Staphylococcus epidermidis is an organism commonly associated with infections caused by biofilms. Biofilms are less sensible to antibiotics and therefore are more difficult to eradicate. Linezolid and N-acetylcysteine (NAC), have demonstrated to be active against gram-positive microorganisms. Therefore and since linezolid and NAC have different modes of action, the main objective of this work was to investigate the single and synergistic effect of linezolid and NAC against S. epidermidis biofilms.
MethodsThis work reports the in vitro effect of linezolid and NAC against S. epidermidis biofilms, treated with MIC (4mgml−1) and 10×MIC of NAC, and MIC (1μgml−1) and peak serum concentration (PS=18μgml−1) of linezolid alone and in combination. After exposure of S. epidermidis biofilms to linezolid and/or NAC for 24h, several biofilm parameters were evaluated, namely the number of cultivable cells [colony forming unit (CFU) enumeration], total biofilm biomass and cellular activity.
ResultsWhen tested alone, NAC at 10×MIC was the most effective agent against S. epidermidis biofilms. However, the combination linezolid (MIC)+NAC (10×MIC) showed a synergistic effect and was the most biocidal treatment tested, promoting a 5log reduction in the number of biofilm viable cells.
ConclusionThis combination seems to be a potential candidate to combat infections caused by S. epidermidis biofilms, namely as a catheter lock solution therapy.
Staphylococcus epidermidis es un organismo comúnmente asociado con infecciones causadas por biofilms. Los biofilms son menos sensibles a los antibióticos y, por lo tanto, más difíciles de erradicar. El linezolid y la N-acetilcisteína (NAC) han demostrado ser activos contra los microorganismos grampositivos. Por lo tanto, y puesto que el linezolid y la NAC tienen diferentes modos de acción, el objetivo principal de este trabajo fue investigar el efecto individual y sinérgico del linezolid y la NAC frente a biofilms de S. epidermidis.
MétodosEste trabajo reporta el efecto in vitro del linezolid y la NAC solos y en combinación contra biofilms de S. epidermidis, tratados con NAC en las concentraciones de MIC (4mg·ml–1) y 10×MIC, y linezolid en las concentraciones MIC (1mg·ml–1) y concentración sérica máxima (PS=18μg ml–1). Después de la exposición de los biofilms de S. epidermidis al linezolid y/o la NAC durante 24h, fueron evaluados varios parámetros del biofilm, a saber, el número de células cultivables (recuento de unidades formadoras de colonias [UFC]), la biomasa total del biofilm y la actividad celular.
ResultadosDurante el ensayo solo, la NAC en la concentración de 10×MIC fue el agente más eficaz contra biofilms de S. epidermidis. Sin embargo, la combinación linezolid (MIC)+NAC (10×MIC) mostró un efecto sinérgico y fue el tratamiento con mayor efecto biocida, promoviendo una reducción logarítmica de 5 en el número de células viables del biofilm.
ConclusiónEsta combinación parece ser un potencial candidato en el combate de las infecciones causadas por biofilms de S. epidermidis, es decir, como una terapia de solución de bloqueo del catéter.
Staphylococcus epidermidis is a commensal inhabitant of the healthy human skin and mucosa. However, this gram-positive bacterium has developed interesting strategies to change into a notorious pathogen,1 being now one of the leading causes of prosthetic device infections. This capacity appears to be due to its ability to colonize both the host's skin as part of the commensal flora and prosthetic materials via its ability to adhere to the surface of indwelling medical devices and to form biofilms.2 This feature is the main virulence factor associated with S. epidermidis infections and is a major clinical problem, mainly due to the high level of biofilm tolerance/resistance to antibiotics.3 In fact, resistance to antibiotics by pathogenic S. epidermidis isolates is an increasing trait.4,5 When in biofilms, cells can be up to 1000 times more tolerant to antibiotics than their planktonic counterparts.6 This coagulase negative staphylococci is the cause of a great number of infections,7,8 namely endocarditis and infections of any type of indwelling medical devices, such as peripheral or central intravenous catheters (CVCs).8
In the beginning of this decade, linezolid (an oxazolidinone) was approved as a new antibiotic, offering a new option for the treatment of, for example, complicated skin and skin structure infections caused by resistant gram-positive pathogens9 and has been claimed to be 100% efficient.10 Linezolid is a protein synthesis inhibitor and is active against gram-positive microorganisms, including enterococci, streptococci, and Staphylococcus aureus.11,12 Nowadays, this synthetic antibiotic plays a very important role against infections caused by multiresistant gram-positive pathogens13,14 and has excellent activity against most staphylococci, although resistance has begun to emerge.15,16 Nevertheless, when tested against S. aureus and S. epidermidis biofilms it was not as efficacious as expected.17
On the other hand, N-acetylcysteine (NAC) is a non-antibiotic drug that decreases/prevents biofilm formation ability and adherence to biomaterials devices of a variety of bacteria18–20 and reduces the production of the extracellular polysaccharide matrix,21 while promoting the disruption of mature biofilm.16,18,20 NAC is widely used in medical practice.23,24
The main goal of this study was to determine the susceptibility of S. epidermidis biofilm cells to a new antibiotic (linezolid) in combination with a non-antibiotic drug (NAC), both with different modes of action, searching for possible synergistic interactions. For that, we assessed the inhibitory effect of these antimicrobial agents through the enumeration of biofilm viable cells, metabolic activity and total biofilm biomass.
MethodsBacterial strains and growth conditionsTwo clinical isolate strains of S. epidermidis, 9142 and 1457 were used in this study because they produce very thick biofilms with an abundant amount of extracellular matrix.25,26 Both strains were provided by Dr. G. B. Pier, Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, USA.
The culture media used, tryptic soy broth (TSB) and tryptic soy agar (TSA), were prepared according to the manufacturer's instructions. All strains were inoculated into 15ml of TSB from TSA plates not older than 2 days and grown for 18 (±2) h at 37°C in an orbital shaker at 130rpm. Cells were harvested by centrifugation (for 5min at 9500×g and 4°C) and resuspended in TSB with the final suspension adjusted to an optical density (640nm) equivalent to 1×109CFUml−1, to be used in the subsequent assays. The experiments were performed with S. epidermidis cultures in the log-phase of growth.
The stock solution of linezolid was prepared in Milli-Q water and diluted in TSB in order to be used as working solution (at MIC and peak serum concentrations). The working solutions of NAC (MIC and 10×MIC) were prepared in TSB. Both antimicrobial agents tested were pure substances (Sigma–Aldrich, St. Louis, MO).
Minimum inhibitory concentration (MIC)The minimum inhibitory concentration was determined by the microbroth dilution technique. MIC determination of the tested agents and for both S. epidermidis strains (9142 and 1457) was performed by the serial two fold dilution method at concentrations ranging from 0.007μgml−1 to 16μgml−1, and 0.007mgml−1 to 16mgml−1, for linezolid and NAC, respectively. The controls were cells not exposed to the antimicrobial agents tested. All experiments were carried out in triplicate and repeated three times.
Biofilm formation and susceptibility/synergy testsBiofilms were formed in 96 well tissue culture plates (Sarstedt, Newton, NC, USA) containing 200μl of S. epidermidis cell suspension (1×106CFUml−1) in TSB supplemented with 0.25% of glucose per well to promote biofilm formation. Plates were incubated at 37°C with orbital shaking at 130rpm for 24h. At the end, the supernatant was carefully removed and the biofilm was washed twice with 200μl of saline solution [0.9% NaCl (Merck)]. The biofilm was then incubated for 24h, in fresh nutrient medium (TSB) containing linezolid (at MIC 1μgml−1 and PS 18μgml−1) or NAC (MIC 4000μgml−1 and 10×MIC 40,000μgml−1) or a combination of both. Enumeration of colony forming units (CFU), crystal violet staining (CV) and XTT reduction assays was performed after 24h of exposure to the antimicrobial agents tested, either alone or in combination. At time 0 (before exposure to antibiotics) the initial biofilm cellular concentration of (∼2×108CFUml−1) was determined.
To assess the number of viable cells after antibiotic treatment, the planktonic cells were carefully removed and then the biofilms were washed with 250μl of saline solution. Every well was resuspended in 0.9% NaCl, thoroughly scraped and biofilm cells transferred to centrifuge tubes and centrifuged for 10min at 9500×g and 4°C. The pellet was resuspended in 0.9% NaCl and washed twice, followed by 20s of sonication at 22W to homogenize the suspension. Viable cells were determined by performing 10-fold serial dilutions in saline solution and plating in TSA. Colonies were counted after 24h incubation at 37°C.
Crystal violet (CV) staining was used as an indicator of total biofilm biomass. After exposure to the treatment agents, biofilms were washed with 250μl of saline solution. Then, 250μl of methanol was added and allowed to act for 15min. Afterwards, methanol was removed and crystal violet 1% (v/v) was added (5min). The wells were washed with distilled water and finally, acetic acid 33% (v/v) was added and the absorbance of the solution was measured at 570nm.
Another colorimetric method, based on the reduction of XTT ({2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide}), was applied to assess cellular activity (XTT is converted to a colored formazan salt in the presence of metabolic activity).27 After exposure to antimicrobial agents, biofilms were washed with 250μl of saline solution, followed by the addition of 250μl of a solution containing 200mgl−1 of XTT and 20mgl−1 of PMS (phenazine methosulfate) (Sigma–Aldrich, St. Louis, MO) to each well. The microtiter plates were incubated for 3h at 37°C in the dark. The absorbance was measured at 490nm.
A control was performed with biofilm cells not exposed to the antibiotics tested. All experiments were carried out in triplicate and repeated three times.
Statistical analysisData were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey method for pairwise multiple comparisons using SPSS software (Statistical Package for the Social Sciences). p<0.05 was considered statistically significant.
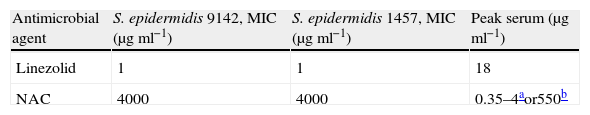
ResultsTable 1 presents the MIC values of linezolid and NAC (for planktonic cells) determined in this study and peak serum concentrations obtained from the literature for linezolid28 and NAC after oral29 and intravenous dose.30
Overall, linezolid showed the best activity against both S. epidermidis planktonic strains, with MIC=1μgml−1 (Table 1) and both agents were equally effective against the two strains assayed.
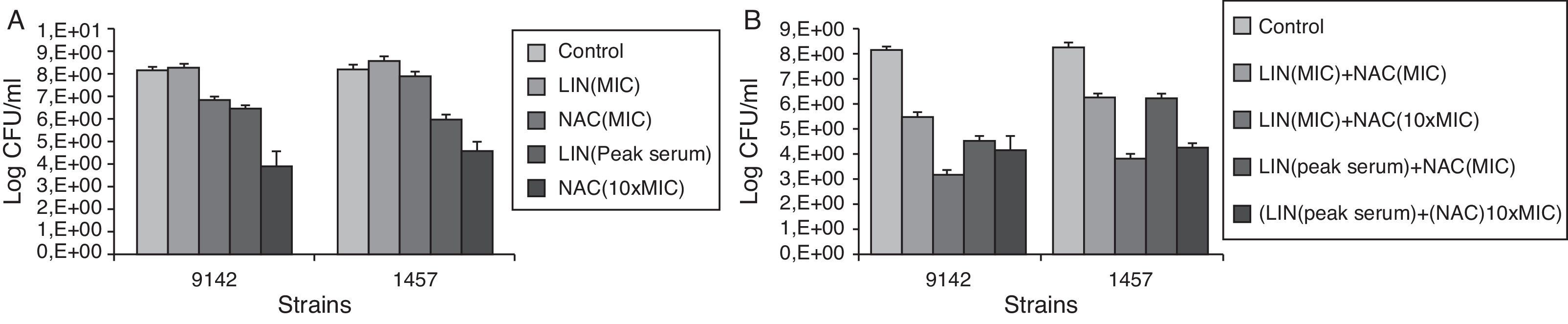
Fig. 1 presents the effect of linezolid and NAC (Fig. 1A) and the association linezolid+NAC (Fig. 2B) against S. epidermidis biofilm cells.
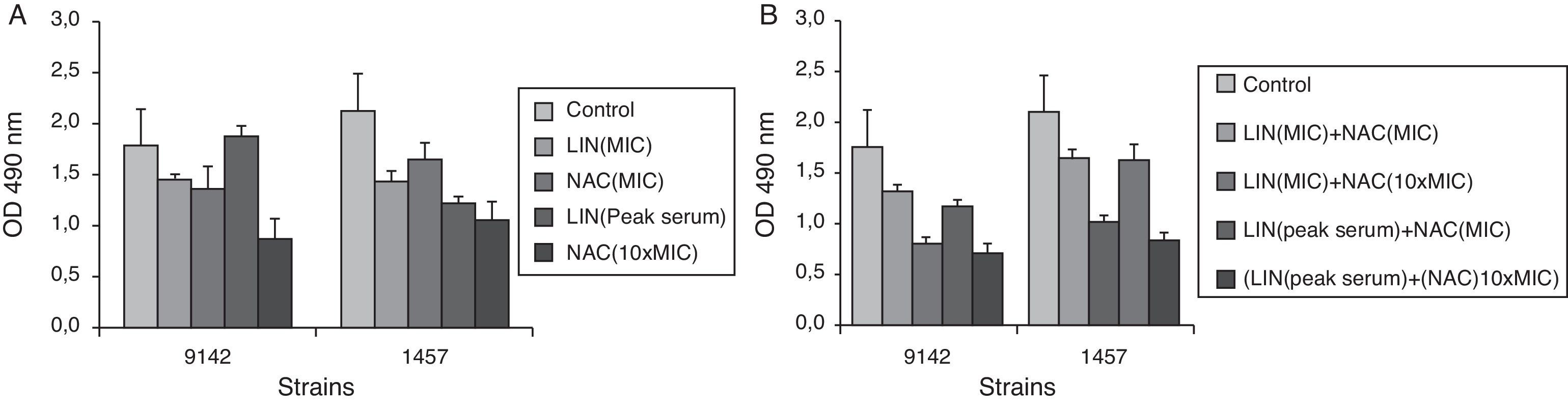
Effect of linezolid (LIN) and/or NAC on the metabolic activity of biofilm cells of S. epidermidis 9142 and 1457, after 24h of contact with linezolid (MIC and PS) and NAC (4mgml−1 and 40mgml−1), alone (A) and in combination (B), after 24h of treatment expressed as XTT absorbance (cellular activity). Error bars represent standard deviation.
A bactericidal effect was defined as a reduction of ≥3log10 of the initial bacterial inoculum. A lesser reduction in bacterial count (<3log10) was defined as a bacteriostatic effect.31,32
Regarding the effect of each agent alone, NAC at 10×MIC showed the highest bactericidal activity against biofilm cells with a significant inhibitory effect (p<0.05), causing a reduction of approximately 4log in CFU retrieved from the biofilm. Although linezolid at peak serum concentration cannot be considered an effective bactericidal agent against S. epidermidis biofilms, it demonstrated a significant inhibitory and bacteriostatic effect, promoting a CFU reduction of ∼2log (p<0.05). However, linezolid at MIC combined with NAC either at MIC or 10×MIC displayed a synergistic effect against both strains in biofilm form. Linezolid at peak serum concentration plus NAC at MIC also had a synergistic effect against the S. epidermidis strain 9142. Overall, the most effective combination was linezolid (MIC)+NAC (10×MIC) causing a CFU reduction of approximately 5log. There was no synergistic or additive effect when linezolid at peak serum concentration was combined with NAC at 10×MIC.
The results expressing the decrease in metabolic activity measured by the XTT reduction assay after treatment with linezolid or NAC alone and in combination are presented in Fig. 2. These results are in accordance with the results obtained by CFU enumeration confirming that linezolid (MIC)+NAC (10×MIC) promoted a very significant reduction in cellular metabolic activity. It should be stressed that the results obtained with the XTT reduction assay are more qualitative than quantitative because it is very difficult to find a direct relation between the number of cells and their metabolic activity in a biofilm, where the upper cell layer is in environmental conditions much different from more inner layers, that can be deprived of some nutrients.
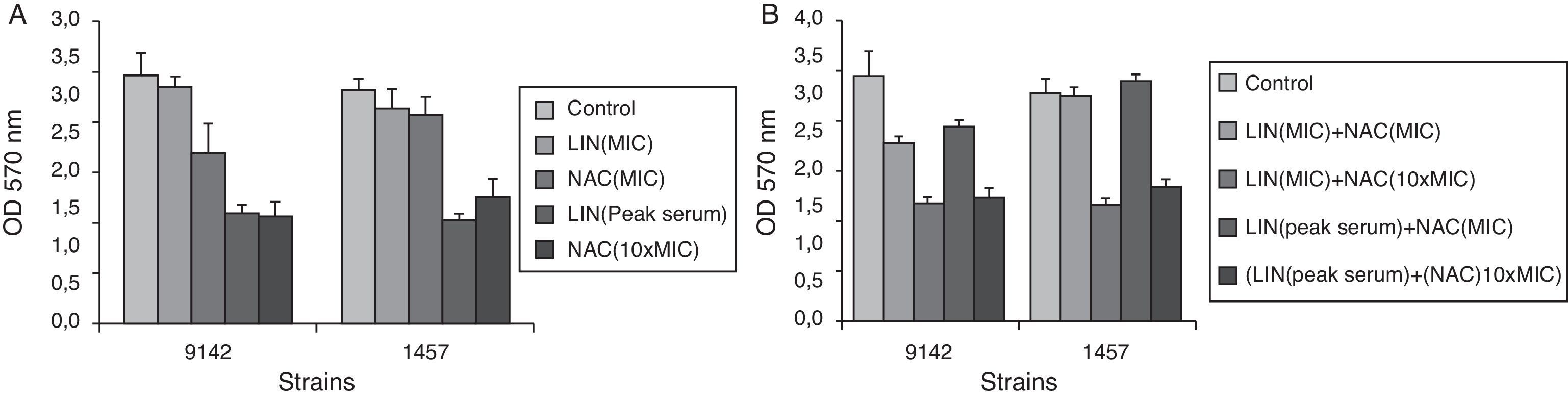
The variation in total biofilm biomass, assessed by CV staining, also confirms the effect of the antimicrobial agents tested. As it can be seen in Fig. 3, linezolid (MIC)+NAC (10×MIC) caused a significant reduction in biofilm biomass of S. epidermidis (p<0.05).
Effect of linezolid (LIN) and/or NAC on total biofilm biomass of S. epidermidis 9142 and 1457, after 24h of contact with linezolid (MIC and PS) and NAC (4mgml−1 and 40mgml−1), alone (A) and in combination (B), after 24h of treatment expressed as CV absorbance (total biofilm biomass). Error bars represent standard deviation.
The results obtained demonstrate that linezolid is a very active agent against S. epidermidis planktonic cells being the minimal concentration necessary to inhibit the staphylococci growth (MIC) 4000-fold inferior than that of NAC (Table 1). However, these two drugs have an opposite behavior when applied to biofilms, which can be related, partly, to diffusional limitations imposed by the biofilm matrix, since the molecular weight of linezolid (337.35) is approximately twice that of NAC (163.19) but mostly due to the lower metabolic activity displayed by biofilm cells. Linezolid works by inhibiting the bacterial protein synthesis at the initiation step, by binding to the 23S portion of the 50S subunit (the center of peptidyl transferase activity),33 and for that it has to be up taken by the cells and this process is hindered in biofilm cells on account of their low metabolic activity or even dormancy.34 On the other hand, NAC, well recognized as a mucolytic agent, acts by disrupting the biofilm structure through the splitting of disulfide bonds linking the proteins present in the extracellular matrix.18,21 Other authors also suggest that the mechanism for the antibacterial effect of NAC may be that it acts by competitively inhibiting amino acid (cysteine) utilization or, by virtue of possessing a sulfhydryl group, may react with bacterial cell proteins.35
Previous studies have shown that NAC could decrease biofilm formation by a variety of bacteria such as Escherichia coli, S. epidermidis, Pseudomonas aeruginosa, etc.18–20,35 Zhao and Liu35 concluded that NAC has antibacterial properties against P. aeruginosa, dispersing and detaching P. aeruginosa biofilms. NAC also inhibited bacterial adherence, reduced the production of extracellular polysaccharide matrix,21 while promoting the disruption of mature biofilms, and reduced sessile cell viability.18–21
Therefore, this detachment and dispersive action of NAC in biofilms may make the biofilm-associated bacteria more susceptible to other antimicrobial agents,22 as in the present association with linezolid. NAC alone, in this case at a concentration of 40mgml−1, also presented a very high inhibitory effect, promoting a 4log reduction in cultivable cells, and a significant reduction of total biofilm biomass and cellular metabolic activity. However, to the author's knowledge, NAC bactericidal mechanism of action is still not known, more probably consisting of a physical cleavage of the bacterial cell envelope. Our results are in accordance with those obtained in previous studies20–23,35: NAC decreased sessile cell viability translated by the reduction of CFU; promoted biofilm detachment and dispersion translated by the reduction of biofilm biomass.
Therapy almost always fails to eradicate the bacteria in biofilms. So, the search of effective drugs able to inhibit biofilm formation and/or disrupt/eradicate established biofilms or that may work in synergy with other antimicrobial agents, is essential for treating biofilm associated-diseases.
Overall, a general promotion of biofilm weakness may be a potential help to human immune system in the combat of S. epidermidis biofilm associated-infections. However, the combination of linezolid (MIC=1μgml−1) with NAC (10×MIC=40mgml−1) demonstrated a synergistic effect, and is therefore more effective than NAC (10×MIC) alone. This combination was able to reduce the number of CFU from 8log to ∼3log, a nearly 5log reduction. Apart from being more active, the combination linezolid (MIC)+NAC (10×MIC) can reduce the probability of the onset of resistance. Other authors have already demonstrated a synergistic effect of NAC with tigecycline, another recent antibiotic, against S. aureus and S. epidermidis.22 However, they have used higher concentrations of both agents, 1mgml−1 of tigecycline (1000×MIC) and 80mgml−1 of NAC, to obtain a similar 5log reduction in viable cells of S. epidermidis biofilm, although at half of the contact time, 12h against the 24h that we have used.
NAC is widely used in medical practice via inhalation and oral and intravenous routes and it has an excellent safety profile.36
Due to intrinsic differences between strains, further studies will be necessary using more clinical and reference strains with the objective of data confirmation. In conclusion, the combination of linezolid with NAC can be a possible therapeutic strategy to eradicate focus of S. epidermidis infections and, even more promisingly, can be further tested as a catheter lock solution.
FundingBruna Leite acknowledges the financial support of ISAC/Program Erasmus Mundus External Cooperation and the IBB-Institute for Biotechnology and Bioengineering, Centre of Biological Engineering, University of Minho, Campus of Gualtar. Fernanda Gomes and Pilar Teixeira fully acknowledge the financial support of Fundação para a Ciência e Tecnologia (FCT) through the grants SFRH/BD/32126/2006 and SFRH/BPD/26803/2006, respectively.
Conflict of interestThe authors have no conflict of interest to declare.