Infections caused by Cryptococcus neoformans are a major cause of fungal mortality in HIV-infected/AIDS patients and in those receiving organ transplants. We evaluated the in vitro activity of tacrolimus and cyclosporine in combination with amphotericin B and fluconazole against C. neoformans.
MethodsMICs were determined against a total of 30 clinical isolates of C. neoformans by the microdilution method following the CLSI M27-A3 guidelines and by the checkerboard method.
ResultsTacrolimus and cyclosporine A showed in vitro activity against cryptococcal isolates. The combination of amphotericin B with cyclosporine A or tacrolimus was synergistic against 90% and 30% of isolates, respectively. Synergism was also observed with the combination of fluconazole with cyclosporine A or tacrolimus, against 70% and 20% of isolates, respectively.
ConclusionsThe synergistic interactions between the calcineurin inhibitors and antifungal drugs against C. neoformans isolates, could potentially have a role in devising novel therapeutic strategies for this opportunistic mycosis.
Las infecciones causadas por Cryptococcus neoformans son la principal causa de mortalidad por hongos en pacientes con infección por el VIH/SIDA o en pacientes trasplantados. Evaluamos la actividad in vitro de tacrolimus y ciclosporina A en combinación con anfotericina B y fluconazol frente a C. neoformans.
MétodosSe determinaron las CMI de ciclosporina A y tacrolimus frente a 30 aislados clínicos de C. neoformans mediante microdilución, según el documento CLSI M27-A3 y por el método del tablero de ajedrez.
ResultadosTacrolimus y ciclosporina A mostraron actividad in vitro frente a C. neoformans. La combinación de anfotericina B con ciclosporina A o tacrolimus fue sinérgica frente al 90 y 30% de aislados, respectivamente. Se observó sinergismo con fluconazol y ciclosporina A o tacrolimus, frente al 70 y 20% de aislados, respectivamente.
ConclusionesLa actividad sinérgica entre inhibidores de la calcineurina y antimicóticos frente a C. neoformans podría ser una nueva estrategia terapéutica para esta micosis.
Cryptococcus neoformans is a fungal pathogen that causes disease mainly in immunocompromised patients, such as human immunodeficiency virus (HIV)-infected/AIDS patients or these receiving organ transplants.1 The availability of antiretroviral therapy has reduced HIV-related mortality; however, deaths due to cryptococcal meningitis are still causing 15% of all HIV-related deaths globally.2 The initial induction treatment for cryptococcal meningitis includes amphotericin B (AMB) combined with flucytosine (5-FC), followed by an azole maintenance therapy.3,4 Although azoles and AMB are currently acceptable therapies for patients with cryptococcal meningitis, the success of these treatments remains suboptimal and Cryptococcus spp. could develop resistance to fluconazole (FLZ).5 Based on this, new therapeutic options are necessary for the treatment of cryptococcal infections. Calcineurin signaling is a highly conserved pathway in eukaryotic microbial pathogens and controls essential virulence traits.6 Compounds inhibiting calcineurin are immunosuppressive drugs, such as tacrolimus (TC) and cyclosporine (CyA), that are commonly used in transplant recipients for immune tolerance of the graft.7 Therefore, the calcineurin cascade could be an attractive target in drug development against eukaryotic pathogens.4,6 The immunosuppressive drugs possess intrinsic antifungal activity against selected fungi, including yeasts, filamentous fungi and dimorphic fungi.8 Of note, solid organ transplant patients receiving calcineurin inhibitors (CIs) were less susceptible to cryptococcosis than those who were not treated with these compounds.9 Cryptococcal infection occurred after rapidly reducing the dose of TC in an umbilical cord blood transplantation recipient who received micafungin prophylaxis during the early phase of transplantation. In this case, the cryptococcal infection was associated with rapid dose-reduction of TC during the early phase of allogeneic hematopoietic stem cell transplantation.10 Based on this, the aim of this study is to evaluate the in vitro activity of TC and CyA in combination with AMB and FLZ against C. neoformans.
Material and methodsA total of 30 C. neoformans clinical strains, obtained from the fungi collection of the Mycological Research Laboratory, from the Department of Microbiology and Parasitology of the Federal University of Santa Maria, Brazil, were used in this study. The strains were recovered from the cerebrospinal fluid of HIV-positive patients. Isolation and identification of the isolates were performed by standard microbiological and molecular techniques.11 All these strains were previously genotyped and deposited at the Genbank database. Molecular analyses were performed to confirm the identity of the species. A DNA fragment comprising an internal transcribed spacer (ITS) was amplified and sequenced using primers ITS1 (5’-GTAGTCATATGCTTGTCTC-3’) and ITS4 (5’-CTTCCGTCAATTCCTTTAAG-3’) for ITS.12 They were genotyped and registered with Genbank access numbers: KJ654322, KJ654324, KJ817892, KJ817893, KJ654325, KJ817894, KJ817895, KJ654326, KJ817896, KJ817897, KJ817898, KJ817899, KJ817900, KJ817903, KJ845678, KJ654327, KJ654328, KJ654329, KJ817901, KJ817902, KJ634265, KJ690944, KJ6990945, KJ690946, KJ743837, KJ743838, KJ743839, KJ743840, KJ743841, KJ743842. The minimal inhibitory concentrations (MICs) for AMB, FLZ, 5-FC, TC and CyA were determined following the Clinical and Laboratory Standards Institute M27-A3 guidelines.13 AMB (Sigma-Aldrich®, St. Louis, USA), FLZ, 5-FC, CyA (Sigma Chemical Co., St. Louis, MO), TC (Janssen-Cilag Pharmaceutica, Belgium) were obtained as standard powders. AMB, CyA, and TC were diluted in dimethyl sulfoxide, whereas FLZ and 5-FC, were diluted in distilled water to generate stock solutions stored at −20°C. The final concentrations were prepared in RPMI plus 0.2% glucose. The final concentration of DMSO in each well was 0.1% or less and the final concentrations tested ranged from 16 to 0.03μg/mL for AMB, 32 to 0.25μg/mL for 5-FC and 64 to 0.5μg/mL for FLZ, TC, and CyA. All isolates were subcultured in liquid YPD medium to ensure optimal growth characteristics. Stock suspensions were prepared in sterile normal saline and adjusted to yield a final inoculum concentration of 1×106 to 5×106cells/ml. The stock solution was then diluted 1:50 in RPMI culture medium to obtain the final test inoculum (dilution of 1×104 to 5×104cells/ml). Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019 and C. neoformans ATCC 90112 were used as quality control strains. The MICs were obtained after incubation at 35°C during 72h. The interaction between AMB, FLZ, 5FC, TC and CyA was evaluated using the microdilution checkerboard method.14 Drug interactions were defined as synergistic, additive, or antagonistic on the basis of the fractional inhibitory concentration (FIC) index. The FIC index was considered to be the sum of the FICs of each of the drugs and defined as the MIC of the drug used in the combination divided by the MIC of the drug when used alone. Drug interactions were considered as synergistic if the lowest FIC index was ≤0.5; FICI >0.5 to ≤4, indifference; FICI >4, antagonism.15 All tests were performed in triplicate in three different days.
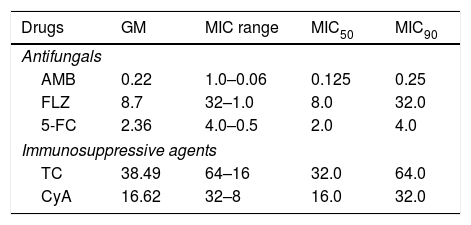
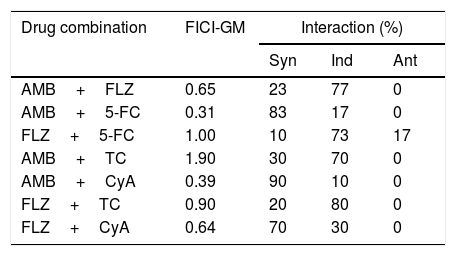
ResultsThe MICs of AMB against the cryptococcal isolates ranged from 0.06 to 1μg/ml (mean, 0.22μg/ml), 5-FC from 0.5 to 4μg/ml (mean, 2.36μg/ml), and those of FLZ from 1 to 32μg/ml (mean, 8.7μg/ml). The MIC of TC ranged from 16 to 64μg/ml (mean, 38.49μg/ml), and for CyA from 8 to 32μg/ml (mean, 16.62μg/ml) (Table 1). The MICs for quality control strains were in agreement with the published ranges for each drug (i.e., AMB: 0.25–2μg/ml; FLZ: 0.12–1μg/ml13). The results of drug combinations are shown in Table 2. The percentages of synergism observed were as follows: AMB+FLZ 7/30 (23%), AMB+5-FC 25/30 (83%), and 5-FC+FLZ 3/30 (10%). To note that an unexpected percentage of antagonism was observed for FLZ+5-FC (17%). Synergistic activity of AMB plus TC was found against 9 (30%) of the 30 isolates, and plus CyA against 27/30 (90%). AMB interactions with these immunosuppressants were additive for the remaining isolates. A synergistic interaction of FLZ+TC was found for 6/30 isolates (20%) and 24/30 (80%) showed additive interactions. For the combination CyA+FLZ the synergism was observed for 21/30 (70%). The remaining isolates (9/30; 30%) demonstrated additive interactions.
Minimum inhibitory concentrations (MICs) of antifungal and immunosuppressive agents against C. neoformans (μg/ml).
| Drugs | GM | MIC range | MIC50 | MIC90 |
|---|---|---|---|---|
| Antifungals | ||||
| AMB | 0.22 | 1.0–0.06 | 0.125 | 0.25 |
| FLZ | 8.7 | 32–1.0 | 8.0 | 32.0 |
| 5-FC | 2.36 | 4.0–0.5 | 2.0 | 4.0 |
| Immunosuppressive agents | ||||
| TC | 38.49 | 64–16 | 32.0 | 64.0 |
| CyA | 16.62 | 32–8 | 16.0 | 32.0 |
MIC50 and MIC90, MIC at which 50% and 90% of the isolates tested were inhibited, respectively; GM, MIC geometric mean; AMB: amphotericin B, FLZ: Fluconazole; 5-FC: 5-Flucytosine; TC: tacrolimus, CyA: cyclosporine A.
Fractional inhibitory concentration index (FICI) and geometric mean (GM) of the interactions of drugs against clinical isolates of C. neoformans.
| Drug combination | FICI-GM | Interaction (%) | ||
|---|---|---|---|---|
| Syn | Ind | Ant | ||
| AMB+FLZ | 0.65 | 23 | 77 | 0 |
| AMB+5-FC | 0.31 | 83 | 17 | 0 |
| FLZ+5-FC | 1.00 | 10 | 73 | 17 |
| AMB+TC | 1.90 | 30 | 70 | 0 |
| AMB+CyA | 0.39 | 90 | 10 | 0 |
| FLZ+TC | 0.90 | 20 | 80 | 0 |
| FLZ+CyA | 0.64 | 70 | 30 | 0 |
Note: Syn, synergism; Ind, indifference; Ant, antagonism.
Infections caused by the fungus C. neoformans have treatment options mainly limited to AMB and FLC or 5-FC.1 Moreover, there is a growing population of drug-resistant cryptococcal isolates.16 Our results show the in vitro antifungal activity of TC and CyA against cryptococcal isolates. Previous in vitro studies have also demonstrated the anti-cryptococcal activity of TC and CyA.7,9 Calcineurin biology has gained significance over the years since it is the target of the immunosuppressive drugs CyA and TC, which inhibit the cellular activity of calcineurin via their interaction with the respective immunophilins, cyclophilin A and FKBP12, proteins required for fungal growth and related to its virulence.4 Specifically, for C. neoformans, the mechanism described for the antifungal activity of CIs is the ability to affect the grow at high temperatures and to inhibit the hyphal elongation during mating and haploid fruiting in fungi.4 Clinical data show that patients receiving a CI agent are less likely to have cryptococcal central nervous system (CNS) involvement and more likely to have infection limited to the lungs.9 Thus, these agents might inhibit fungal calcineurin in strains emerging from the dormant phase and decrease dissemination from lungs and hilar lymph nodes to the CNS.7 In clinical settings, combination therapy has become a potential alternative to treat invasive fungal infections by improving clinical efficacy of existing drugs.1 Studies have shown that CIs, such as CyA and TC, have synergistic interactions when combined with azole antifungal agents, resulting in fungicidal activity.
In this study we observed high percentages of synergism for AMB+CyA (90%) and FLZ+CyA (70%), and an unexpected percentage of antagonism for the combination FLZ+5-FC (17%). This antagonism should be better investigated, since this combination is very useful for the treatment of patients with cryptococcal meningitis. Previously, promising results were observed by combining azoles with CyA.8 A powerful fungicidal effect of the combination of FLZ with CyA has been observed in vitro and in vivo against Candida albicans, C. neoformans, and Candida parapsilosis.8 When tested in vitro against C. parapsilosis, C. albicans, and C. glabrata, CyA presented synergistic interactions with AMB and FLZ.8 Investigators sought to correlate survival in solid organ transplant recipients infected with C. neoformans receiving CIs and the observed in vitro synergy with AMB and FLZ. Overall, in vitro synergy with AMB and FLZ was observed against 90% of the 59 clinical isolates, and patients who received a CI had a significantly higher probability of survival than those who did not receive CI.9,10 In a previous clinical study of C. neoformans infection in solid organ transplant recipients, the 90-day survival rate of recipients who received both an antifungal agent and CIs was higher (91%) than that of those who received an antifungal agent alone (61.5%).7 According to these results, CIs can inhibit growth and virulence of fungi, including C. neoformans.
Based on the increasing incidence of these difficult to treat invasive fungal infections, coupled with the current limited antifungals, the calcineurin pathway is an incompletely explored an attractive novel alternative for antifungal therapy. Although these drugs could not be used at present as antifungal agents in clinical practice, we believe that our findings are valuable. The study of their analogs with low immunosuppressive activity and the mechanism of action would provide deep insight into overcoming antifungal resistance. The limitation of our study consists of the absence of in vivo studies confirming whether these synergistic activities confer a beneficial effect in animal models of cryptococcal infections. Evaluation of the synergistic activity of new antifungal compounds through in vitro susceptibility testing can help to establish guidelines for the potential clinical application of new therapies.
Conflict of interestsThe authors declare that they have no conflict of interest.
Alves SH acknowledge the financial support by Brazilian Agencies FAPERGS (Grant Proc. 2261–12).