Cytomegalovirus (CMV) is regarded as the most common pathogen that negatively influences the overall outcome of hematopoietic stem cell (HSCT) and solid organ (SOT) transplantation.1,2 While CMV causes a benign and often-asymptomatic infection in immunocompetent individuals, it causes severe, morbid, and sometimes fatal illness among those with compromised immunity such as HSCT and SOT recipients.1,2 In the transplant population, the impact of CMV has been categorized into direct effects and indirect effects.1,2 The collective impact of these direct and indirect CMV effects impedes on the overall success of transplantation.1,2 A major goal in the management of transplant recipients is therefore to prevent, or at least minimize, the direct and indirect consequences of CMV infection. Is this goal attainable?
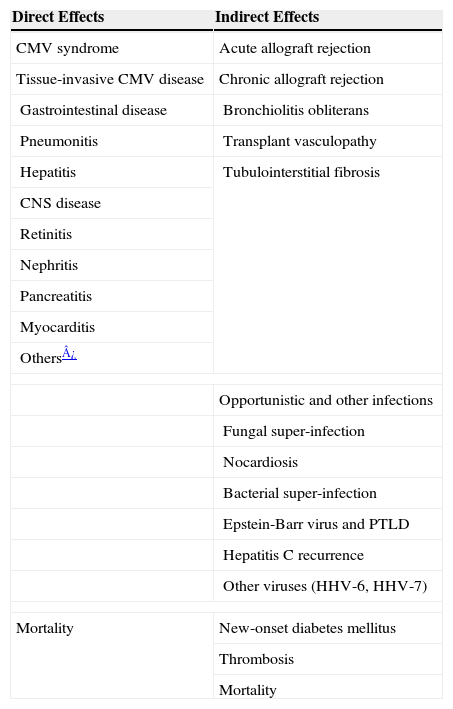
Can we prevent the direct effects of cytomegalovirus?The prevention strategies against CMV have been primarily aimed at reducing the immediate direct effects of the virus. These direct CMV effects are well-characterized among transplant recipients,1,2 and includes a febrile illness accompanied by bone marrow suppression (termed CMV syndrome) or organ-invasive symptoms (termed tissue-invasive CMV disease) (Table 1).3 While CMV may present as an asymptomatic infection after transplantation, the majority of cases are manifested as “CMV syndrome,” which is characterized by fever with some degree of bone marrow suppression.4–9 Less commonly, CMV disease may be manifested as an organ-invasive illness, which most commonly involves any part of the gastrointestinal tract.4–9 A transplant recipient with gastrointestinal tissue-invasive CMV disease typically presents with abdominal pain and diarrhea, and in severe cases, bleeding. Allogeneic HSCT recipients are particularly at high risk of developing CMV pneumonitis, a severe form of tissue-invasive CMV disease that can have a fatal outcome if not detected and treated promptly.2,10–12 Allograft pneumonitis is also a common presentation of CMV disease among lung transplant recipients compared to other SOT recipients.1,2,7 Indeed, CMV infection of the allograft, such as hepatitis, pancreatitis, myocarditis, and nephritis are observed more commonly among liver, pancreas, heart, and kidney transplant recipients, respectively.1,2 Virtually any organ system, however, may be affected directly by CMV to cause retinitis, neuritis (and polyradiculitis), cholecystitis, and epididymitis.1,2,13 If left untreated, CMV disease, especially if this involves the lung and the central nervous system, can rapidly evolve and progress to death in immunocompromised transplant recipients.
Direct and indirect effects of CMV after transplantation
| Direct Effects | Indirect Effects |
| CMV syndrome | Acute allograft rejection |
| Tissue-invasive CMV disease | Chronic allograft rejection |
| Gastrointestinal disease | Bronchiolitis obliterans |
| Pneumonitis | Transplant vasculopathy |
| Hepatitis | Tubulointerstitial fibrosis |
| CNS disease | |
| Retinitis | |
| Nephritis | |
| Pancreatitis | |
| Myocarditis | |
| Others¿ | |
| Opportunistic and other infections | |
| Fungal super-infection | |
| Nocardiosis | |
| Bacterial super-infection | |
| Epstein-Barr virus and PTLD | |
| Hepatitis C recurrence | |
| Other viruses (HHV-6, HHV-7) | |
| Mortality | New-onset diabetes mellitus |
| Thrombosis | |
| Mortality | |
Note: PTLD, post-transplant lymphoproliferative disease; HHV, human herpes virus.
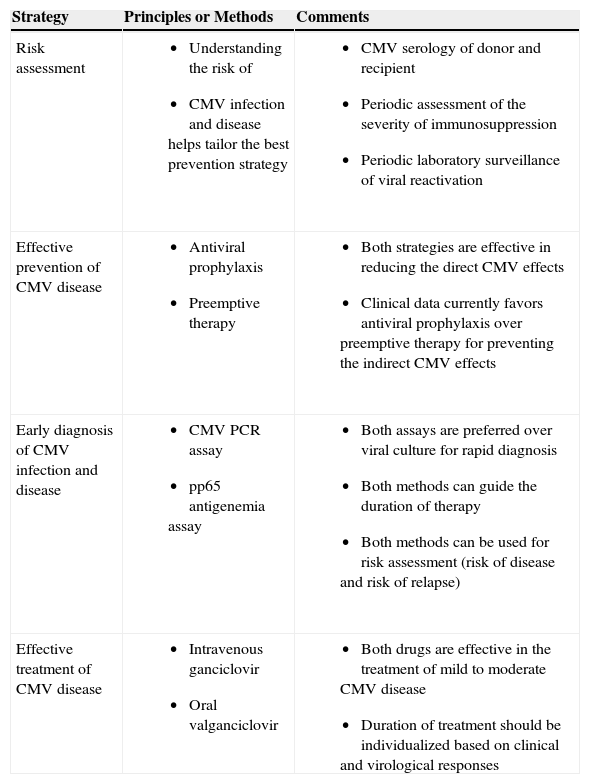
Traditionally, the onset of CMV infection and disease occurs during the first 3 months after HSCT and SOT.1–3 However, this onset has been delayed by the use of antiviral prophylaxis.4–9 It is therefore during this period of time when CMV prevention strategies are implemented. There are several strategies that are aimed to prevent the immediate direct effects of CMV (Table 2).1,2,14 Understanding a patient's CMV risk is the first step in defining the best approach for prevention. For example, a CMV donor-seropositive/recipient-seronegative (D+/R-) SOT recipient and CMV recipient-seropositive (R+) allogeneic HSCT recipient are considered at highest risk of CMV disease and are therefore candidates for aggressive CMV prevention strategy.1,2,10,11,14,15 Likewise, lung, intestinal, and pancreas recipients are considered at relatively higher risk when compared to other SOT recipients.1,2,14 A profound net state of immunosuppression, such as when an allogeneic HSCT recipient receives augmented immunosuppression for prevention or treatment of acute or chronic graft-versus-host disease, is associated with increased risk of CMV disease.10,11 Pharmacologically-induced immunological dysfunction is especially severe when lymphocyte-depleting drugs such as muromonab-CD3 (OKT3) and anti-thymocyte globulin are used.1,2,14 Use of the anti-CD52 alemtuzumab, which causes a prolonged suppression of T cell function, is particularly associated with higher risk of CMV disease, especially when it is used for the treatment of acute rejection.16 Acute rejection by itself is also associated with increased risk of CMV disease.17 Host factors, such as deficiencies in innate and adaptive immune molecules, have been shown to increase the risk of CMV disease.18–20 Finally, viral factors, such as peak viral load or the kinetics of viral replication, may influence the risk of CMV disease.21 A patient with a higher absolute viral load or a rapidly increasing viral load is at increased risk of CMV disease.21
Strategies to Prevent the Direct and Indirect Effects of CMV
| Strategy | Principles or Methods | Comments |
| Risk assessment |
|
|
| Effective prevention of CMV disease |
|
|
| Early diagnosis of CMV infection and disease |
|
|
| Effective treatment of CMV disease |
|
|
Knowledge of these risk factors for CMV disease defines the best strategy for preventing its direct effects. There are generally two antiviral approaches for CMV prevention in SOT and HSCT recipients–preemptive therapy and antiviral prophylaxis.22–27 The strategy of preemptive therapy selectively provides antiviral drugs (most commonly oral valganciclovir or intravenous ganciclovir) to HSCT and SOT recipients with laboratory evidence of CMV replication.21,28 The major goal of this strategy is to prevent the progression of asymptomatic CMV reactivation to full-blown clinical disease. For preemptive therapy to work, one needs a highly-sensitive assay that can detect CMV reactivation as early as possible and prior to the onset of clinical symptoms.29 In this regard, frequent (i.e., weekly) laboratory surveillance for CMV replication is usually performed using CMV polymerase chain reaction (PCR) assay or a pp65 antigenemia assay.29 In this issue of the journal, Torre-Cisneros and colleagues report on their clinical experience using preemptive therapy for CMV infection in allogeneic HSCT recipients.30 Using a CMV PCR assay, thirty-two of 89 HSCT recipients were found to have CMV replication that required preemptive therapy.30 The authors preemptively treated their patients for a fixed duration of 2 weeks (with an additional 2 weeks of maintenance therapy for those with persistent viral load) and they observed that this approach was effective in half of the patients.30 The main factor associated with failure to clear the virus from the blood was a high initial viral load (>20,000 copies/ml), which in turn was associated with graft-versus-host disease (thereby implying a profound state of immunosuppression).30 In retrospect, this study highlights the need to individualize the duration of preemptive therapy, as this should be guided by periodic clinical and virological assessments.14,31,32 It is generally recommended to preemptively treat HSCT and SOT recipients until all evidence of CMV replication has resolved.14,31,32 In recent meta-analyses that evaluated its efficacy, preemptive therapy prevented the direct effects of CMV disease in transplant recipients by as much as 70%.23,24,27
The second major antiviral strategy of CMV prevention is antiviral prophylaxis which entails the administration of an antiviral drug (most commonly valganciclovir) to all at-risk transplant recipients for at least 3 months after SOT and HSCT.6,8,9 This is usually begun immediately after SOT or after engraftment in HSCT recipients. Antiviral prophylaxis is used more often among SOT recipients, especially among those at highest risk of CMV disease. This group includes lung, intestinal, and pancreas recipients, and all CMV D+/R− SOT recipients. In contrast, antiviral prophylaxis is used less often after HSCT since the myelosuppressive effects of ganciclovir-based regimen may impede the optimal engraftment of the stem cells.11 Several clinical trials have demonstrated that antiviral prophylaxis is highly effective in preventing the direct effects of CMV disease.6,8,9 In recent meta-analyses, antiviral prophylaxis reduced the incidence of CMV disease by 60–80%.22,24,27 A placebo-controlled randomized trial demonstrated the reduction in the incidence of CMV disease (from 19% to 5%) in liver transplant recipients who received 98 days of oral ganciclovir prophylaxis.6 Among the high-risk CMV D+/R- liver recipients, oral ganciclovir reduced the incidence of CMV disease from 44% to 15%.6 Similarly, valacyclovir prophylaxis reduced the direct effects of CMV disease from 45% to 16% among CMV D+/R- kidney recipients.8 However, these seminal studies illustrate that, while the direct effects of CMV disease have been markedly reduced, it has not been completely prevented. Up to 30% of CMV D+/R− SOT recipients will develop the direct effects of CMV disease soon after they complete a standard 3-month course of oral ganciclovir, valacyclovir or valganciclovir prophylaxis.4–6,8,9,17 In a recently reported multicenter study of CMV D+/R− kidney transplant recipients, prolonging valganciclovir prophylaxis from 100 days to 200 days further reduced the incidence of CMV disease from 37% (with 100 days of prophylaxis) to 16% (with 200 days of prophylaxis).33 Hence, while the majority of direct CMV effects have been prevented by antiviral prophylaxis, the degree of prevention is not yet absolute since CMV D+/R− SOT recipients who have not developed CMV-specific immunity will continue to remain at risk after cessation of prophylaxis (termed delayed-onset primary CMV disease).34 Further reduction in the direct CMV effects should remain a primary goal of post-transplant care since the occurrence of CMV disease, even at a delayed onset, continues to negatively impact transplant outcome.5,35
Can we prevent the indirect effects of cytomegalovirus?In addition to the direct effects, CMV appears to trigger many clinically-relevant indirect effects after transplantation.1,2,14 While these indirect effects have been observed most commonly among SOT recipients,1,2,14 there is increasing appreciation of these indirect effects in HSCT recipients as well.36,37 These indirect effects are generally attributed to the immunomodulating property of CMV38 and its persistence, even at low levels, in the transplanted allograft.39,40
One of the well-recognized indirect effects of CMV is an increased predisposition of CMV-infected patients to develop other opportunistic infections (such as fungi, other viruses, and opportunistic bacteria including Nocardia spp.).14,16 Patients who developed primary CMV infection after allogeneic HSCT were more likely to develop invasive bacterial and fungal infections;37 this association has previously been observed in SOT recipients.5,41 Likewise, SOT recipients who developed CMV disease were more likely to develop Epstein-Barr virus associated post-transplant lymphoproliferative disease,42 or develop co-infections with other viruses including human herpes virus.6,43 The association between CMV and an accelerated hepatitis C virus recurrence after liver transplantation has been described in several studies.44,45
Through various mechanisms, including the upregulation of alloantigens, CMV may facilitate the development of acute and chronic allograft rejection (among SOT patients), which may eventually lead to chronic allograft dysfunction.39,40 The relationship between chronic allograft nephropathy (and tubulointerstitial fibrosis) and CMV disease has been suggested,39 and in one study, CMV persistence in the kidney allograft was associated with lower creatinine clearance.39 Bronchiolitis obliterans, which is a form of chronic lung allograft failure, was reportedly more common among lung recipients who developed CMV disease.46,47 CMV seropositivity (and the lack of anti-CMV prophylaxis) was significantly associated with negative vascular remodeling and greater loss of vascular lumen after heart transplantation, leading to accelerated cardiac allograft vasculopathy.48 CMV seropositivity was also found to be a predictor of vasculopathy in kidney recipients.49 Recently, the potential association between CMV and new-onset diabetes after transplantation was described, although the underlying mechanism remains undefined.50 Finally, CMV is described as an independent predictor of mortality after SOT and HSCT.5,15,35,36,51
Preventing these indirect CMV effects is believed to be intimately related to the prevention of the direct effects of CMV infection. It is postulated that if one can prevent the direct effects of CMV, a downstream benefit is the prevention of the immediate and long-term indirect effects. Indeed, several studies have now demonstrated that antiviral prophylaxis (which is primarily intended to prevent direct CMV effects) have the added benefit of reducing the incidence of at least some of the indirect effects of CMV.6,8,22,52 For example, a clinical trial in kidney transplant recipients demonstrated a significantly lower 6-month incidence of biopsy-proven acute allograft rejection with valacyclovir prophylaxis (26% versus 52% among placebo recipients).8 Another study demonstrated that acute rejection occurred in 58% of patients who developed CMV disease compared to 12% among those who received valacyclovir prophylaxis.53 These observations mirror the findings observed with oral ganciclovir prophylaxis after liver transplantation54. Likewise, a study in heart transplant recipients demonstrated reductions in the indirect effects of CMV, particularly allograft rejection and vasculopathy, by strategies of CMV prevention.55 Effective CMV-specific immune reconstitution has also been associated with lower incidence of acute rejection and vasculopathy.56 In addition, oral ganciclovir prophylaxis has been significantly associated with a lower incidence of bacteremia after liver transplantation.41 Recent meta-analyses further demonstrated reductions in the incidence of bacterial and protozoal infections in transplant recipients who received antiviral prophylaxis.22 These meta-analyses of antiviral prophylaxis and preemptive therapy have also demonstrated a significant reduction in acute allograft rejection.24 These meta-analyses also demonstrated that the indirect effect of CMV on all-cause mortality was reduced with antiviral prophylaxis but not with preemptive therapy.22,24,27 A prospective clinical study further demonstrated that antiviral prophylaxis was associated with a significantly better long-term kidney allograft survival at 4 years compared with preemptive therapy.52 Collectively, these data suggest that the efforts primarily directed at preventing the direct effects of CMV disease may consequently prevent the indirect CMV effects. Current data suggests that antiviral prophylaxis may be a better approach, although this conclusion is based mainly on small prospective studies, retrospective studies, and meta-analyses.
Finally, preventing the indirect effects of CMV necessitates early diagnosis and treatment of CMV infection and disease. The use of rapid and sensitive methods of CMV detection facilitates in achieving this goal.29 In this regard, CMV PCR assays (and pp65 antigenemia assays) have demonstrated superiority over virus culture in the early diagnosis of CMV.29 Moreover, the quantitative property of viral PCR assays have the added advantage of guiding clinicians in assessing the risk of disease, in defining the duration of treatment, and in predicting the risk of treatment failure or relapse.14,29,32 The clinical study by Torre-Cisneros and colleagues in HSCT recipients clearly illustrates this point.30 Peak viral load was significantly correlated with the success of treatment. This observation is similar to a recent study of SOT recipients with CMV disease, wherein the viral load at start of treatment was significantly associated with the outcome of treatment.31 Patients with higher initial viral load (>20,000 copies/ml in the study by Torre-Cisneros30) would therefore require a longer duration of antiviral treatment.14,30–32 The persistence of the virus at the end of therapy is associated with a higher risk of clinical relapse.32,57 Hence, the duration of antiviral treatment should not be for a fixed duration, but instead should be guided by CMV PCR assay. It is generally recommended that multiple (at least two) weekly negative CMV PCR should be demonstrated before antiviral therapy can be discontinued.58
ConclusionsThe management of CMV infection and disease after HSCT and SOT has improved remarkably over the years. What was once a commonly fatal infection is now a treatable disease, as long as it is diagnosed early. The improvement in outcome of post-transplant CMV infection is the product of multiple advances in the field of diagnostics (PCR and pp65 antigenemia assay) and therapeutics (ganciclovir and valganciclovir). The study by Torre-Cisneros exemplifies these advances.30 In the contemporary era, the majority of the direct CMV effects have been prevented with the use of effective antiviral prophylaxis and preemptive therapy. Remarkably, improvements in CMV prevention have also translated into reductions in the indirect effects of the virus. With effective CMV prevention, one now anticipates the downstream beneficial effect of reducing opportunistic infection and acute rejection as well as improving patient and allograft survival. However, there is still work to be done since a considerable number of high-risk HSCT and SOT recipients remain at increased risk of CMV disease as soon as the antiviral prevention strategy is discontinued. As we continue to optimize our efforts to prevent the direct effects of CMV, we should also anticipate further reductions in the indirect effects of CMV, thereby improving overall transplant outcomes. Indeed, eliminating both the direct and indirect CMV effects should be considered as intertwined goals of CMV prevention. Data from recent clinical studies suggest that this goal of preventing the direct and indirect effects of CMV should be attainable.