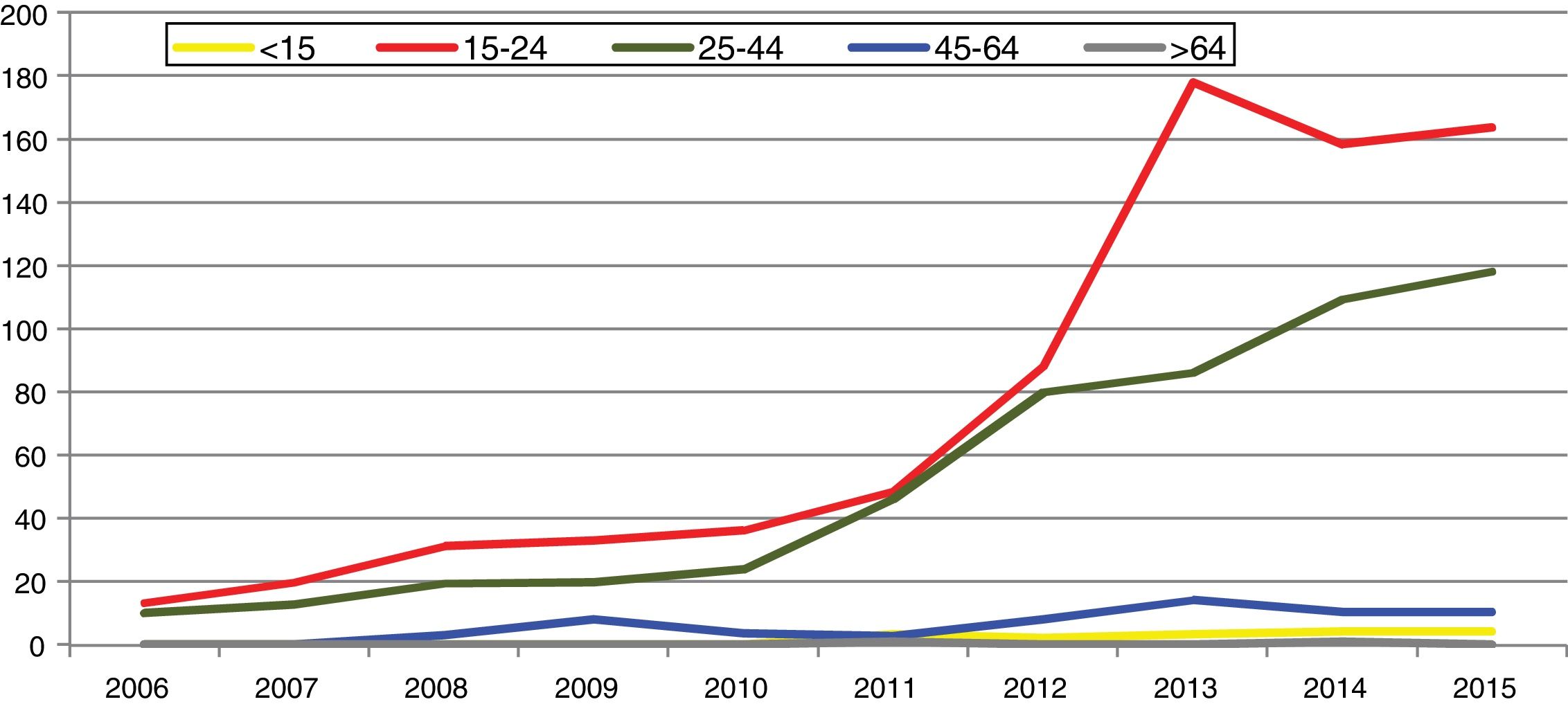
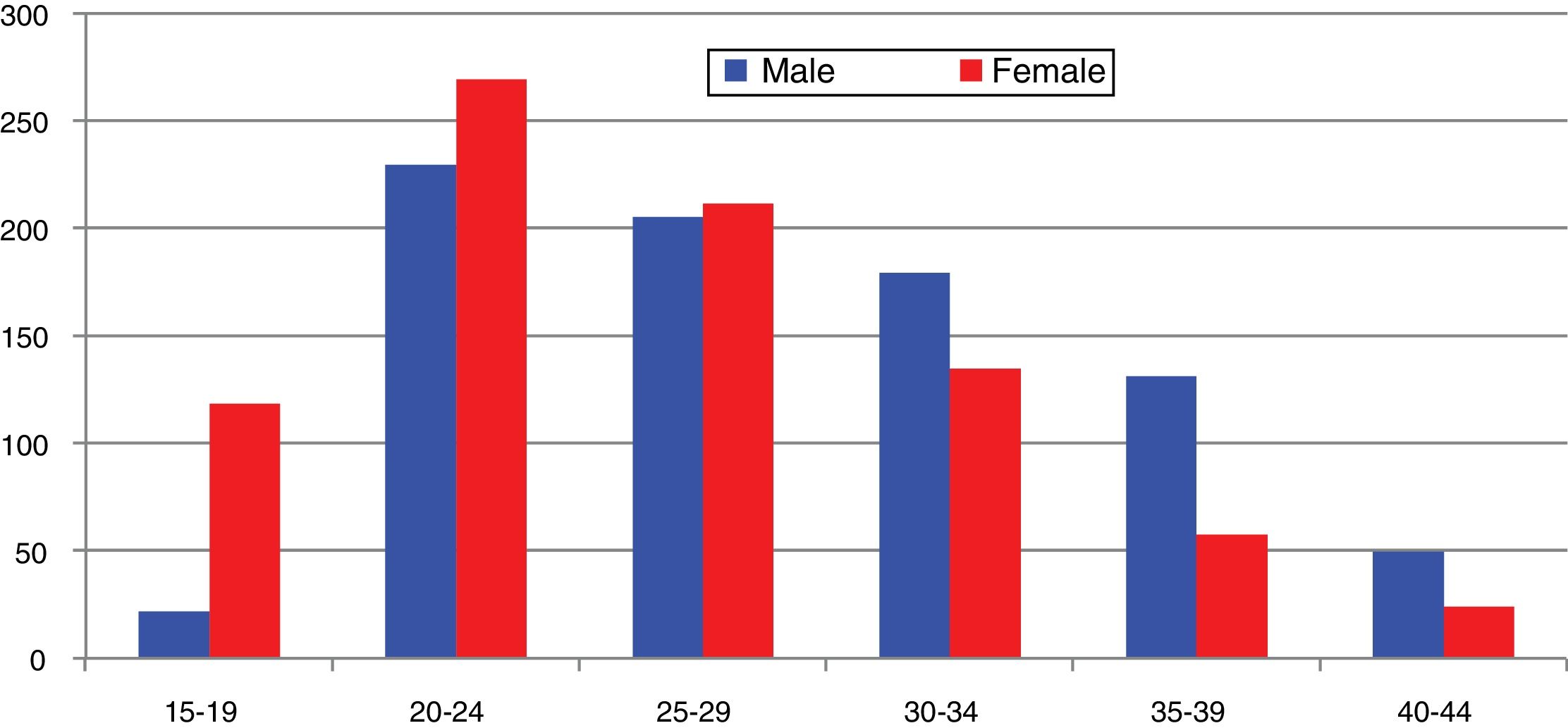
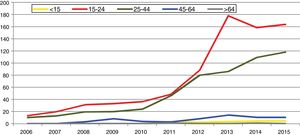
In Spain, reporting of C. trachomatis infection has been compulsory since 2016. This recently published initial report shows a lack of data for some autonomous communities and a low overall reporting rate (15.48/100,000), with significant differences between the reporting communities due to underreporting (≤1/100,000) in several communities.5 Compliance with and improvement of epidemiological surveillance of this infection will allow changes in its incidence to be analysed to ensure that information obtained is comparable between the different autonomous communities and countries. Incidence data recorded in Gipuzkoa over recent years indicate a rising trend, especially among young age groups, with the highest rates reported among women aged 20–24 years (269/100,000) (https://addi.ehu.es/handle/10810/20049, Figs. 1 and 2).
Knowing the prevalence of C. trachomatis infection among the general population, especially in infants, young people and high-risk groups, is essential for designing infection control programs in each geographical area. The prevalence observed in studies conducted in different countries ranges from 1 to 10% and the highest prevalence is reported among sexually active young women (>10% among those under the age of 20–22 years). In Spain, the overall prevalence among mothers from Gipuzkoa (2011–2015) was 1%, with a prevalence of 6.4% among women under the age of 25 and 1.9% among immigrants.6 In another study conducted in Asturias (November 2010–December 2011), the prevalence among young people aged 20–24 years was also high: 4.8% in women and 4.5% in men.7
Other data of interest relating to the epidemiology of C. trachomatis infection are its seasonality (higher diagnostic demand and more positive cases after holiday periods) and its genotype distribution according to sexual behaviour. In line with other European studies, in Spain, genotypes E and F are more common among heterosexual women and men, while genotypes D, G, J and L2 are more common among MSM.8 Despite genotype E being the most common (41%), detection of the new variant of C. trachomatis, nvCt (a genotype E strain with a 377-pb deletion in the cryptic plasmid that emerged in Sweden in 2006), in our country has been anecdotal.
SymptomsDue to the special characteristics of this intracellular microorganism (biphasic division cycle, slow metabolism) and its interaction with the host's immune response, C. trachomatis infection may often go unnoticed (up to 50% of cases in men and 70–80% in women).9 The main clinical manifestations of infection are cervicitis in women and urethritis in men, and symptoms usually start 2–6 weeks after infection. Discharge is normally mucoid and less abundant and purulent than with N. gonorrhoeae infection. In women, dysuria and pollakiuria are uncommon, but these may be present in up to 50% of cases in men. These men may experience pain radiating to the epididymis, while women may experience lower abdominal pain which may be due to pelvic inflammatory disease (PID). Depending on the individual's sexual behaviour, other localised symptoms (pharyngeal, rectal, conjunctival) may be present. Genotypes A, B and C are primarily associated with trachoma (chronic follicular conjunctivitis), although they have occasionally been reported in genital infections.10
Without an accurate, rapid diagnosis, untreated infections (asymptomatic or symptomatic) can easily spread among the sexually active population. Furthermore, in 50% of cases, infections are present for months, promoting the development of reproductive health complications and sequelae as a result of immune response.9 In men, infections can cause prostatitis, vesiculitis, epididymo-orchitis and finally sterility. In women, they can cause endometritis, salpingitis or PID. The risk of PID in women with untreated C. trachomatis infection is estimated to be around 15%.11 The non-specific nature of the symptoms of this disease means that several doctor appointments may be necessary to reach a diagnosis, and some cases may go undiagnosed. PID can lead to sequelae in 10–20% of patients, mainly infertility and ectopic pregnancy.12
C. trachomatis can also occasionally cause HLA-B27-associated reactive arthritis (sometimes accompanied by eye and/or skin symptoms) and perihepatitis. Finally, during gestation, these infections have been associated with premature rupture of membranes, premature labour, low birth weight and miscarriage. Women can infect the newborn as it passes through the birth canal, causing conjunctivitis, nasopharyngitis and pneumonia.13 Postpartum endometritis can also be associated with C. trachomatis infection.
In our experience, infection may often go undetected due to the presence of few symptoms or manifestations shared with other infections. Therefore, we want to emphasise that the value of its diagnosis can be improved greatly if investigation into the usual suspected cases of STIs is combined with an active search for infection in sexually active patients presenting with dysuria with leukocyturia and a negative urine culture (men), abdominal pain possibly of gynaecological origin (probably underdiagnosed PID) and conjunctivitis that does not heal following the usual antibiotic therapy (not only adults but also neonatal conjunctivitis). In studies involving the sexually active population of Gipuzkoa, C. trachomatis was detected in 44% of men with initial signs of urinary tract infection and leukocyturia with a negative urine culture and in 17% of women with signs of PID (https://addi.ehu.es/handle/10810/20049).
DiagnosisAlthough there is a wide variety of methods available for detecting C. trachomatis, NAATs are currently recommended due to their enhanced sensitivity, specificity and fast turnaround time and should replace earlier antigen or cell culture-based tests used by clinical laboratories. Several different commercial NAATs are available, which are generally based on multiplex real-time PCR methods, allowing rapid, simultaneous testing for various STI-causing microorganisms. Different types of samples, based on the site of infection (urethral, cervical, conjunctival, pharyngeal, rectal swabs, etc.), can be used for diagnosis purposes, although some are not validated in all commercial tests. Thanks to the high sensitivity of NAATs, in cases where urethral or cervical swabs cannot be obtained, or for screening, other samples that can easily be obtained by the patient, such as self-obtained vaginal or urine samples (first 10–30ml without the patient having urinated for at least 2h) can be used, with no significant differences in sensitivity or specificity observed.14 The latest NAATs use specific primers and probes against 2 different targets, which allows both the wild-type strain and the Swedish variant, nvCt, and often also plasmid-free strains to be detected (one platform detects the 2 targets in the cryptic plasmid, and therefore the 1% of plasmid-free strains cannot be identified).
The need for rapid diagnosis of C. trachomatis infection in order to treat the individual case and prevent the infection from spreading means that the sites treating these patients must have a rapid response laboratory equipped with NAATs where it can send the samples or a point-of-care (POC) test to be able to quickly and appropriately respond to at least the most symptomatic cases. Earlier POC tests, based on immunological methods (detection of lipopolysaccharide), had poor sensitivity and specificity. POC tests based on molecular methods that detect C. trachomatis and N. gonorrhoeae are currently available, but at a high cost. The benefit and cost-effectiveness of using these tests properly will encourage the development of new POC tests that will include other causes of STI.15
Finally, other more specific molecular techniques, such as genotyping by sequencing of the ompA gene which encodes the major outer membrane protein (MOMP) or multi-locus sequence typing which allows for greater discrimination and differentiation between strains, may help increase knowledge regarding the molecular epidemiology of C. trachomatis infection.8,10
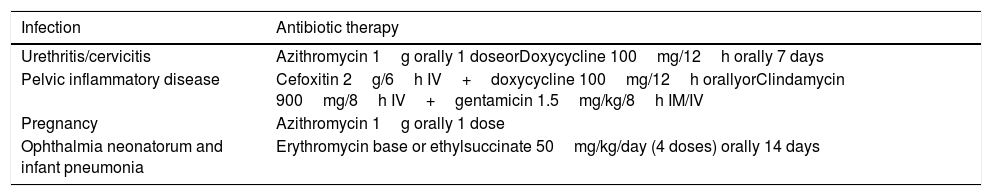
TreatmentStandard guidelines for the treatment of uncomplicated lower genital tract infections recommend a single dose of azithromycin or two daily doses of doxycycline for 7 days (treatments recommended in other situations are also shown in Table 1). Both regimens have microbial cure rates higher than 95% in C. trachomatis, and although the doxycycline regimen may be somewhat more efficacious, compliance is lower. Treatment with doxycycline for 7 days (one 200mg dose/day) might be as effective and safe as the usual regimen (100mg/12h) and improve treatment compliance.2 Other alternatives, such as erythromycin and levofloxacin, are associated with a higher rate of adverse effects.
Recommended antibiotic therapy regimens for C. trachomatis infections.
| Infection | Antibiotic therapy |
|---|---|
| Urethritis/cervicitis | Azithromycin 1g orally 1 doseorDoxycycline 100mg/12h orally 7 days |
| Pelvic inflammatory disease | Cefoxitin 2g/6h IV+doxycycline 100mg/12h orallyorClindamycin 900mg/8h IV+gentamicin 1.5mg/kg/8h IM/IV |
| Pregnancy | Azithromycin 1g orally 1 dose |
| Ophthalmia neonatorum and infant pneumonia | Erythromycin base or ethylsuccinate 50mg/kg/day (4 doses) orally 14 days |
Source: Workowski et al.2
After starting treatment, patients must abstain from sexual intercourse for 7 days. Test-of-cure is not routinely recommended if the index case has been adequately treated. However, it is recommended in pregnant women and when therapeutic compliance is in question, reinfection is suspected or symptoms persist. When a test-of-cure is performed, NAATs must be used 3–5 weeks after completion of treatment since nucleic acids from C. trachomatis may persist in cells for up to 3 weeks. Nevertheless, as reinfection is common among people with a previous C. trachomatis infection, retesting approximately 3 months after completing treatment is recommended.2
Reinfection is the main cause of treatment failure and no genetic mutations that confer stable antibiotic resistance phenotypes have been documented. Since C. trachomatis is an obligate intracellular bacterium, it is unlikely to accommodate horizontally acquired resistance genes from other bacteria. There are only a few studies on antimicrobial susceptibility in C. trachomatis, with limited isolates from resistant strains: strains (n=8 clinical isolates) with decreased susceptibility to azithromycin and doxycycline in patients with recurrent infections16 and identified mutations in a 23S rRNA gene of strains with phenotypic resistance to macrolides (n=4 clinical isolates)17 and in strains exposed to laboratory conditions at sub-inhibitory concentrations of macrolides18 have been reported. These strains mostly present characteristics of heterotypic resistance, affecting <1–10% of the potentially resistant bacterial population (probably due to both their reticulate bodies and intracellular shape), and often disappear with propagation of the bacteria (possibly due to reduced bacterial fitness), but might encourage treatment failure in patients with a high Chlamydia burden.19
The need to monitor the development of resistance in C. trachomatis is hindered by the lack of standardised antimicrobial susceptibility tests, which currently require cell culture. New studies in select cases with treatment failure designed to test both phenotypic antibiotic susceptibility using cell culture techniques and, if resistance is found, possible associated genetic mutations identifiable using molecular techniques from the direct sample, may be necessary.
PreventionIn addition to being easily treatable with antibiotics, C. trachomatis infection is also preventable. Given that an effective vaccine has not yet been developed, and considering the reported increase in high-risk sexual behaviour among certain groups of the population, it is necessary to improve primary prevention through health information and educational campaigns on safe sex (regular and correct use of condoms) in order to control the incidence of infection. Such campaigns are essential to increase awareness of STIs among the sexually active population.
Secondary prevention by opportunistic or systematic screening is still the most important intervention for limiting the adverse effects of C. trachomatis infection on reproductive health.20 The US Centers for Disease Control and Prevention recommend screening all sexually active women <25 years and older women at increased risk for Chlamydia on an annual basis (pregnant women during the first and third trimester).2 Screening in men has not proven to be cost-effective and, although this practice could prevent many infections in women, its impact on disease burden in women as a result of testing young men or specific high-risk groups is controversial.
In order to be able to reduce transmission, such screening programs should cover 80% of the population, with figures >70% acceptable.21 Unfortunately, coverage does not tend to exceed 60% and therefore strategies offering confidentiality, simplicity and a perceived health benefit are necessary to improve acceptance among the target population. Studies with mathematical models show that screening reduces the prevalence of infection.22 However, the effectiveness of screening cannot be measured only in terms of a reduction in infection prevalence (it may even increase with adequate coverage upon detecting new cases) but also in terms of the prevention of longer-term complications.23 Screening programs have actually been shown to reduce rates of PID among women.2,9 It is currently estimated that, optimally, retesting should be performed at least once a year or with every partner change. Consequently, prompt diagnosis and treatment (tertiary prevention) help reduce the duration of the infection.
In various countries, such as the US, Canada, Australia, Japan, Taiwan, South Korea, United Kingdom, Belgium, Luxembourg, Denmark, Estonia, Iceland, Lithuania, Norway and Sweden, different screening policies and strategies have been used or are currently in use, partly due to differences in local epidemiology and disease burden, although in some countries these efforts are not enforced nationally and are only in use in specific regions. Screening in pregnant women improves infection control since it includes a substantial proportion of the target population, helps achieve better coverage since it is perceived as beneficial, helps avoid pregnancy-related (and postnatal) complications due to the infection and prevents perinatal transmission to the neonate.2
Lymphogranuloma venereumC. trachomatis genotypes L1–L3 cause a disease known as LGV, characterised by invasion and inflammation of the lymphatic tissue, unlike all other genotypes, in which inflammation is limited, almost exclusively, to the infection site. As a result of the outbreak described in the Netherlands among MSM caused by the new L2b variant, there has been a permanent review of the epidemiology, symptoms and treatment of LGV cases since 2003.
EpidemiologyPrior to 2003, the epidemiology of LGV indicated that this disease was uncommon in developed countries but prevalent in tropical developing countries. Therefore, most of the cases described in Europe were sporadic import cases. However, imported outbreaks associated with troop movements from areas where LGV cases were common were first described during the last century.24 In 2003, a new sporadic LGV case affecting a bisexual male was described.25 However, 92 more cases were diagnosed in just 17 months, becoming the largest outbreak of LGV reported in Europe.26 Similar cases were soon reported in other European countries, such as Germany, France or the United Kingdom, and the disease was gradually reported in practically all European countries. According to the latest ECDC report, more than 2000 cases were reported in 22 countries.27 The countries that detected the first cases of LGV (the Netherlands, France and the United Kingdom) currently account for 86% of all cases. However, the ECDC figures are underestimated since some countries, like Spain, have not reported any cases. The current outbreak is still very much out of control and LGV infection could become a permanent problem for the European population. Several epidemiological factors make its eradication particularly difficult. Firstly, greater bacterial diversification has been observed over the years. The current outbreak was first associated with a unique variant designated L2b. The co-circulation of 2 variants, L2 and L2b, was described for the first time in Spain28 and, as the outbreak has gradually progressed, new variants which are transmitted very well have been selected, leading to a complex epidemiological scenario.29 Second, the current outbreak was initially associated with a well-defined epidemiological pattern, the MSM population and rectal infection. Although this pattern is still very typical, LGV variants have progressively been detected at other sites, such as the urethra, pharynx and cervix. Third, the purely European outbreak has continued to spread to other geographic regions.
What has caused this outbreak to spread when others in the past did not? Arguments put forward include the atypical presentation of the infection, the fact that the infection mimics other non-infectious conditions, such as Crohn's disease, or the high percentage of asymptomatic infections.30,31 All of these arguments result in diagnostic delay and therefore more opportunities for transmission. Most of the cases described in men are rectal infections with proctitis, while the most classic symptom of the infection is actually inguinal adenopathy (buboes). However, prior to 2003, rectal infections were described in >25% of cases, but in women.24 On the other hand, diagnostic mimicry between LGV infection and other bowel diseases was also described prior to the current outbreak.30,32 Finally, the high proportion of asymptomatic LGV detected cast doubt on whether the new diagnostic platforms were specific enough to identify LGV33 since infection had to meet specific symptoms. However, 40% of cases were asymptomatic prior to the implementation of molecular techniques.24 Causes that may explain the adaptive success of the genotype associated with the current outbreak are unknown but do not appear to be related to biological and genetic features of the bacterium. The causes appear to be more closely related with changes in sexual behaviour, denser networks between individuals and improved diagnostic capabilities due to the generalisation of molecular techniques.
SymptomsInfection caused by LGV-associated genotypes has three stages. The primary infection, which tends to appear 3–15 days after exposure to the source, is characterised by painless genital ulcers that resolve spontaneously, which may be compatible with other STIs, such as syphilis or HSV-2. The second stage, which starts 2–6 weeks later, is characterised by invasive spread of the microorganism to the lymph nodes closest to the site of primary infection (usually the inguinal or femoral lymph nodes). Involvement of the inguinal lymph nodes is associated with the characteristic classic presentation of buboes in males, which are often unilateral and painful, while an anorectal syndrome is characterised by an inflammatory mass in the rectum with a wide variety of symptoms, ranging from no symptoms at all to rectal ulcers, lower abdominal pain, bleeding and tenesmus, accompanied by fever, malaise and weight loss. If the infection persists, haemopurulent proctocolitis appears, which is why it is sometimes confused with inflammatory bowel disease. The chronification of LGV infection produces fibrosis that can cause lymphatic obstruction (stenosis) of the anogenital or genital tract (elephantiasis).
As mentioned in the previous section, there have been reports of infections caused by LGV-associated genotypes in extragenital sites, such as oropharyngeal infection with ulcerative glossitis and secondary cervical lymphadenitis, which are associated with oral sex,34 although most cases are asymptomatic. One case of supraclavicular lymphadenopathy secondary to a case of pneumonitis has also been reported. Reactive polyarthropathy, with or without conjunctivitis, has been observed in several cases, with the wrist, knee, ankle or elbow being the most affected joints.
DiagnosisClinical limitations that lead to diagnostic delay have been described in the previous sections (high proportion of asymptomatic patients, confusion with other inflammatory diseases, atypical presentation, etc.). Let's now look at laboratory limitations affecting molecular detection of LGV-associated genotypes.
Firstly, there is no FDA-validated system for specific molecular detection of LGV-associated genotypes. Although there may be CE-marked platforms for the specific detection of LGV genotypes, these are limited to ulcers as part of multi-target strategies that do not include C. trachomatis. Except as described above, C. trachomatis genotyping to identify invasive genotypes (L1–L3) is always performed after initial detection of C. trachomatis in the sample tested. Only a few research groups have developed their own systems for the detection of invasive genotypes in samples using positive amplification tests for C. trachomatis. This limited availability of NAATs further delays LGV diagnosis. These diagnostic strategies are based on the intragenic deletion of 36pb of the pmpH gene.35 The strategy based on a 9-bp deletion in pmpH to differentiate between the L2/L2b variants has failed.33
Is it necessary to identify the LGV genotypes in all C. trachomatis-positive cases? At present, 10–20% of all C. trachomatis-positive cases among MSM and/or individuals who practise anal sex correspond to LGV-associated genotypes; therefore, genotyping should be recommended in all samples in which C. trachomatis is detected. In this scenario, different types of samples can be used: primary anogenital lesion (ulcer exudate), rectal swab (when anorectal LGV is suspected), bubo aspirate (when inguinal LGV is suspected) or urine when inguinal lymphadenopathy is suspected. However, the low prevalence of infections at other sites is not currently cost-effective and this would be limited to specialised centres. Nevertheless, differentiating the different L2/L2b variants or other highly transmissible L variants has no clinical impact since it does not affect the therapeutic decision and differentiation is only of epidemiological interest.
TreatmentThere are various treatment options for eradicating LGV-associated genotypes. Treatment with 100mg of doxycycline every 12h for 21 days is generally the treatment of choice. The second widely accepted option is azithromycin (first choice in pregnant women) 1g weekly for 21 days. In the few examples of treatment failure, moxifloxacin was used (400mg/day for 21 days) with good results. The recommended 21-day treatment regimen, compared to 7 days, which is the standard treatment for non-LGV genotype infections, is based on a meta-analysis published in 200736 but confirmed in an experimental study in 2009.37 However, the 3-week treatment to eradicate LGV38 was questioned in 2015 and a trial involving 7–14 day treatments with doxycycline was carried out in 2018, achieving bacterial eradication rates similar to those observed with conventional treatment.39 The use of shorter doxycycline-based treatments could mitigate adverse gastrointestinal effects, such as dyspepsia or nausea. Other studies have compared the efficacy of doxycycline and azithromycin to eradicate the microorganism. In rectal infections, doxycycline has better eradication rates than azithromycin (95.5% and 78.5%, respectively), while in genital samples, the two antibiotics are equally effective (95.5% and 93.5%, respectively). There is less experience with moxifloxacin than with the other two drugs. Although it shows very high intrinsic activity, LGV-associated genotypes can develop in vitro resistance to quinolones.
Antibiotic resistance in invasive C. trachomatis genotypes has been described several times40; however, there is no genetic evidence of such failures and recent studies indicate that this failure potential may be more closely related to high bacterial loads and slower bacterial eradication.41
PreventionIn these infections which have a high proportion of asymptomatic individuals, there is a direct relationship between the screening model implemented and the infection rate among the population. Those countries with opportunistic and symptom-based screening have low or very low rates of Chlamydia infections (15cases/100,000), while countries with systematic screening (either by selection of risk or universal groups) have infection rates of about 300–400cases/100,000.42 Secondly, it may be better to establish protocols to identify infections that, due to their asymptomatic nature, may help perpetuate the outbreak. These cases would include rectal infections in MSM or pharyngeal infections (although spontaneous resolution is high). Both models are excellent examples of bacterial reservoir. Therefore, the more generalised the screening and detection of invasive genotypes, the closer we will be to a real view of the LGV outbreak.
Some STI units are choosing to perform screening in pooled samples (pharyngeal, urethral/cervical and rectal samples from the same patient) in individuals with compatible sexual behaviour.
Mycoplasma genitaliumEpidemiologyM. genitalium is an emergent sexually-transmitted pathogen first described in 1980. It is clearly related to non-chlamydial non-gonococcal urethritis in men and has also been associated with urethritis, cervicitis, endometritis and PID in women.43 The reporting of M. genitalium is not compulsory, which limits, to a certain point, knowledge of its epidemiology. Information on the prevalence of M. genitalium may be conditioned by the introduction and improvement of diagnostic techniques, including the incorporation of multiple targets into NAATs. Continued use of azithromycin to treat C. trachomatis and N. gonorrhoeae infections may also have influenced the epidemiological history of M. genitalium. According to pooled data published in the European guideline on M. genitalium infections, it is estimated that this infection contributes to 10–35% of non-chlamydial non-gonococcal urethritis in men.44 The prevalence of M. genitalium is increasing, especially among high-risk groups, such as MSM with or without HIV. In one study conducted in HIV-infected MSM in the US, published in 2018, the prevalence of urethral and rectal M. genitalium was 10.8% and 6.4%, respectively.45 There are few population-based studies, with some methodology-related limitations, in which M. genitalium is detected in 1–3% of the general population.44 However, this figure could be unreliable since asymptomatic infections (people who are carriers) may be more common in STI centre users. In Spain, studies based on series of cases diagnosed at STI centres and A&E departments have been published. Prevalences of 9% and 13% in men and women, respectively, were reported in Barcelona in 2014.46 However, in Madrid, incidence rates were 6.6–0.96% in men and women, respectively, in 2015.47 One study conducted with samples from different departments in Donostia in 2014–2017 found an incidence rate of 3.9%.48 One noteworthy fact, however, is the high frequency of infection found among the asymptomatic population treated at an STI centre in Barcelona in 2017: in a total of 1403 samples taken from different anatomical sites as part of a pilot study, a prevalence of 7.4% was detected.49 These data, although influenced by the selection of cases, indicate the possible impact of M. genitalium in our country.
In addition to its pathogenesis, treatment of M. genitalium is a challenge due to the development of antimicrobial resistance. Macrolide resistance rates vary significantly among different geographical areas. In those regions where a single dose of azithromycin 1g is used for the treatment of non-gonococcal urethritis, resistance is generally found in 30–45% of the isolates.44 In our country, macrolide resistance rates of 16–35% have been reported.46–48 Resistance is higher among men than among women (22.2% vs. 4.6%) and among MSM compared with heterosexual men (32.5% vs. 17.6%).48 With regard to fluoroquinolone resistance rates, values of 8% have been reported in our country.46,48
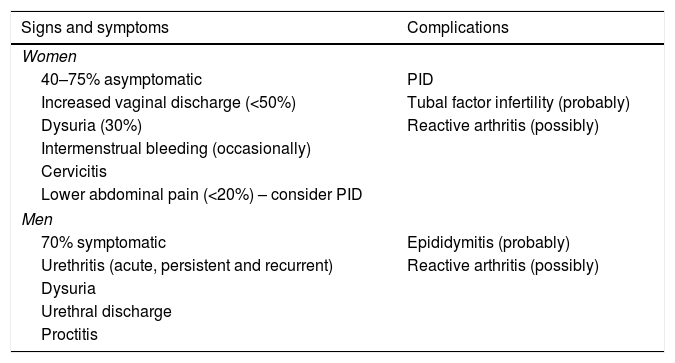
SymptomsTransmission is primarily by direct genital-genital and also genital–anal mucosal contact. Clinical features in women and in men are summarised in Table 2. It is estimated that M. genitalium infections are less symptomatic in women than in men. In women, due to urethral and cervical involvement, the main symptoms are leukorrhoea, dysuria and intermenstrual or postcoital bleeding. PID should be suspected in the event of lower abdominal pain. In men, the main symptom is subacute urethritis, accompanied less commonly by dysuria, discomfort and mucosal or mucopurulent, or occasionally purulent, urethral discharge. M. genitalium is probably the causative agent involved most commonly in persistent (>21 days) and recurrent urethritis, which is related to the absence of systematic detection and to baseline empirical treatment of urethritis, especially when azithromycin is used.1 Although its link to proctitis has not been established, M. genitalium was detected in 12% of MSM with symptoms of proctitis.50
Clinical features of M. genitalium infection in women and in men.
| Signs and symptoms | Complications |
|---|---|
| Women | |
| 40–75% asymptomatic | PID |
| Increased vaginal discharge (<50%) | Tubal factor infertility (probably) |
| Dysuria (30%) | Reactive arthritis (possibly) |
| Intermenstrual bleeding (occasionally) | |
| Cervicitis | |
| Lower abdominal pain (<20%) – consider PID | |
| Men | |
| 70% symptomatic | Epididymitis (probably) |
| Urethritis (acute, persistent and recurrent) | Reactive arthritis (possibly) |
| Dysuria | |
| Urethral discharge | |
| Proctitis | |
Adapted from Jensen et al.44
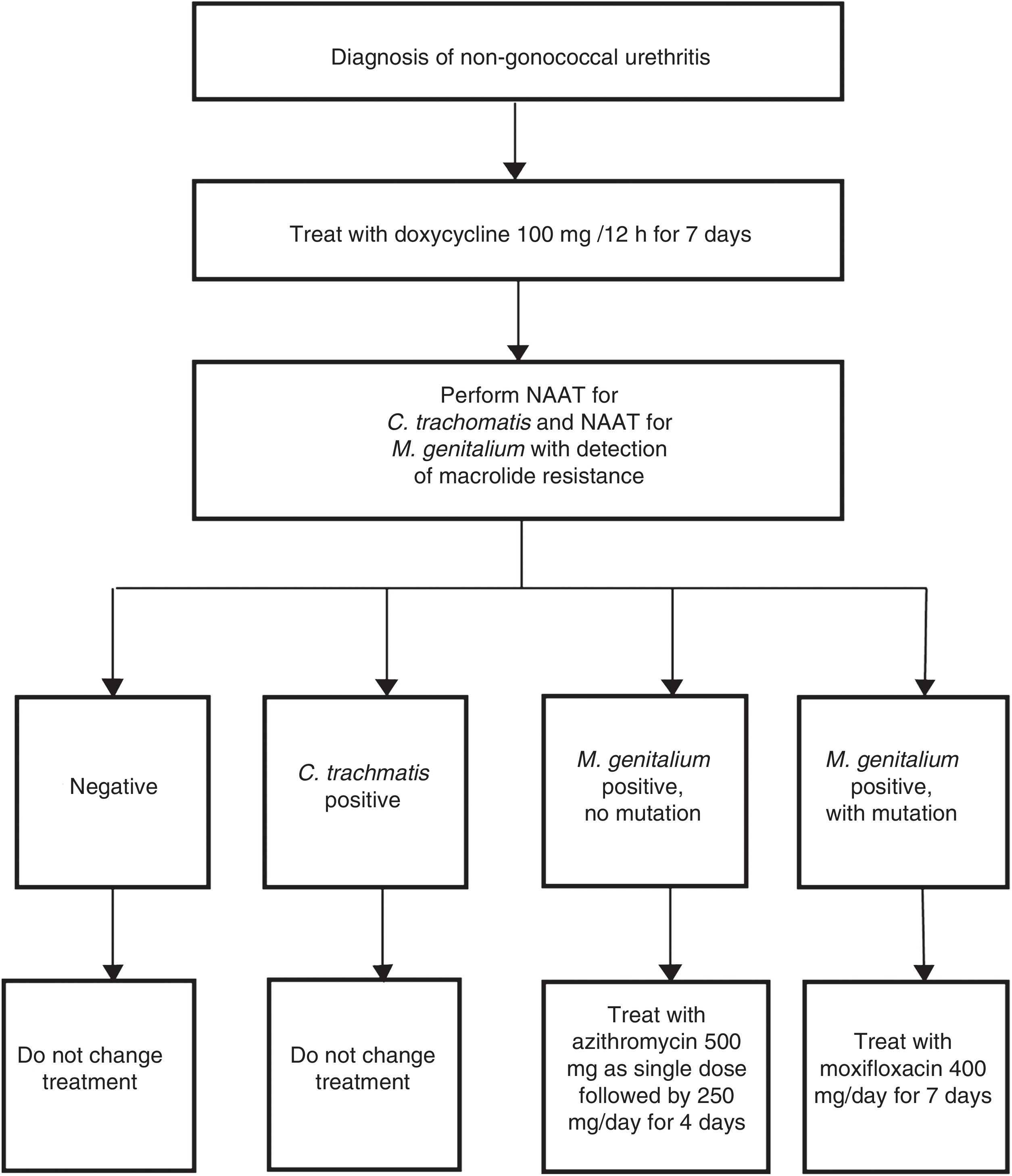
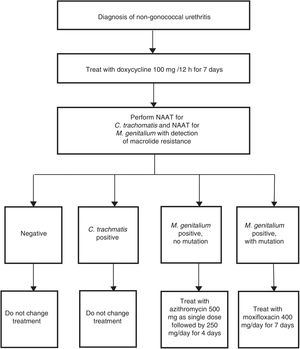
Indications for the detection of M. genitalium are for those patients with the symptoms mentioned in Table 2 and their sexual contacts.44 In these cases, if an STI is suspected, it is necessary to highlight the importance of systematic detection of M. genitalium, especially in cases of persistent or recurrent symptoms. The type of sample and the procedures used are the same as those used for the detection of C. trachomatis. The characteristics of this bacterium (no cell wall and a small genome) make it difficult and slow (weeks) to grow in culture media. Therefore, NAATs that identify M. genitalium DNA are the only useful techniques for diagnosis of this infection. It is recommended that the laboratory be asked about sample processing-related aspects and the features of the technique used, whether this be a commercial or in-house method. Furthermore, due to the high antimicrobial resistance rates reported, a rapid genotyping assay for macrolide susceptibility testing is recommended in order to be able to prescribe targeted treatment in positive cases.44 Both in-house48 and commercial51 techniques are available for this purpose. Implementation of these methods is conditioned by the susceptibility of the technique and physical aspects of the laboratory. Australia is one example where this strategy has been routinely adopted (Fig. 3).52
Example of diagnostic algorithm for the treatment of non-gonococcal urethritis including targeted treatment of M. genitalium based on macrolide susceptibility. Adapted from Australian STI Management guidelines.52
Azithromycin is highly effective for the treatment of macrolide-susceptible M. genitalium. Nevertheless, mass use of a single dose of 1g of azithromycin for the management of non-gonococcal urethritis has led to increased reports of treatment failure in M. genitalium, associated with the development of mutations at positions 2058 and 2059 in domain V of the 23S rRNA gene. Although the cure rate for M. genitalium with doxycycline is 35%, this treatment may help decrease bacterial load and make subsequent treatment with the recommended azithromycin regimen more effective once M. genitalium has been detected. The recommended treatment regimen2,44 adopted by the SEIMC [Spanish Society for Infectious Diseases and Clinical Microbiology]53 is oral azithromycin 500mg as a single dose on day one followed by 250mg/day for 4 consecutive days, with oral moxifloxacin 400mg a day for 7–14 days used as an alternative or in cases of recurrence or complicated infection. The third-line antibiotic therapy is pristinamycin 1g/6h for 10 days or doxycycline 100mg/12h for 14 days.44 In addition to macrolides, M. genitalium may be resistant to other antibiotics and therefore the treatment of multi-resistant strains may become more complicated. Post-treatment testing at 3 weeks is indicated in all cases.
PreventionTreated patients must abstain from sexual intercourse during treatment until their sexual contacts have been tested, symptoms have disappeared and post-treatment testing is negative. All sexual contacts of the index case from the last three months must be tested. In addition to preventing transmission, the aim of treatment is to prevent reinfection and detect persistent infections (due to the possible development of resistance during treatment or selection of minority strains that are initially carriers of resistance-associated mutations). The detection of other STIs must also be included in diagnosed cases of M. genitalium infection.
Unlike other STIs, the need for systematic screening for M. genitalium in asymptomatic individuals has not been established as a preventive strategy. Although M. genitalium has a similar prevalence to C. trachomatis, screening cannot be routinely recommended. Little is still known about the natural history of M. genitalium, which causes doubts regarding the effectiveness of screening and also represents a problem due to increased resistance and the toxicity of treatment.46,54,55 Likewise, the identification of asymptomatic individuals in order to treat them and test their sexual contacts is not recommended.56
FundingSome of the data reported in the C. trachomatis section are from a study partially funded by a grant received from the Fondo de Investigación Sanitaria (FIS PI10/02191).
Authors’ contributionsIntroduction: Luis Piñeiro, Juan Carlos Galán and Martí Vall-Mayans; Chlamydia trachomatis: L. Piñeiro; Lymphogranuloma venereum: J.C. Galán; Mycoplasma genitalium: M. Vall-Mayans.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Piñeiro L, Galán JC, Vall-Mayans M. Infecciones por Chlamydia trachomatis (incluye linfogranuloma venéreo) y Mycoplasma genitalium. Enferm Infecc Microbiol Clin. 2019;37:525–534.
Note: Section accredited by Consell Català de Formació Continuada de les Professions Sanitàries. See questions on each article at http://www.eslevier.es/eimc/formacion.