Group B streptococcus (GBS) neonatal sepsis prevention is based in the intrapartum antibiotic treatment of colonised women.1 It is recommended to carry out microbiological colonisation studies between 35 and 37 weeks of gestation for GBS detection. These studies involve the inoculation from rectum and vaginal swabs on to enriched, selective and/or pigment-producing media, according to the Centers for Disease Control and Prevention (CDC).2 The selective and differential Granada agar medium has been recommended by several authors in the screening of GBS, with good results.3–5 Over the last few years, different models of this medium have been marketed with the purpose of improving some parameters, such as the detection time, ease of operation, and storage and preservation conditions. Liquid biphasic and instant liquid biphasic Granada media are examples of this variation.
The aim of this study was to evaluate the diagnostic effectiveness of instant liquid biphasic Granada medium (Biphasic Granada™ culture broth, bioMérieux® Spain S.A.) and compare it with the standard medium for GBS detection.
Two vagino-rectal samples were collected from each patient between 35 and 37 weeks of gestation and placed into Amies transport medium before processing. A total of 502 patients were included in this study. Swabs were dipped in parallel, both into Todd-Hewitt broth with nalidixic acid (15μl/ml) and gentamicin (8μg/ml) (Biomedics, Spain) (standard method) and into instant biphasic Granada medium, swirled briefly, broken and left in the tubes. They were incubated at 37°C. Instant biphasic Granada tubes were inspected at 24h and 48h. The appearance of orange-pigmented colonies in this medium was indicative of the presence of GBS. After 24h of incubation, Todd-Hewitt broths were subcultured onto blood agar plates, which were examined at 24h and 48h of incubation at 37°C in a 5% CO2 atmosphere. Beta-haemolytic colonies were identified as GBS using the automatic system, Vitek2 (bioMérieux® Spain S.A.).
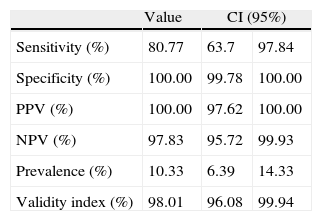
Group B streptococci were identified after 24h of incubation, in 52 of 502 pregnant women (10.35%) using the standard method, and in 42 cases (8.36%) by instant biphasic Granada media. A sensitivity of 81%, compared to the standard method, was observed using this last procedure. All the GBS detected by Granada media were confirmed using the reference method with 100% specificity. Avidity values of diagnostic tests are shown in Table 1.
Operation features of instant liquid biphasic Granada medium compared with the standard method.
| Value | CI (95%) | ||
| Sensitivity (%) | 80.77 | 63.7 | 97.84 |
| Specificity (%) | 100.00 | 99.78 | 100.00 |
| PPV (%) | 100.00 | 97.62 | 100.00 |
| NPV (%) | 97.83 | 95.72 | 99.93 |
| Prevalence (%) | 10.33 | 6.39 | 14.33 |
| Validity index (%) | 98.01 | 96.08 | 99.94 |
PPV, positive predictive value; NPV, negative predictive value.
The most widely used enrichment medium for detection of GBS is Todd-Hewitt broth in the presence of antibiotics. Moreover, this culture broth has been recommended by CDCs.2,6,7 The highest sensitivity for GBS screening is obtained using an enrichment broth followed by a subculture in a solid medium, i.e. blood agar or Granada media.8 Data from the present study showed a excellent specificity with instant liquid biphasic Granada medium, in full agreement with previous works.8,9 Nevertheless, only 81% of GBS in pregnant women were detected using this medium alone. Sensitivities between 80% and 93% using similar liquid Granada media were reported by others authors,8,9 so data obtained in the present work are located in the lower limit of sensitivity reported by the literature consulted. On the other hand, a study performed by Carvalho et al. reported an excellent sensitivity in the GBS screening study. However, this study did not reproduce real conditions, as it was performed with GBS typified strains, and in ideal conditions, using known concentration bacterial inoculi, and without samples collected from patients.10 In conclusion, according to data in the present study, the use of instant liquid Granada medium as the only method for the screening of GBS from colonised pregnant women is not the procedure of choice, due to its limited yield. Nevertheless, the advantages of this medium, such as, short time in detection, ease of manipulation and simplicity in the identification of the typical colonies, could make it useful in combination with other standard methods.