The objective of this study is to estimate the economic impact associated with the optimisation of triple antiretroviral treatment (ART) in patients with undetectable viral load according to the recommendations from the GeSIDA/PNS (2015) Consensus and their applicability in the Spanish clinical practice.
MethodsA pharmacoeconomic model was developed based on data from a National Hospital Prescription Survey on ART (2014) and the A-I evidence recommendations for the optimisation of ART from the GeSIDA/PNS (2015) consensus. The optimisation model took into account the willingness to optimise a particular regimen and other assumptions, and the results were validated by an expert panel in HIV infection (Infectious Disease Specialists and Hospital Pharmacists). The analysis was conducted from the NHS perspective, considering the annual wholesale price and accounting for deductions stated in the RD-Law 8/2010 and the VAT.
ResultsThe expert panel selected six optimisation strategies, and estimated that 10,863 (13.4%) of the 80,859 patients in Spain currently on triple ART, would be candidates to optimise their ART, leading to savings of €15.9M/year (2.4% of total triple ART drug cost). The most feasible strategies (>40% of patients candidates for optimisation, n=4,556) would be optimisations to ATV/r+3TC therapy. These would produce savings between €653 and €4,797 per patient per year depending on baseline triple ART.
ConclusionImplementation of the main optimisation strategies recommended in the GeSIDA/PNS (2015) Consensus into Spanish clinical practice would lead to considerable savings, especially those based in dual therapy with ATV/r+3TC, thus contributing to the control of pharmaceutical expenditure and NHS sustainability.
El objetivo de este estudio es estimar el impacto económico en España de la optimización del tratamiento antirretroviral (TAR) triple en pacientes con carga viral suprimida según las recomendaciones GeSIDA/PNS (2015) y su aplicabilidad en la práctica clínica.
MétodosA partir de los datos de prescripción del TAR de la encuesta hospitalaria 2014, siguiendo las recomendaciones de GeSIDA/PNS de optimización de TAR con grado de evidencia A-I, se desarrolló un modelo farmacoeconómico. Las pautas de optimización, la voluntad de optimización y demás asunciones y resultados del modelo fueron validados por un panel de expertos en la infección por VIH (infectológos y farmacéuticos hospitalarios). El análisis se realizó desde la perspectiva del SNS, considerando el coste farmacológico anual, precio de venta del laboratorio notificado, deducción RD-Ley-8/2010 e IVA.
ResultadosEl panel seleccionó 6 estrategias de optimización y estimó que en España de los 80.859 pacientes actualmente en TAR triple, 10.863 (13,4%) serían candidatos a optimizar su TAR según estas estrategias, generando ahorros de 15,9M€/año (2,4% del coste farmacológico del TAR triple). Las estrategias más factibles (>40% del total de pacientes candidatos a optimizar, n=4.556) y asociadas a mayores reducciones del gasto (ahorro entre 653 y 4.797€/paciente-año según el TAR triple de partida) serían las optimizaciones a ATV/r+3TC.
ConclusiónLa aplicación a la práctica clínica española de las principales estrategias de optimización recomendadas en el documento GeSIDA/PNS (2015) generaría ahorros sustanciales, especialmente aquellas basadas en biterapia con ATV+3TC, contribuyendo así al control del gasto farmacéutico y a la sostenibilidad del SNS.
It is estimated that around 150,000 people are infected with the human immunodeficiency virus (HIV) in Spain,1 with numbers increasing by about 4,000 new cases each year.2 The epidemiological situation, social impact and repercussions for the economy currently make HIV one of Spain's biggest public health problems.1 Since the availability of highly active antiretroviral therapy (ART), morbidity and mortality have been drastically reduced, and it has even modified the natural course of HIV infection.3–5 The aim of ART is to achieve sustained viral load (VL) suppression,6,7 with the combination of two nucleoside analogue reverse transcriptase inhibitors (NRTIs) and a third drug from another family being the standard of treatment.
The improvement in the quality of life and survival of patients with HIV thanks to the efficacy and safety of triple ART,4,5 combined with the high incidence of HIV in Spain2 and the current consensus recommendation to initiate ART in all patients independent of CD4 levels,3,6,7 increases the number of patients to be treated annually.
Although it has been shown to be cost-effective, the fact that triple ART has to be administered for life, and is associated in the medium and long term with a certain level of toxicity,8 means the economic cost is high.9,10 Spending on ART in Spain reached €968 M in 2015, representing 12% of hospital pharmacy expenditure.11
Since the early 2000s, the search for simpler and less toxic treatments as alternatives to triple ART in patients with undetectable VL has been one of the main challenges in HIV,12,13 as discussed by the Grupo de Estudio de Sida/Plan Nacional sobre el Sida (GeSIDA/PNS) [Expert Study Group on AIDS/Spanish National AIDS Plan]3 in their recommendations and, to a lesser extent, by the European7 and North American6 guidelines.
The aim of optimising ART is to improve the patient's quality of life and their adherence to the therapy, while maintaining its efficacy (virus control and immune response).3,6,7,13 Optimisation may be desirable to reduce the complexity of the treatment (reducing the number of antiretroviral drugs [ARD], doses/day, food restrictions, etc.), to adapt the treatment to the requirements and particularities of the patient, or to prevent, reduce and/or reverse the toxicity of the ART.3,6,7,13
As a consequence, the cost of ART and the possibility of finding therapeutic options that guarantee efficacy and safety with lower associated costs become increasingly important.14
Since 2011, cost-effectiveness analyses of the ART initiation regimens recommended in the current GeSIDA/PNS consensus document15 have been carried out annually. However, no evaluation has yet been carried out of the economic impact of the implementation of the ART optimisation recommendations on the Spanish National Health Service (SNHS). Therefore, the aim of this analysis is to estimate the economic impact of triple ART optimisation according to the recommendations with the highest level of evidence (AI) in the GeSIDA/PNS consensus document (2015),16 currently considered most applicable in clinical practice in Spain.
MethodsStudy designA model was designed for an analysis over one year of the budgetary impact of the application in clinical practice of the A-I evidence recommendations in the GeSIDA/PNS consensus document (2015)16 regarding the optimisation of triple ART in adults with HIV with undetectable VL.
To design and carry out the study, a panel of six experts with extensive experience in the management of HIV in Spain (three specialists in infectious diseases and three hospital pharmacists) was set up. In parallel, in order to identify the main parameters to be included in the analysis, we carried out a review of the national scientific literature. The initial approach, such as the main inputs and assumptions, were collected through a structured electronic questionnaire designed ad hoc for individual completion by each expert. Subsequently, two face-to-face meetings were held where the parameters and assumptions of the model were agreed on and the results of the analysis were discussed, evaluating the main strengths and limitations of the study.
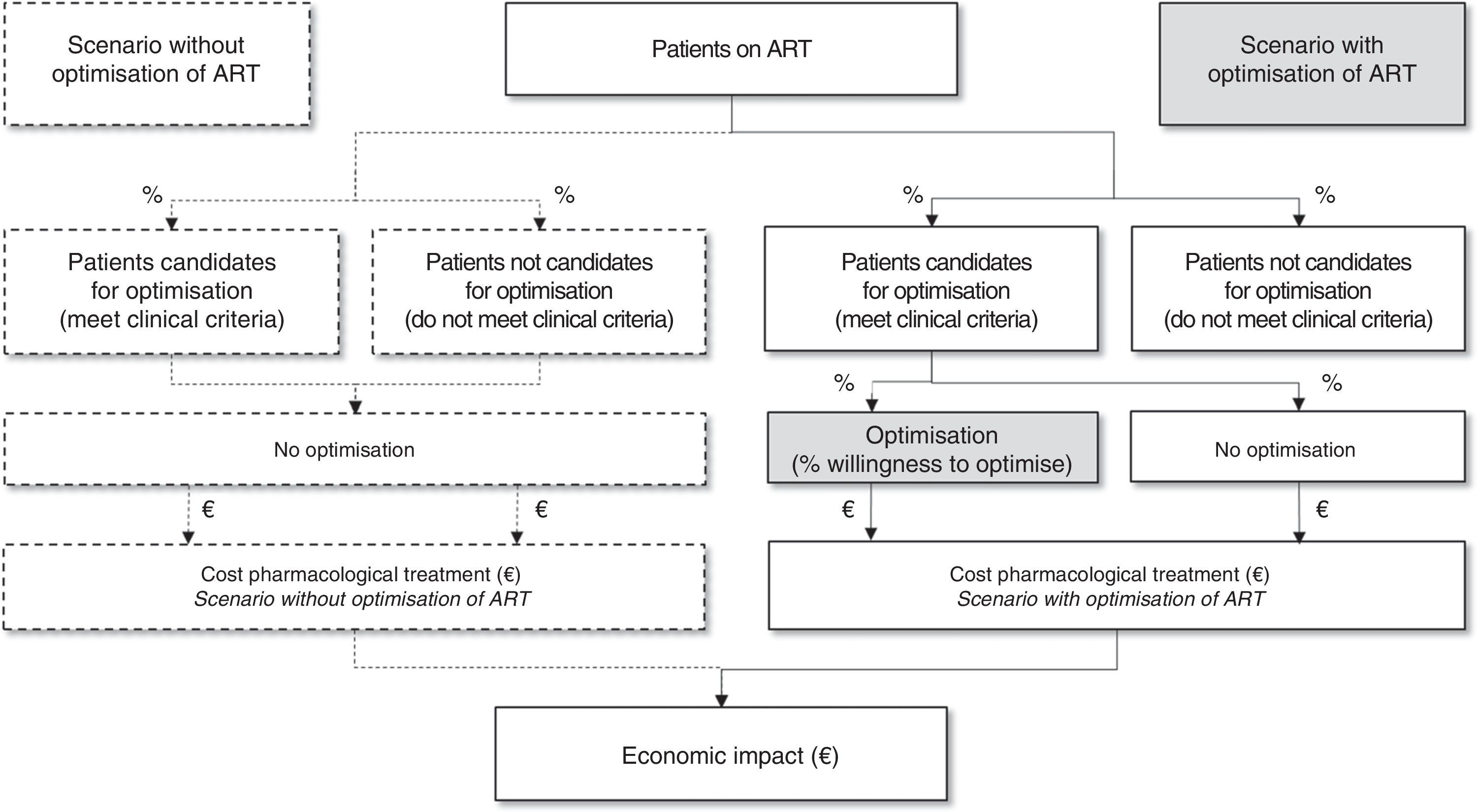
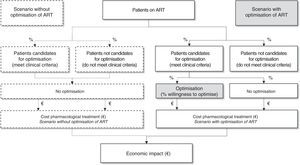
From there, taking the Spanish population on triple ART and the optimisation recommendations selected, they identified a candidate population for optimisation of their ART. As the optimisation of triple ART depends on multiple factors, the panel of experts estimated the applicability of these recommendations in clinical practice through the percentage of patients who would be potential candidates to optimise ART. Lastly, the budgetary impact was estimated as the difference between the costs of the scenario without optimisation (regardless of their suitability, the patients with undetectable VL would not optimise their ART) against the costs of the scenario with optimisation as estimated would be applied in current clinical practice in Spain (Fig. 1).
Optimisation strategiesOf the recommendations for changing triple ART in patients with undetectable VL in the GeSIDA/PNS consensus document (2015),16 we ruled out strategies with baseline ART that included drugs not recommended for the initiation of ART (zidovudine, stavudine and enfuvirtide), as they represented a marginal proportion of patients who were candidates to receive evaluable therapeutic changes in our analysis. We also ruled out the optimisation of two NRTI + EFV to TDF/FTC/EFV, considering that, by now, the majority of patients on that baseline ART would already have been optimised to receive a single-tablet regimen which was authorised in Spain in 2007 and widely used. Additionally, we decided to split the recommendation of change from two NRTI + LPV/r or ATV/r to LPV/r + 3 TC or ATV/r + 3 TC into: a) two NRTI + LPV/r to 3 TC + LPV/r; and b) two NRTI + PI/r or NNRTI or INI to ATV/r + 3TC, because, despite being in line with the design of the OLE study,17 where all patients received LPV/r as part of baseline, the original recommendation does not reflect the baseline treatment of patients included in the SALT study.18 Along similar lines, for the sake of being faithful to the methodology established for the study, we ruled out the optimisation of two NRTI + one INI, two NRTI + NVP or TDF/FTC/RPV to ATV/r + 3 TC, as these regimens were either completely, or at least fairly, unrepresentative of baseline ART in the SALT study.18
PopulationThe population receiving ART in Spain and, therefore, candidate for having it optimised, was obtained from the Informe de evaluación del plan multisectorial de VIH/sida [Evaluation Report of the Multisectoral HIV/AIDS Plan] (2012).19 The proportion of patients on triple ART was extracted from the data from the Encuesta hospitalaria de pacientes con VIH/sida [Hospital Survey of Patients with HIV/AIDS] (2014).20 In the absence of published data on the distribution of the different ART regimens in Spain, the percentages of patients in each starting triple regimen were estimated according to the median of the values provided by the panel of experts in the questionnaire, which reflected the actual healthcare provided by their respective hospital areas.
Proportion of patients to be optimisedFor each optimisation strategy, we estimated: a) eligibility to optimise: proportion of patients who would meet the clinical conditions stipulated in the GeSIDA/PNS consensus document (2015); and b) willingness to optimise: proportion of patients who, meeting clinical criteria, would finally optimise their triple ART considering aspects such as the availability of other options (therapeutic preference), the degree of resolution or prevention of toxicity (therapeutic adaptation) or the economic consequences (therapeutic cost). The final proportion of patients to be optimised for each of the analysed strategies was calculated as the eligibility to optimise multiplied by the willingness to optimise.
CostsThe analysis was performed for a time horizon of one year, from the perspective of the SNHS, considering only the pharmacological costs of ART. The total cost of the treatment was estimated taking into account the official notified wholesale price,21 the corresponding Spanish Royal Decree-Law (RDL) 8/2010 deduction in each case and 4% VAT. Only pharmaceutical presentations with marketing authorisation were considered,21 and all costs were valued in euros in 2015. The posology of each ART was established taking into account the usual dose recommended in the GeSIDA/PNS consensus document (2015).16
Sensitivity analysisTo assess the robustness of the results, and identify the parameters with greatest uncertainty and impact on the study conclusions, a series of sensitivity analyses were carried out using the extreme values obtained from consultation with the panel of experts. These were the willingness to optimise and the price of the ARD. The price of antiretrovirals can vary depending on the hospital or healthcare region. As it was not feasible to perform a sensitivity analysis that would reflect all the scenarios, a tool was enabled to allow the analysis to be reproduced by applying the specific prices of each healthcare area, available at: https://ira.redbms.es/CalcOpt
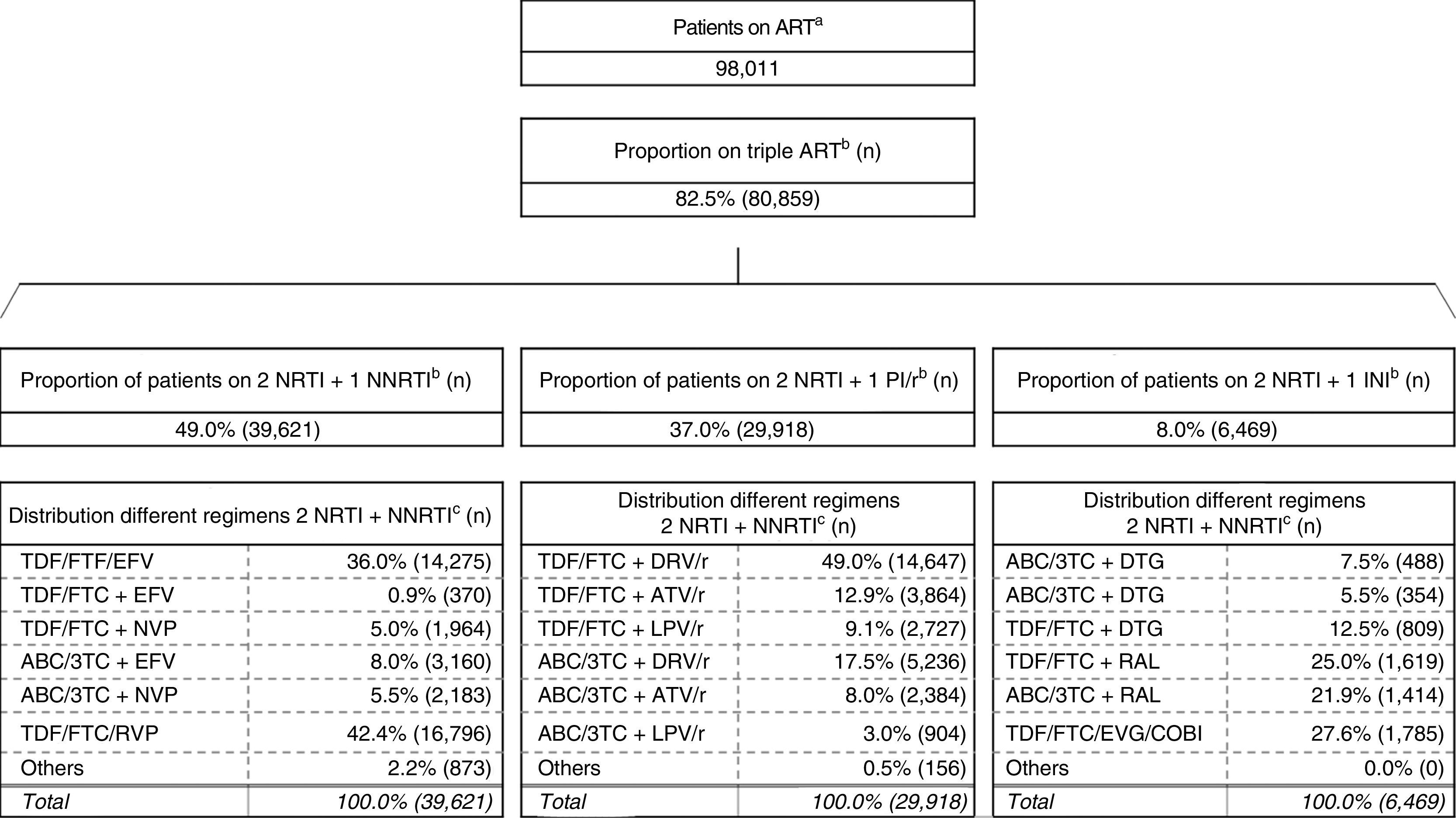
ResultsOutcome on patientsUsing the methods described above, we obtained 80,859 patients on triple ART, the most commonly used combination being two NRTI + one NNRTI (49.0%), followed by two NRTI + one PI/r (37.0%) and two NRTI + one INI (8.0%) (Fig. 2).19,20
Estimation of the distribution of the use of the different ART regimens in Spain.
ABC: abacavir; ART: antiretroviral therapy; ATV: atazanavir; COBI: cobicistat; DTG: dolutegravir; DRV: darunavir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; INI: integrase inhibitor; LPV: lopinavir; NRTI: nucleoside or nucleotide analogue reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NVP: nevirapine; PI/r: ritonavir-boosted protease inhibitor; r: ritonavir; RAL: raltegravir; RPV: rilpivirine; TDF: tenofovir; 3 TC: lamivudine.
aInforme de evaluación del plan multisectorial de VIH/sida [Evaluation report of the multisectoral HIV/AIDS plan] (2012).
bEncuesta hospitalaria de pacientes con VIH/sida [Hospital Survey of Patients with HIV/AIDS] (2014). The existence of triple ART based on entry inhibitors, fusion inhibitors or without specifying accounts for the remaining 6% of the different triple-ART regimens considered.
c Panel of experts median.
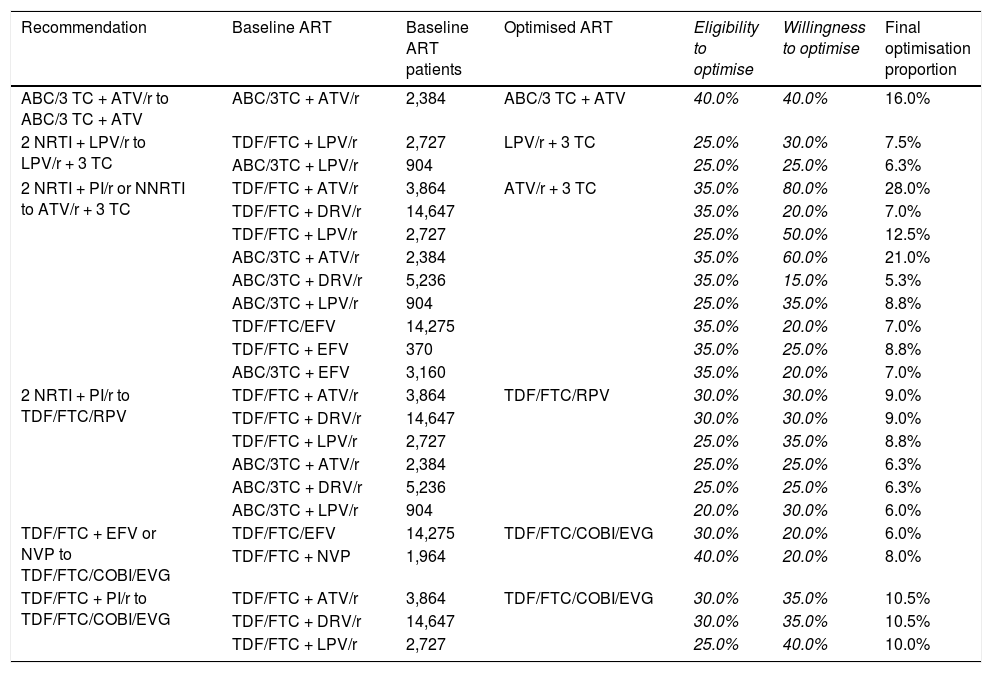
From the recommendations for switching ART in patients with undetectable VL in the GeSIDA/PNS consensus document (2015),16 we finally selected six optimisation recommendations with A-I evidence involving 23 baseline triple ART regimens. Table 1 shows the selected strategies and the consensus optimisation percentages. Based on the eligibility to optimise and willingness to optimise the final optimisation ratio varied from a maximum of 28.0% when optimising from TDF/FTC + ATV/r to ATV/r + 3 TC to a minimum of 5.3% when optimising from ABC/3 TC + DRV/r to ATV/r + 3 TC. On aggregate, it was estimated that 10,863 (13.4%) patients would be optimised for their ART according to the applicability in clinical practice of the selected recommendations.
Selection of A-I evidence recommendations for optimisation of ART from the GeSIDA/PNS (2015) consensus document in patients with undetectable VL in Spain.
| Recommendation | Baseline ART | Baseline ART patients | Optimised ART | Eligibility to optimise | Willingness to optimise | Final optimisation proportion |
|---|---|---|---|---|---|---|
| ABC/3 TC + ATV/r to ABC/3 TC + ATV | ABC/3TC + ATV/r | 2,384 | ABC/3 TC + ATV | 40.0% | 40.0% | 16.0% |
| 2 NRTI + LPV/r to LPV/r + 3 TC | TDF/FTC + LPV/r | 2,727 | LPV/r + 3 TC | 25.0% | 30.0% | 7.5% |
| ABC/3TC + LPV/r | 904 | 25.0% | 25.0% | 6.3% | ||
| 2 NRTI + PI/r or NNRTI to ATV/r + 3 TC | TDF/FTC + ATV/r | 3,864 | ATV/r + 3 TC | 35.0% | 80.0% | 28.0% |
| TDF/FTC + DRV/r | 14,647 | 35.0% | 20.0% | 7.0% | ||
| TDF/FTC + LPV/r | 2,727 | 25.0% | 50.0% | 12.5% | ||
| ABC/3TC + ATV/r | 2,384 | 35.0% | 60.0% | 21.0% | ||
| ABC/3TC + DRV/r | 5,236 | 35.0% | 15.0% | 5.3% | ||
| ABC/3TC + LPV/r | 904 | 25.0% | 35.0% | 8.8% | ||
| TDF/FTC/EFV | 14,275 | 35.0% | 20.0% | 7.0% | ||
| TDF/FTC + EFV | 370 | 35.0% | 25.0% | 8.8% | ||
| ABC/3TC + EFV | 3,160 | 35.0% | 20.0% | 7.0% | ||
| 2 NRTI + PI/r to TDF/FTC/RPV | TDF/FTC + ATV/r | 3,864 | TDF/FTC/RPV | 30.0% | 30.0% | 9.0% |
| TDF/FTC + DRV/r | 14,647 | 30.0% | 30.0% | 9.0% | ||
| TDF/FTC + LPV/r | 2,727 | 25.0% | 35.0% | 8.8% | ||
| ABC/3TC + ATV/r | 2,384 | 25.0% | 25.0% | 6.3% | ||
| ABC/3TC + DRV/r | 5,236 | 25.0% | 25.0% | 6.3% | ||
| ABC/3TC + LPV/r | 904 | 20.0% | 30.0% | 6.0% | ||
| TDF/FTC + EFV or NVP to TDF/FTC/COBI/EVG | TDF/FTC/EFV | 14,275 | TDF/FTC/COBI/EVG | 30.0% | 20.0% | 6.0% |
| TDF/FTC + NVP | 1,964 | 40.0% | 20.0% | 8.0% | ||
| TDF/FTC + PI/r to TDF/FTC/COBI/EVG | TDF/FTC + ATV/r | 3,864 | TDF/FTC/COBI/EVG | 30.0% | 35.0% | 10.5% |
| TDF/FTC + DRV/r | 14,647 | 30.0% | 35.0% | 10.5% | ||
| TDF/FTC + LPV/r | 2,727 | 25.0% | 40.0% | 10.0% |
ABC: abacavir; ART: antiretroviral therapy; ATV: atazanavir; COBI: cobicistat; DRV: darunavir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NRTI: nucleoside or nucleotide analogue reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NVP: nevirapine; PI/r: ritonavir-boosted protease inhibitor; r: ritonavir; RPV: rilpivirine; TDF: tenofovir; 3 TC: lamivudine.
Numbers in italics: original values validated by the panel of experts and used to calculate the “Final optimisation proportion”.
Based on the number of patients on triple ART (Fig. 2) and the annual pharmacological cost of each treatment regimen, it was estimated that the cost of triple ART in Spain would be slightly above €671 M (without considering discontinuations of therapy or regimens described as “others”).
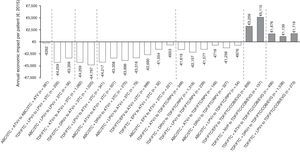
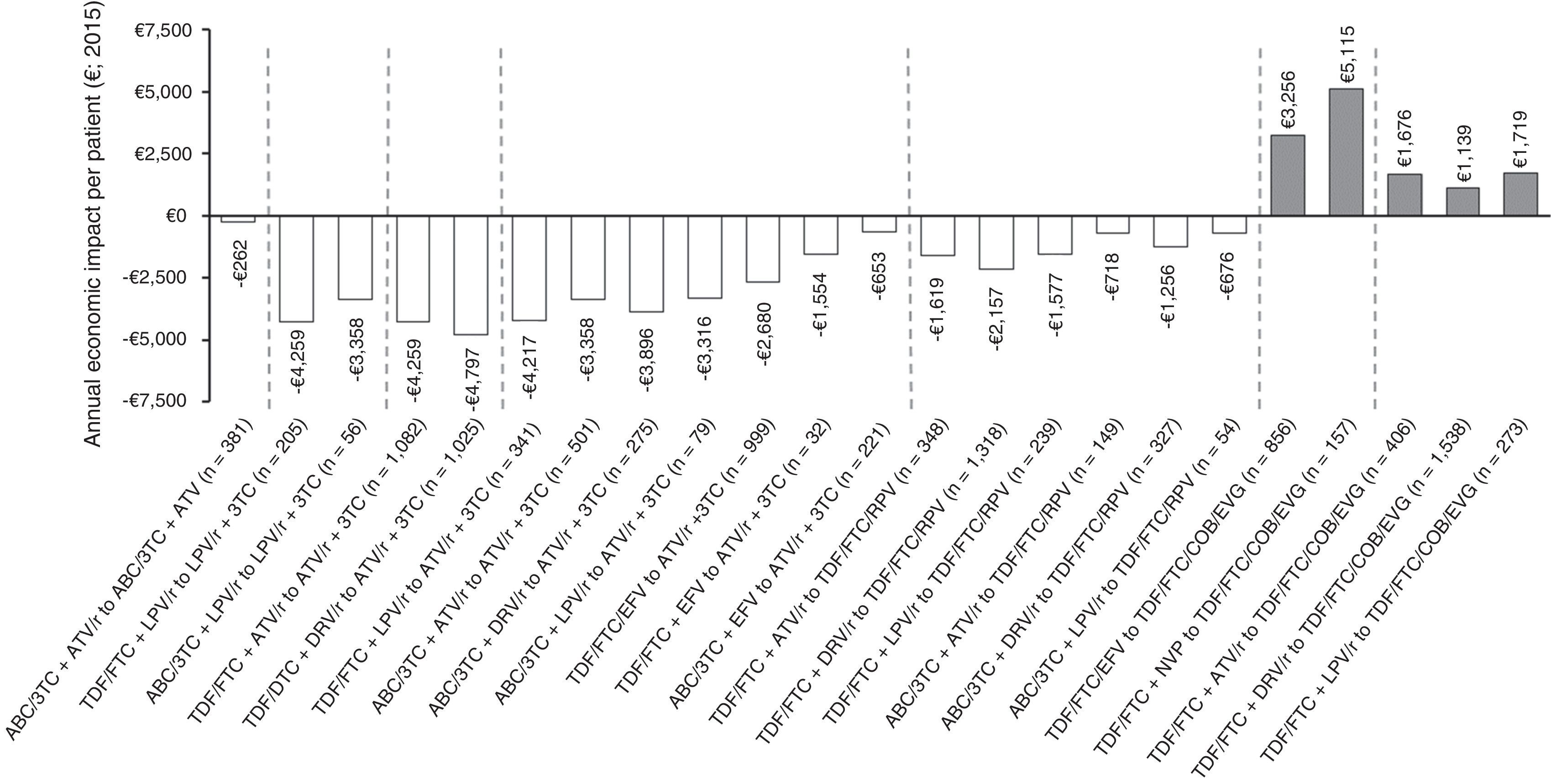
Figure 3 shows the economic impact per patient/year of the optimisation strategies evaluated. Most would involve savings in pharmacological costs, varying from €262 to €4,797 per patient/year. All the optimisation recommendations for PI/r + 3 TC dual therapy would lead to savings, with the change to dual therapy with ATV/r + 3 TC from TDF/FTC + DRV/r being the optimisation with biggest savings in terms of pharmaceutical expenditure per patient/year (€4,797). In contrast, the optimisation based on the withdrawal of ritonavir from ABC/3 TC + ATV/r would mean the lowest savings per patient/year (€262). All optimisation recommendations to TDF/FTC/COBI/EVG would mean increases in drug spending (official notified wholesale price), with the change from TDF/FTC + NVP to TDF/FTC/COBI/EVG being the optimisation with the biggest increase in terms of pharmaceutical expenditure per patient/year (€5,115).
Annual economic impact per patient of the 25 A-I evidence ART optimisation strategies from the GeSIDA/PNS (2015) consensus document in patients with undetectable VL in Spain.
ABC: abacavir; ATV: atazanavir; COBI: cobicistat; DRV: darunavir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NVP: nevirapine; r: ritonavir; RPV: rilpivirine; TDF: tenofovir; 3 TC: lamivudine.
Of the 10,863 patients whose triple ART would be optimised, 70.3% (7,633) would optimise to a cheaper alternative (mean saving of €2,928 per patient/year) and 29.7% (3,230) to a more expensive alternative (mean increase of €2,010 per patient/year).
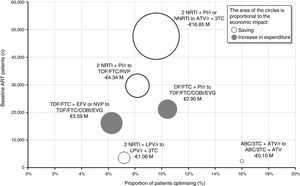
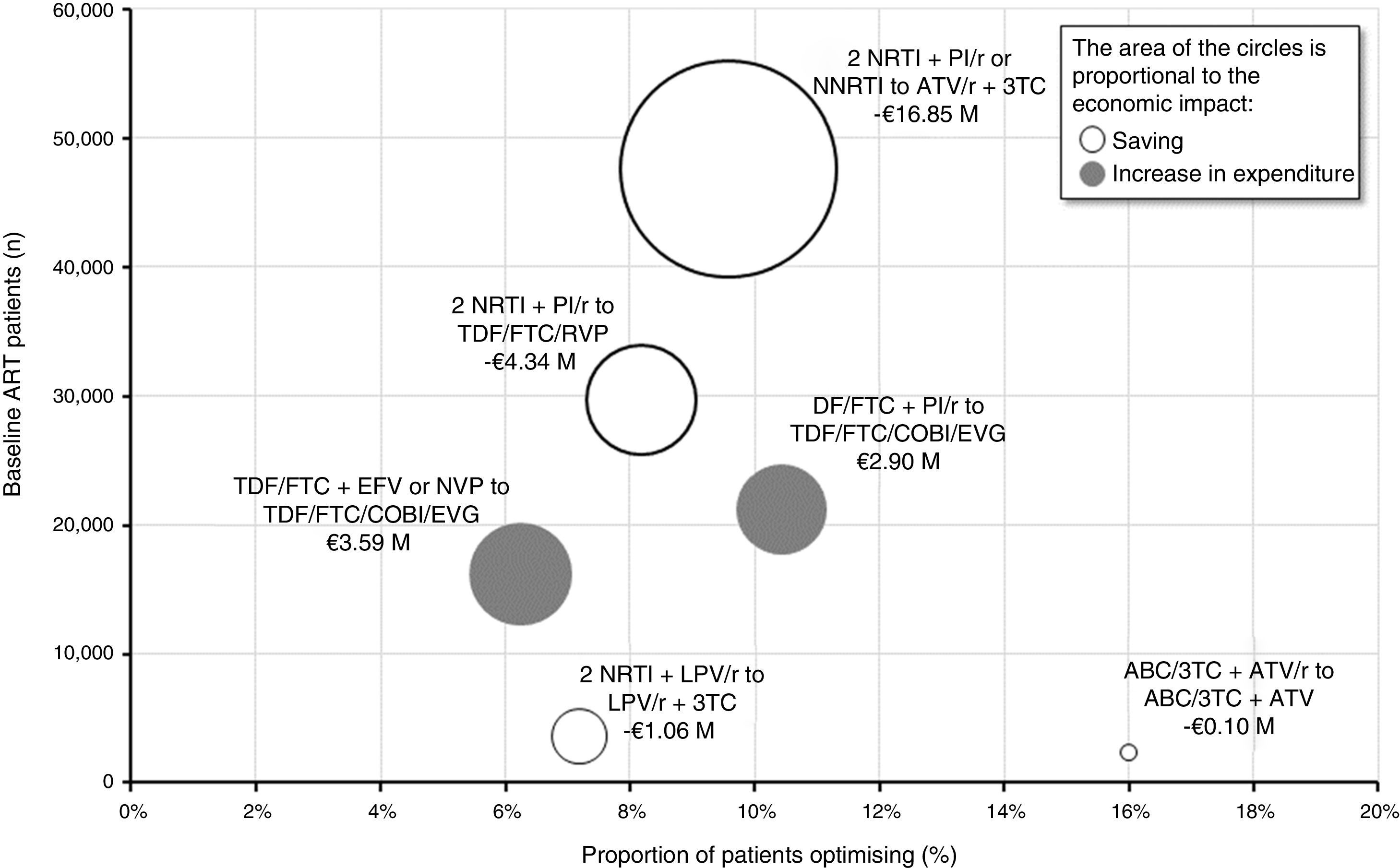
Figure 4 provides an overview of the proportion of patients who would have their triple ART optimised, the number of patients on baseline ART and the impact (saving or increase in expenditure) of each of the six optimisation recommendations. The optimisation to dual therapy based on ATV/r + 3TC would involve the largest number of patients on baseline triple ART (47,567) and apply to 9.6% (4,556) of those patients. Considering a saving per patient/year of between €653 and €4,797 based on triple baseline ART, finally, the budgetary impact of this optimisation would reach €16.85M per year, equivalent to an average saving per patient/year of €3,699. Conversely, the change from TDF/FTC + EFV or NVP to TDF/FTC/COBI/EVG would be the recommendation associated with the biggest increase in pharmacological expenditure (€3.59M per year). Overall, the implementation in clinical practice of the recommendations of the GeSIDA/PNS consensus document (2015) would generate an average saving for the SNHS of €1,460 per patient/year, corresponding to a total of €15.86M annually.
Economic impact grouped by optimisation recommendation.
ABC: abacavir; ART: antiretroviral therapy; ATV: atazanavir; COBI: cobicistat; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NRTI: nucleoside or nucleotide analogue reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NVP: nevirapine; PI/r: ritonavir-boosted protease inhibitor; r: ritonavir; RPV: rilpivirine; TDF: tenofovir; 3 TC: lamivudine.
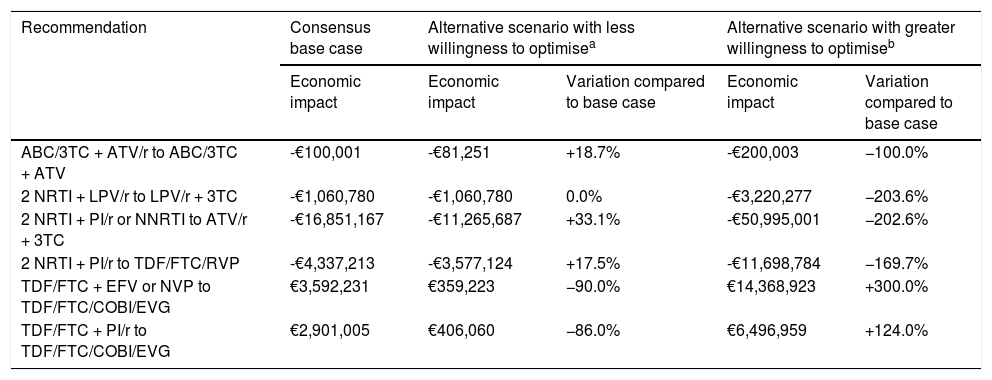
Based on the extreme values for the willingness to optimise (supplementary Table 1), the economic impact of the optimisation strategies would vary from an average reduction of 40.9% (0.0-90.0%) to an average increase of 183.3% (100.0-300.0%) with respect to the base case (Table 2). Also, the savings provided by the optimisation to dual therapy based on ATV/r + 3 TC would be reduced to €11.3 M if we consider the minimum willingness to optimise and increase to €51.0 M considering the maximum willingness to optimise. Moreover, a lower willingness to optimise to TDF/FTC/COBI/EVG would reduce the increase in pharmaceutical expenditure to €0.36-0.41M, while a higher willingness to optimise would increase spending to €6.5-14.4M, depending on the baseline ART. Overall, even taking into account the lower level of willingness to optimise in all cases, the ART optimisation recommendations assessed would provide savings for the SNHS of €15.2M per year.
Variation of the main results based on the sensitivity analyses for willingness to optimise.
| Recommendation | Consensus base case | Alternative scenario with less willingness to optimisea | Alternative scenario with greater willingness to optimiseb | ||
|---|---|---|---|---|---|
| Economic impact | Economic impact | Variation compared to base case | Economic impact | Variation compared to base case | |
| ABC/3TC + ATV/r to ABC/3TC + ATV | -€100,001 | -€81,251 | +18.7% | -€200,003 | −100.0% |
| 2 NRTI + LPV/r to LPV/r + 3TC | -€1,060,780 | -€1,060,780 | 0.0% | -€3,220,277 | −203.6% |
| 2 NRTI + PI/r or NNRTI to ATV/r + 3TC | -€16,851,167 | -€11,265,687 | +33.1% | -€50,995,001 | −202.6% |
| 2 NRTI + PI/r to TDF/FTC/RVP | -€4,337,213 | -€3,577,124 | +17.5% | -€11,698,784 | −169.7% |
| TDF/FTC + EFV or NVP to TDF/FTC/COBI/EVG | €3,592,231 | €359,223 | −90.0% | €14,368,923 | +300.0% |
| TDF/FTC + PI/r to TDF/FTC/COBI/EVG | €2,901,005 | €406,060 | −86.0% | €6,496,959 | +124.0% |
ABC: abacavir; ART: antiretroviral therapy; ATV: atazanavir; COBI: cobicistat; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NRTI: nucleoside or nucleotide analogue reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NVP: nevirapine; PI/r: ritonavir-boosted protease inhibitor; r: ritonavir; RPV: rilpivirine; TDF: tenofovir; Triple ART: triple therapy; 3 TC: lamivudine.
The results of our study show that most of the recommendations with the highest level of evidence (A-I) on optimisation of ART in the GeSIDA/PNS consensus document (2015)16 would generate savings for the SNHS; globally, an average saving of €1,460 per patient/year, corresponding to €15.86M, equivalent to 2.4% of the expenditure on triple ART in Spain. The magnitude of these savings depends largely on the baseline situation (starting ART), with the main vector for increase being the potential to apply these recommendations in clinical practice (willingness to optimise). In this context, the optimisation of ATV/r + 3 TC dual therapy is the recommendation that would involve the biggest savings for the SNHS in terms of pharmacological expenditure (€16.85M). In addition to the willingness to optimise and the high economic differential resulting from the withdrawal of an ARD from triple ART, another reason for this impact is the wide potential for optimisation deriving from the design and results of the SALT18 study, which explains the significant number of patients on triple ART who are candidates to be optimised.
Studies conducted at a national level in Spain point in the same direction, and show that having been validated as appropriate clinically, optimisation is an attractive alternative from an economic point of view.12,22–25 Although cost-effectiveness analyses on the ART initiation regimens from the GeSIDA/PNS15 consensus document have been disseminated and updated since 2011, nobody has inquired about the degree of applicability in clinical practice and the budgetary impact of the ART optimisation recommendations.
Nonetheless, strategic plans for the control of HIV infection,1 recommendations to improve adherence to ART26 and technical reports27 all emphasise the optimisation of ART, and point to it as one of the measures responsible for reducing drug expenditure per patient.1
The reflection of current clinical practice in the management of HIV in Spain is one of the strengths of this study. In the absence of recent studies on Spanish clinical practice regarding the degree of optimisation of triple ART in patients with undetectable VL according to the recommendations of the GeSIDA/PNS consensus document, we turned to the panel of experts to estimate the eligibility for optimisation and the willingness to optimise for each recommendation analysed. The sensitivity analysis showed that: a) even taking into account lower percentages of willingness to optimise, as a whole, the ART-optimisation recommendations continue generating savings for the SNHS; and b) the base case is remarkably closer to the scenario with less willingness to optimise. This would reflect the restrictive environment in terms of pharmacological expenditure in the hospital setting, and highlight the wide margin for achieving higher levels of willingness to optimise.
Lastly, we recommend interpreting the results of the analysis corresponding to the scenario with the greatest willingness to optimise separately, as they represent the maximum potential of each strategy, without considering the existence of other therapeutic alternatives with the same recommendation.
Among the limitations of this analysis, the budgetary impact is highly dependent on the national distribution of the different triple-ART regimens; it was not feasible to perform sensitivity analyses because of the lack of sufficiently robust and representative alternative information.
Moreover, the possible existence of prices other than the notified official wholesale prices, deriving from confidential agreements, is a limitation when interpreting and extrapolating the results of this study to different healthcare environments. However, the interactive tool facilitated allows the economic impact to be adapted to different particular situations.
The analysis used as a starting point for the distribution of the use of ART in 2015 was based on the data published in the Encuesta hospitalaria de pacientes con VIH/sida [Hospital Survey of patients with HIV/AIDS] (2014)20 for 2014. The marketing of new ARD and implementation of the recommendations of the GeSIDA/PNS consensus document on starting and changing ART mean that this structure is highly dynamic, and that affects the results and conclusions of this study. For that reason, and given that the GeSIDA/PNS consensus document is updated annually, we decided, on a conservative basis, that carrying out the analysis for time horizons of more than one year would generate a high degree of uncertainty.
In this context, review of the treatment recommendations and the future marketing authorisation for new co-formulated ART may modify the willingness to optimise values, especially in the case of recommendations whose main purpose is to reduce the complexity of ART. We therefore recommend continuing to assess the economic consequences of the new recommendations as they are implemented in clinical practice.
This analysis has allowed us, on a national level in Spain, to identify the optimisation strategies which are applicable to a larger number of patients on triple ART, and would therefore generate a bigger economic impact. We believe our results can contribute to decision-making processes for healthcare professionals, managers and health officials involved in the management of HIV infection, especially in situations where healthcare resources are scarce or restricted,15,22 when the efficient use of these resources is fundamental to guaranteeing the sustainability of the SNHS.1,28,29
In conclusion, the application to Spanish clinical practice of the main optimisation strategies recommended in the GeSIDA/PNS (2015) consensus document16 would generate substantial savings, especially those based on dual therapy with ATV/r + 3 TC, thus contributing to controlling pharmaceutical spending and the sustainability of the SNHS.
FundingThis study was sponsored by Bristol-Myers Squibb. Oblikue Consulting S.L. provided technical support for the development of the pharmacoeconomic model.
AuthorshipJosé Manuel Martínez-Sesmero and Javier Sánchez-Rubio contributed in equal measure to this work; the contribution of Celia Roldán and Beatriz Hernández-Novoa was at the time the study was carried out.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To Eduardo Villa, Market Access Department at Bristol-Myers Squibb, for his contribution to the conducting of this study and revision of the manuscript.
Please cite this article as: Ribera E, Martínez-Sesmero JM, Sánchez-Rubio J, Rubio R, Pasquau J, Poveda JL, et al. Impacto económico asociado a la implementación de las recomendaciones con grado de evidencia A-I del documento de consenso de GeSIDA/PNS (2015) relativas a la optimización del tratamiento antirretroviral en adultos infectados por virus de la inmunodeficiencia humana con carga viral suprimida en España. Enferm Infecc Microbiol Clin. 2018;36:157–164.





![Estimation of the distribution of the use of the different ART regimens in Spain. ABC: abacavir; ART: antiretroviral therapy; ATV: atazanavir; COBI: cobicistat; DTG: dolutegravir; DRV: darunavir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; INI: integrase inhibitor; LPV: lopinavir; NRTI: nucleoside or nucleotide analogue reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NVP: nevirapine; PI/r: ritonavir-boosted protease inhibitor; r: ritonavir; RAL: raltegravir; RPV: rilpivirine; TDF: tenofovir; 3 TC: lamivudine. aInforme de evaluación del plan multisectorial de VIH/sida [Evaluation report of the multisectoral HIV/AIDS plan] (2012). bEncuesta hospitalaria de pacientes con VIH/sida [Hospital Survey of Patients with HIV/AIDS] (2014). The existence of triple ART based on entry inhibitors, fusion inhibitors or without specifying accounts for the remaining 6% of the different triple-ART regimens considered. c Panel of experts median. Estimation of the distribution of the use of the different ART regimens in Spain. ABC: abacavir; ART: antiretroviral therapy; ATV: atazanavir; COBI: cobicistat; DTG: dolutegravir; DRV: darunavir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; INI: integrase inhibitor; LPV: lopinavir; NRTI: nucleoside or nucleotide analogue reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NVP: nevirapine; PI/r: ritonavir-boosted protease inhibitor; r: ritonavir; RAL: raltegravir; RPV: rilpivirine; TDF: tenofovir; 3 TC: lamivudine. aInforme de evaluación del plan multisectorial de VIH/sida [Evaluation report of the multisectoral HIV/AIDS plan] (2012). bEncuesta hospitalaria de pacientes con VIH/sida [Hospital Survey of Patients with HIV/AIDS] (2014). The existence of triple ART based on entry inhibitors, fusion inhibitors or without specifying accounts for the remaining 6% of the different triple-ART regimens considered. c Panel of experts median.](https://static.elsevier.es/multimedia/2529993X/0000003600000003/v1_201803080424/S2529993X1830025X/v1_201803080424/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)