*Members of the HIV SOT Working Group at the end of manuscript.
Introduction
Until a few years ago, infection by the human immunodeficiency virus (HIV) was an absolute contraindication to any type of transplant. The prognosis and the fear that transplant-associated immunosuppression could speed up the progression to AIDS or increase the risk of opportunistic infection meant that the measure was ruled out 1. From 1996 on, after the introduction of highly active antiretroviral therapy (HAART), the situation of HIV-infected patients has changed radically, and morbidity due to opportunistic infections and global mortality in AIDS patients has fallen drastically 2,3. This, in turn, has meant that there is now sufficient time for chronic conditions to evolve to terminal failure (hepatic, renal, cardiac), which can only be treated by transplant.
The problem is particularly urgent in the case of chronic liver disease due to the hepatitis B and C viruses. In Spain, there are now estimated to be between 60,000 and 80,000 people co-infected by HIV and HCV and about 5,000-10,000 by HIV and HBV. Approximately 5-7% of Spanish HIV-positive patients are co-infected by HBV and 45-50% by HCV 4-6. Furthermore, terminal liver disease has now become one of the main causes of hospital admission and the first cause of death among these patients 7-12. This problem may be particularly severe in the case of HCV, since progression of liver disease is accelerated in the HIV patient 13 and the response to HCV therapy with interferon and ribavirin are lower than those of non-HIV-infected patients 14-19.
The spectacular improvement in prognosis observed in HIV patients after the introduction of HAART has meant that transplant has now been reconsidered in patients with a terminal heart, liver or kidney disease, so that, at present, HIV infection is no longer a formal contraindication to transplant 20. Fortunately, we have recently been witnessing a change in attitude towards the problem as there is more information on the outcome of HIV infection and more experience in organ transplantation in this population 21-26. Different groups are studying the real need for liver transplant in these patients and are working towards harmonizing criteria and actions in order to optimise this new therapeutic strategy 27,28.
Aware that donor organs are scarce and must be distributed fairly, the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) has tried to clarify the indication of transplant in HIV-infected patients and provide recommendations on post-transplantation management. Therefore, in 2000, the AIDS Study Group (GESIDA) and the Transplant Infection Study Group (GESITRA) began a consensus process which culminated in the approval of this document at the meeting held in Bilbao on 18th May 2004, within the framework of the 11th Congress of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). The general lines of the document are based on the transplant criteria approved in 2001 by the Catalonian Transplant Organization (OCATT) and the AIDS Program of the Health Department of the Generalitat de Catalunya. In the year 2003, the consensus was presented to those in charge of the National AIDS Plan and the National Transplant Organization (Spanish Ministry of Health), to representatives of the different transplant groups, to other scientific societies and some citizens' groups. During the third quarter of 2004, the final document was shown on the web pages of the supporting scientific organizations and, during the last quarter of 2004, the suggestions received, both from healthcare professionals and NGOs in the HIV field, were evaluated. This document does not aim to provide an exhaustive review of the matter at hand, which has already been amply studied in two recent reviews in Spain 6,29, rather it aims to give a brief overview of the subjects agreed on by study groups whose main field is HIV infection. Our objective was neither to establish transplant criteria related to organ dysfunction, nor to provide strict norms on post-transplantation management, which should be drawn up by each group. This consensus is conceived as a basic document for other scientific societies in the transplant field and/or local transplant groups to draw up specific protocols which can make it easy for transplants to be made available to those HIV-infected patients who need one. Finally, this field is evolving continuously and the indications for transplant or management of these patients may change as more evidence becomes available. Therefore, this Committee undertakes to provide periodic updates of this document.
Criteria for including HIV-infected patients on the solid organ transplant list
The consensus criteria are described below (table 1).
Organ condition leading to transplant:
The same criteria as for HIV-negative patients will be used.
HIV infection
Clinical criteria
Ideally, the patient should not have suffered previously from AIDS-defining diseases, since there may be a greater risk of reactivation. Nevertheless, this consensus considers as exceptions tuberculosis, oesophageal candidiasis and Pneumocystis jiroveci pneumonia (previously known as P. carinii pneumonia). Tuberculosis is highly prevalent in Spain and sometimes occurs without marked immunosuppression. Furthermore, its recurrence is rare in correctly treated patients. Oesophageal candidiasis can occur with immunosuppression which is not excessively severe and is sometimes due to concomitant antibiotherapy. Moreover, it is not a life-threatening condition and recurrence is easily controlled. Finally, HIV infection is often revealed by P. jiroveci pneumonia (PCP), which can be treated efficaciously and prevented. Studies on immune restoration with HAART have shown that secondary prophylaxis can be safely withdrawn when the CD4 lymphocyte count is sustained above 200 cells/μL 30,31. In any of these cases, the patient should be evaluated 6-12 months after the PCP episode.
Patients with early-stage dementia-AIDS complex should not be excluded from SOT, because HAART and secondary immune recovery of the immune system control the condition in most cases and prognosis for survival is very good. Similarly, the noticeably improved prognosis thanks to HAART means that some authors are in favour of withdrawing exclusion criteria for other opportunistic infections which can be efficaciously treated and prevented 26,28,32-34. Nevertheless, this committee considers that experience with other HIV-infected opportunistic infections and tumours is still too limited to make any recommendations. In the case of tumours which are not related to HIV infection, the same criteria will be followed as for the general population when evaluating organ transplant.
Immunological criteria
This section has fixed different minimum CD4+ lymphocyte counts depending on whether the transplant is a liver or other solid organ transplant 28,35. This is because patients with chronic liver disease often suffer from lymphopenia due to hypersplenism, which leads to a lower absolute CD4+ count, despite high CD4 percentages and good virological control of HIV. Therefore, it has been agreed that the CD4+ lymphocyte figure should be above 200 cells/μL for any transplant and greater than 100 cells/μL for a liver transplant. However, this committee considers that, in the case of a patient eligible for liver transplant with previous tuberculosis, oesophageal candidiasis or PCP, the CD4 lymphocyte count (at least until more experience is available), should be above 200 cells/μL, due to the additional risk of reactivation.
These cut-offs are reasonable if we take into account the fact that most opportunistic infections appear in HIV patients when the CD4+ lymphocyte count is below 200 cells/ mL 36. Furthermore, experience has shown us that HIV-infected patients with no previous AIDS criteria who underwent liver transplant with a CD4+ lymphocyte count of between 100 and 200 cells/μL did not have a greater risk of opportunistic infections in the post-transplant period 37-39. In the HIV-negative patient who undergoes liver transplant, it has been shown that the risk of opportunistic infections is only on the increase in patients whose CD4+ lymphocyte count is under 100 cells/μL 40 and that the CD4 count does not influence rejection or survival rates 41. Furthermore, the nadir CD4+ (or lowest figure) should not be an exclusion criterion for a transplant candidate. It is generally accepted that, if the patient has managed to increase a low nadir while taking HAART and this response is durable, there is no risk of developing new opportunistic infections.
Virological criteria
The basic criteria for SOT is that the patient must be able to have effective and lasting antiretroviral therapy during the post-transplant period 26,28,33,34,42.
The ideal situation is one in which the patient tolerates HAART before transplant and is ready for the transplant with an HIV viral load in plasma which is undetectable by ultra-sensitive techniques (< 50 copies/mL). Nevertheless, this is not always possible for several reasons:
1.In some patients with end-stage liver disease, it may be difficult to have an undetectable HIV viral load in plasma because they often experience intolerance or toxicity related to antiretroviral drugs 10 and these must be stopped. In these cases, and to avoid resistance, it is better to leave antiretroviral therapy for the post-transplant period.
2.Some patients remain viremic with antiretroviral therapy. In these cases, it is essential to carry out antiretroviral sensitivity testing (genotypic or phenotypic resistance testing) 43,44 to ascertain the real therapeutic options. The evaluating team and HIV experts will evaluate whether the patient has effective and durable rescue therapy.
3.Some patients do not have an indication for HAART as they are long-term non-progressors (LTNP) or do not have immunological criteria (CD4+ lymphocyte count above 350 cells/μL) or clinical criteria to start HAART 42 and, therefore, they have viremia which is detectable in plasma. In this setting, it is unknown whether and when (pre-transplant or post-transplant) it would be beneficial to initiate HAART, in order to reach an undetectable HIV viral load in plasma.
Psychiatric criteria
To be included on a transplant waiting list, a candidate must have a favourable psychiatric evaluation. The patient's ability to understand and comply with the recommendations received will be evaluated, as will any pre-transplant psychiatric condition. Psychiatric evaluation will also be relevant in patients with a past history of drug abuse (heroin, cocaine, alcohol) to detect the presence of previous or current psychiatric alterations. A history of psychiatric illness which has been cured should not contraindicate transplant, at least in absolute terms.
Patients who actively consume drugs will be excluded. This consensus recommends a consumption-free period of two years for heroin and cocaine 45 and six months without addiction for other drugs (cannabis, benzodiazepines, designer drugs and alcohol) 46,47.
In the case of heroin and cocaine, this consensus recommends a consumption-free period of two years, as these patients will be understood to be in sustained total remission 45. Occasionally, these patients can be included on the waiting list before this two-year period is up depending on the individual psychiatric evaluation. Patients who are on stable methadone maintenance programmes must not be excluded from transplant and can continue taking it after the transplant 48. After transplant, some patients may require small modifications to their dose of methadone.
The consumption of cannabis or benzodiazepines should not be an exclusion criterion in itself. Benzodiazepines must be on prescription only.
In the case of alcohol, the highest rate of relapse is observed during the first six months 46,47, and this consumption-free period allows us to see whether liver function improves in alcoholic liver transplant candidates.
Social criteria
As is the case with any transplant candidate, patients must show an appropriate degree of social stability so that follow-up and the necessary minimum care are viable in the post-transplant period.
Special considerations for solid organ transplant in HIV-infected patients
The details set out above, the complexity of HIV transplant patient management and the range of difficulties which may arise mean that SOT in these patients requires a multidisciplinary approac. Therefore, it is very important for centres wishing to carry out transplants in HIV-positive patients to set up a multidisciplinary team which can periodically evaluate these patients during the pre- and post-transplant period. The team must include two representatives from the transplant team (medical and surgical), an infectious diseases specialist with expert knowledge of transplant patients and HIV/AIDS, a psychologist/psychiatrist, an expert on alcoholism and drug dependence, and a social worker.
Pre-transplant period
Criteria for selection of donor
With respect to the type of donor to be used in HIV-infected patients, most solid organ transplants were carried out using cadaver donors 27,35,37-39,49. In recent years, and as a consequence of the increased demand for organs, the number of living donors has increased 50,51. Nevertheless, the benefits of this technique have yet to be demonstrated in the HIV-infected population. In the case of liver transplant, where many patients are HIV-HCV co-infected, HCV re-infection in mono-infected patients is likely to be worse in live transplant recipients than in cadaver transplant recipients 52. Furthermore, this technique is not free from complications in donors, who have up to 15% morbidity and a mortality of at least 0.2% 53. In the case of renal transplant, survival in the medium term (5-7 years) of HCV mono-infected patients who receive a live-donor kidney is similar to that of patients who receive a cadaver transplant 54. Nevertheless, there is little information in this setting on the outcome of HIV-HCV co-infected patients 29. Therefore, at present, this Committee feels that there is not enough experience to enable us to make a recommendation on living donors.
Finally, some authors have proposed the use of other HIV-infected patients as donors. This option must not be admitted due to the possibility of re-infection by HIV after transplant, and the fact that this may be more virulent or generate new resistance problems.
Pre-transplant evaluation of infection in donor and recipient
We recommend following the GESITRA-SEIMC recommendations for non-HIV-infected patients 55,56.
Antiretroviral therapy
Terminal patients in need of a transplant often find it difficult to correctly maintain antiretroviral therapy. This problem is habitual in patients with decompensated cirrhosis, in whom it is often necessary to suspend HAART owing to liver complications or hepatotoxicity secondary to antiretroviral therapy 10. This makes HIV infection more difficult to control, with the subsequent deterioration in immune function and risk of opportunistic infections. The complications associated with terminal illness of an organ often involve treatment interruptions which could favour the appearance of resistance. Therefore, in patients who do not achieve an undetectable viral load in spite of HAART, it is advisable to carry out resistance testing 43,44 to ensure that there are valid treatment options in the post-transplant period. Testing should be carried out while the patient is taking antiretroviral therapy because, in the absence of this type of therapy, the wild-type replaces the mutant virus after a few weeks, as its replicative capacity is greater. Resistance tests would not reflect the patient's mutations.
Waiting-list mortality in End-Stage Liver Disease (ESLD)
Waiting list mortality is particular worrying in the case of liver transplant. We are witnessing a progressive increase in the mean time on the list and, consequently, an increase in waiting-list mortality, since there is no possibility of artificial support. The problem is made worse in HIV-infected patients, since, once the liver disease is decompensated, survival is much lower than in the HIV-negative patient 57,58. In a study by Miró et al, HIV-infected patients with end-stage liver disease had a median survival of 22 months. If the patient had an advanced Child-Pugh stage (B or C) or under 200 total CD4+ lymphocytes, survival fell to seven months. If both factors coincided, survival was four months 57. A similar study 58 found a very low survival (under 9 months) in HIV patients after the first episode of hepatic decompensation. In this study, survival was similar when comparing the pre-HAART era and the HAART era. Other Spanish groups have recently reported similar data 59,60. Recently, García-García et al 61 have shown that the outcome of cirrhosis after the first decompensation in HIV-HCV co-infected patients is much worse than in the mono HCV-infected population. Survival at one, two and five years for both populations was 54/74%, 40/61% and 25/44%, respectively 61.
In order to limit the problem, this Committee recommends physicians attending HIV-infected patients with cirrhosis of the liver to carry out prospectively and jointly with the transplant team an evaluation for transplant after the first clinical decompensation of the liver disease: ascitis, encephalopathy, variceal bleeding or jaundice. Similarly, patients whose cirrhosis is compounded by a hepatocellular carcinoma should also be evaluated.
Ethics
Organ transplantation in HIV-infected patients raises ethical problems which have not yet been completely resolved 62. Organs are scarce and demand increases as the number of indications expands. This has raised questions over who should receive the organ, the patient most in need or the patient who can profit most from it. Even though HIV infection is not considered an absolute contraindication for transplant, some authors think that it is still very experimental and should not be subject to the same transplant protocols as other patients. Nevertheless, the members of this consensus document feel that HIV-infected patients should receive the same treatment as other patients and be included on waiting lists under the same conditions 62.
Post-transplant period
Problems of adherence to therapy
After transplant, patients must receive a large quantity of medication, and this can compromise adherence. In addition to HAART, which they may be accustomed to, they must take immunosuppressors and the habitual prophlaxis against opportunistic infections. Patients on methadone programmes must continue with this and HCV co-infected patients may require therapy with interferon and ribavirin. Therefore, these patients must have a lot of support at all times, and understand the importance of correct adherence to all their treatment schedules.
HAART must be administered again as soon as the patient begins to receive food orally. Regimens will be on an individual basis, but will generally be subject to the general recommendations for antiretroviral therapy in the adult 42. The regimens chosen should have a low potential for pharmacological interactions with immunosuppressors and anti-HCV drugs, lower liver and renal toxicities, and they should be easier to adhere to 6,29,42.
Pharmacological interactions between antiretroviral therapy and immunosuppressors
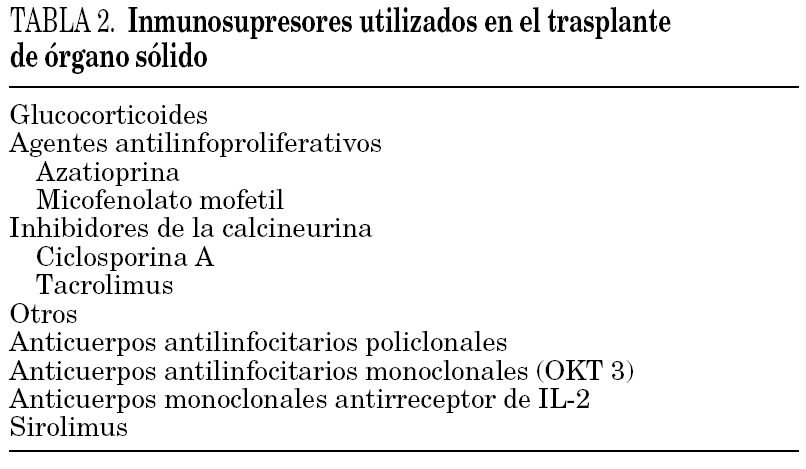
Table 2 sets out the main immunosuppressors used in transplanted patients. There are no specific regimens for HIV-infected patients, and each centre can use the same regimens as for HIV-negative patients. In some studies, the rejection rates were higher than in the HIV-negative population. The cause of this phenomenon is unknown, and it is particularly noticeable in kidney transplants 29.
Many immunosuppressors and antiretrovirals have pharmacological interactions which may occasionally be clinically relevant 42,63-70. Cyclosporine A, tacrolimus and sirolimus are metabolised in the liver using cytochrome P-450, whereas mycophenolate mofetil is glucuronidated in the liver. Antiretrovirals can act as inhibitors or inducers of these enzymatic systems. When they act as inhibitors, they increase concentrations of immunosuppressors and can lead to toxicity. On the other hand, when they act as inducers, they reduce their levels and can trigger rejection. Therefore, it is important to know well the possible interactions and closely monitor the plasma levels of immunosuppressors. Enzymatic induction is a slow process which usually requires days or weeks. Enzymatic inhibition, however, is fast: it occurs in hours and its effects are immediate.
Protease inhibitors act mainly as inhibitors of CYP3A (a component of cytochrome P-450) by increasing the blood levels of cyclosporine, tacrolimus and sirolimus. Ritonavir is the strongest of the protease inhibitors and, even though it is not used alone, it boosts most protease inhibitors 68. There are pharmacokinetic trials with nelfinavir and cyclosporine which have shown how the simultaneous administration of both drugs increases the area under the curve of cyclosporine, making it necessary to adjust the dose (generally, it is necessary to reduce the dose of cyclosporine to 25% of the usual dose). Nelfinavir levels increase quickly but they later stabilise and it is not necessary to modifiy the dose 66,70. In general, we can conclude that, if protease inhibitors are used in an antiretroviral schedule, the doses of cyclosporine, tacrolimus and sirolimus must be reduced, and their levels carefully monitored. The importance of correct adherence to therapy must be stressed for the patient, as not taking medication or uncontrolled modifications can be dangerous 35,38.
Non-nucleoside reverse transcriptase inhibitors also have problems of interaction with immunusuppressors, although they are generally easier to manage 67,69,70,71. Both nevirapine and efavirenz are cytochrome CYP3A inducers (nevirapine is also a CYP2B inducer) and this leads to a reduction in the levels of immunosuppressors (cyclosporine, tacrolimus and sirolimus). This interaction is expressed slowly (days or weeks) and means that drug levels must be monitored. Existing pharmacokinetic studies show that levels of nevirapine are barely modified in the presence these immunosuppressors 71.
Nucleoside and nucleotide reverse transcriptase inhibitors have few pharmacological interactions. In vitro, mycophenolate mofetil antagonizes the antiviral effect of AZT and d4T by inhibition of thymidine kinase. On the other hand, it increases the effects of ddI, abacavir and tenofovir, through inhibition by its metabolite, mycophenolic acid, of an intracellular enzyme involved in dGPT synthesis 72.
There are reports of severe toxicity with antiretroviral therapy in post-transplant patients 73, although this possibility does not justify the reintroduction of HAART after transplant. It is advisable, however, to select drugs with a lower potential for liver and renal toxicities 29,42,74. Given the speed with which new antiretrovirals and immunosuppressors appear and thus generate unknown interactions, physicians are recommended to consult updated databases on interactions (http://www.interaccionesHIV.com) 70.
Effect of transplant and associated immunosuppression on HIV infection
One of the classic fears when considering organ transplant in the HIV-positive patient was that immunosuppressor therapy could lead to progression to AIDS and related death, thus aggravating the prognosis of these patients. It is true that azathioprine has been associated with an increased replication of HIV in vitro and that the use of corticoids can exacerbate opportunistic infections. The use of antilymphocyte antibodies has also been associated with an exacerbation of HIV. Nevertheless, experience to date suggests that the standard immunosuppressor schedule used in solid organ transplant does not increase susceptibility to opportunistic tumours or infections, and there is even evidence to the contrary 23,26,39,75,76. Some immunosuppressors, such as cyclosporine A and tacrolimus, can improve the control of HIV by inhibition of the synthesis of interleukin 2 and therefore the replication of its dependent T cells 26,77. These drugs have also been shown to directly inhibit HIV replication (as does mycophenolate mofetil) 24,26,78,79. Mycophenolate mofetil inhibits HIV reverse transcriptase by inhibiting viral replication 80. Other evidence which supports the fact that immunosuppressor therapy does not worsen HIV evolution is:
1.Immunosuppressors reduce cell-to-cell transmission of HIV.
2.The growth of HIV infected cell lines is inhibited in vitro in the presence of immunosuppressors.
3.Immunosuppressors produce a fall in CD4 molecular expression and apoptosis in HIV-infected cells 79.
During the HAART era, these fears are even more unfounded, since post-transplant antiretroviral therapy controls HIV in most patients.
Opportunistic infections and tumours
There is no evidence that the HIV-infected patient has a greater risk of developing opportunistic infections or tumours after transplant 26,39. Therefore, the same prophylaxis schedules as for HIV-negative patients must be followed. All patients must receive prophylaxis against PCP 81. Periodic surveillance and early diagnosis of these patients is important, especially in the case of infections caused by the herpes family virus (herpes simple and zoster, cytomegalovirus, Epstein-Barr, human herpes virus 6 and human herpes virus 8) and human papillomavirus, since both these entities can modify immunosuppression and play an important role in the development of tumours and in rejecting the graft 26,28.
Relapse of HCV infection
Management of HCV relapse will depend on the type of organ transplant, since each one is managed differently.
In the case of liver transplant, HCV relapse is universal, regardless of whether the patient is infected by HIV or not 23,37,39,71,82-85. At present, it is not known whether relapse is worse in the HIV-positive patient than in the HIV-negative patient 37. Neither is there sufficient experience on the efficacy and safety of therapy with interferon and ribavirin in HIV-HCV co-infected transplant patients 86. Furthermore, the possible interactions between antiretrovirals and interferon and ribavirin must be taken into account 42,86.
In the case of kidney transplant, a series of factors affecting the HCV mono-infected population must be taken into account in HIV-HCV co-infected patients 87:
1.Kidney transplant is the main risk factor for progression of chronic HCV liver disease to cirrhosis, owing to the fact that post-transplant immunosuppressor therapy modifies the natural history of liver disease, activates viral replication and accelerates progression.
2.HCV infection can cause several varieties of glomerulonephritis in kidney recipients, which could affect the survival of the graft.
3.Treatment with interferon is contraindicated in kidney recipients, due to the risk of triggering an acute rejection and/or acute interstitial nephropathy. For these reasons, correct valuation and possible antiviral treatment of chronic HCV hepatitis must be prescribed during dialysis.
4.Post-transplant studies show that HCV-RNA pre-transplant values which become negative are not only accompanied by a better course of chronic liver disease, but also by a lower incidence of post-transplant glomerular diseases. Management of HCV co-infection in HIV-infected patients in dialysis will follow the recommendations made by the Viral Infections in Haemodialysis Working Group of the Spanish Nephrology Society 87.
Finally, and although there is little experience in heart transplant in HCV-infected patients, therapy with interferon is also contraindicated during the post-transplant period and should be carried out before transplant, if there are no contraindications, for the same reasons as in kidney transplant.
Interactions between interferon and antiretroviral drugs. No significant reactions have been reported between interferon and antiretroviral drugs. The combination of efavirenz and interferon has been thought to increase neurotoxicity, and the combination of zidovudine and interferon could increase the risk of myelotoxicity 42,86.
Interactions between ribavirin and antiretroviral drugs. The main problems stem from the combination of ribavirin and other nucleoside analogues used in HIV therapy 42,86. Ribavirin is a guanosine analogue which, in vitro, reduces the phosphorylation necessary for the intracellular antiviral activity of AZT 88 and stavudine (d4T). Nevertheless, there have been no reports of this reaction being clinically relevant. With ddI and abacavir, the interaction is the opposite: ribavirin increases the phosphorylation of these drugs by increasing their levels and, therefore, their toxicity 89. The most problematic combination is ddI-ribavirin, and there is a risk of up to a five-fold increase in mitochondrial toxicity in patients who use these drugs simultaneously 90,91. Some cases of symptomatic hyperlactaemia with(out) pancreatitis have been fatal, with the result that the FDA has recommended that this combination be avoided.
There is also an increased risk of mitochondrial toxicity when ribavirin is combined with nucleoside analogues, although it is smaller.
There have been reports of excessive weight loss in cirrhotic co-infected patients treated simultaneously with interferon and ribavirin and HAART (mainly with d4T), as yet another expression of mitochondrial toxicity 92. Finally, AZT can increase the risk of ribavirin-induced anemia 42,86.
Relapse of HBV infection
The recurrence of HBV infection is very high in patients with active replication markers (detection of HBeAg and/or HBV DNA) before transplant, and this is accompanied by a significant increase in mortality 93,94. Therefore, most groups require negative values of HBV DNA before including a patient in a liver transplant programme. Patients who are not viremic before liver transplant can also have a recurrence of HBV (approximately 30%). To prevent this, HBV-specific immunoglobulin is combined with lamivudine in the same way as with HIV-negative patients. This regimen is generally very efficacious, renders HBV re-infection very uncommon and leads to a better prognosis compared with HCV-infected transplant recipients 85. Adefovir and tenofovir have proven useful against HBV and could be used in cases of resistance to lamivudine 95. HIV-positive patients who require antiretroviral therapy and have a chronic HBV infection can use lamivudine (or emtricitabine) and tenofovir as part of triple HIV therapy 42,95,96. Finally, it is important to remember that if there are changes in antiretroviral therapy in HIV-HBV co-infected patients, the drug which is active against HBV must be maintained to avoid the acute and severe exacerbations observed in these cases 95.
Conclusions
Experts in HIV infection and transplant infection, together with the experts in solid organ transplant of this Working Group agree that, today, solid organ transplantation is perfectly acceptable in HIV-infected patients with a terminal dysfunction of the organ in question. Our experience to date shows that short and medium-term survival in liver and kidney transplant is the same as that of HIV-negative patients and that HIV infection can be controlled with antiretroviral therapy during the post-transplant period. Interactions between immunosuppressors and antiretrovirals, especially protease inhibitors and, to a lesser extent, non-nucleoside reverse transcriptase inhibitors, are important and require close monitoring of immunosuppressor plasma levels. These patients do not have a greater risk of opportunistic infections or de novo tumours, and therefore should follow the same prophylaxis protocols as the general population. In transplant patients with HCV cirrhosis, relapse of the HCV infection is universal during the post-transplant period. It is unknown whether this re-infection has a worse outcome than in the HIV-negative patient and there is insufficient experience with pegylated interferon and ribavirin in this population. Outcome of patients who have received a transplant due to HBV cirrhosis seems to be better than HCV cirrhosis, since there is an efficacious prophylaxis against relapse (HBV-specific immunoglobulin and lamivudine). There is much less experience in kidney transplant in the HIV-HBV/HCV co-infected population. The same is true for heart transplant.
Addendum
To date (1st May 2005), 37 liver transplants in 36 infected VIH patients (eight of them have been published 97-100) and seven kidney transplants (one of them was published 101) have been carried out in 42 Spanish HIV-infected patients. No heart transplants have been performed.
Acknowledgements
This document is dedicated to all our patients and has come about thanks to the collaboration of many people and institutions. In this sense, we would like to thank the State Coordinator of Associations for the Fight against AIDS (CESIDA) and the Spanish Treatment Activists Forum (FEAT) for their suggestions, some of which have been added to the final version. We are also grateful to Thomas O'Boyle and FIPSE (Fundación para la Investigación y la Prevención del Sida en España) for their help in the English translation of this manuscript. Dr. José M. Miró was a recipient of a Research Grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona (Spain).
Members of the Spanish HIV SOT Working Group
The members of this Working Group are from: Organización Nacional de Trasplante (ONT; National Transplant Organization): B. Miranda, G. Garrido and J. Canón; Organización Catalana de Trasplante (OCATT; Catalonian Transplant Organization): J. Vilardell, M. Anguera and M. Sansromà; Organización Andaluza de Trasplantes (OAT; Andalusian Transplant Organization): M. Alonso-Gil; Grupo de Estudio de Sida (GESIDA; AIDS Study Group) de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC; Spanish Society of Infectious Diseases and Clinical Microbiology): J.M. Miró, A. Rivero, P. Miralles, K. Aguirrebengoa, M. Laguno, C. Quereda, J. González, J.A. Iribarren, R. Rubio and S. Moreno; Grupo de Estudio Infecciones en Trasplantados (GESITRA; Infection in Transplant Study Group) de la SEIMC: M. Montejo, G. Rufí, A. Moreno, J. Fortún, A. Pahissa, J.M. Cisneros, and J. Torre-Cisneros; Comisión de la Guía de Actuación ante Enfermedades Víricas en Hemodiálisis de la Sociedad Española de Nefrología (Committee of the "Action Guidelines for Viral Diseases in Haemodialysis" of the Spanish Society of Nephrology): G. Barril and J.M. Campistol; Sección de Insuficiencia Cardíaca y Trasplante de la Sociedad Española de Cardiología (Transplant and Cardiac Insufficiency Study Group of the Spanish Heart Society): M. Crespo and L. Alonso-Pulpón; Members of the Sociedad Española de Gastroenterología y Hepatología (Spanish Society of Gastroenterology and Hepatology): A. Rimola, V. Vargas, X. Xiol A. Valdivieso, R. Bárcena and R. Bañares; Members of the Sociedad Española de Trasplante Hepático (Spanish Society of Liver Transplantation): Drs. A. Rafecas, C. Margarit, L. Grande, J. Fabregat, E. de Vicente, J. Ortiz de Urbina and A. Valdivieso; Members of the Sociedad Española de Nefrología (Spanish Society of Nephrology): A. Mazuecos; Members of the Sociedad Española de Urología (Spanish Society of Urology): Dr. F.J. Burgos; Programa para la Prevención y la Asistencia del Sida en Cataluña (Programme for the Prevention and Care of AIDS in Catalonia): J. Colóm, A. Giménez and E. Buira; Secretaría del Plan Nacional del Sida (SPNS; National AIDS Plan Secretariat) del Ministerio de Sanidad y Consumo (MSC; Spanish Ministry of Health): F. Parras, L. Guerra, L. Chamorro and R. Polo; Delegación del Gobierno para el Plan Nacional sobre Drogas del MSC (Government Delegation for the National Drugs Plan): J.A. Salvador and L. de la Fuente; and to the following members of the Hospital Clínic-IDIBAPS, University of Barcelona, Barcelona (Spain): JL Blanco, N. de Benito, J. Blanch, JC García-Valdecasas, J. Mallolas, CA Mestres, M. Monrás, F. Oppenheimer, D. Paredes F.J. Pérez-Villa, E. Roig and to Mrs. C. Lanaspa.