To characterize the clinical manifestations of Rickettsia japonica (R. japonica) infection and to generate evidence facilitating early diagnosis and targeted treatment.
MethodsWe retrospectively reviewed the clinical data of five patients with R. japonica infection who were treated in the Emergency Department, Xiling Campus, Yichang Central People's Hospital, between January 2023 and December 2024.
ResultsAll patients were residents of Yichang City, Hubei Province, aged 58–70 years, and 80% (4/5) were farmers. The onset of illness occurred exclusively between May and September, and all patients reported a definite history of outdoor exposure. The predominant clinical manifestations were fever, rash, and eschar. Laboratory findings revealed thrombocytopenia, elevated aspartate aminotransferase (AST) and creatine kinase (CK), as well as increased inflammatory markers including C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6). R. japonica nucleic acid was detected in all patients by metagenomic next-generation sequencing (mNGS) of blood samples. Three patients initially received empirical doxycycline therapy, which was subsequently adjusted to a standard regimen after diagnostic confirmation. Defervescence occurred at a median of two days (range, 1–7 days), followed by gradual resolution of rash and alleviation of systemic symptoms. All patients achieved complete clinical recovery and were discharged without complications.
ConclusionThis study highlights the importance of heightened clinical awareness of R. japonica infection, emphasizing the integration of epidemiological context with hallmark clinical features – particularly fever, rash, and eschar – during peak transmission seasons in endemic areas. Early recognition allows the timely initiation of doxycycline therapy, which is essential for achieving favorable outcomes. Moreover, metagenomic next-generation sequencing (mNGS) provides the definitive identification of pathogens and guides targeted antimicrobial therapy.
Caracterizar las manifestaciones clínicas de la infección por Rickettsia japonica (R. japonica) y generar evidencia que facilite el diagnóstico temprano y el tratamiento dirigido.
MétodosSe analizaron retrospectivamente los datos clínicos de cinco pacientes con infección por R. japonica atendidos en el Departamento de Emergencias del Campus Xiling del Hospital Central de Yichang, entre enero de 2023 y diciembre de 2024.
ResultadosTodos los pacientes eran residentes de la ciudad de Yichang, provincia de Hubei, con edades comprendidas entre 58 y 70 años; el 80% (4/5) eran agricultores. El inicio de la enfermedad ocurrió exclusivamente entre mayo y septiembre, y todos los pacientes referían antecedentes claros de exposición al aire libre. Las manifestaciones clínicas predominantes fueron fiebre, exantema y escara. Los hallazgos de laboratorio mostraron trombocitopenia, elevación de aspartato aminotransferasa (AST) y creatina quinasa (CK), así como aumento de marcadores inflamatorios, incluyendo proteína C reactiva (PCR), procalcitonina (PCT) e interleucina-6 (IL-6). Tres pacientes recibieron inicialmente tratamiento empírico con doxiciclina, el cual se ajustó posteriormente a un régimen estándar tras la confirmación diagnóstica. La defervescencia se alcanzó a una mediana de dos días (rango: 1–7 días), seguida de la resolución gradual del exantema y la mejora de los síntomas sistémicos. Todos los pacientes lograron recuperación clínica completa y fueron dados de alta sin complicaciones.
ConclusiónEste estudio resalta la importancia de una mayor conciencia clínica sobre la infección por R. japonica, integrando el contexto epidemiológico con las características clínicas distintivas—especialmente fiebre, exantema y escara—durante las temporadas de máxima transmisión en áreas endémicas. El reconocimiento temprano permite la iniciación oportuna de la terapia con doxiciclina, fundamental para alcanzar resultados favorables. Además, la secuenciación metagenómica de nueva generación (mNGS) permite la identificación definitiva de los patógenos y guía la terapia antimicrobiana dirigida.
Rickettsiae are Gram-negative, obligate intracellular bacteria transmitted by arthropod vectors, causing zoonotic diseases with natural endemic foci.1Rickettsia japonica, a member of the spotted fever group rickettsiae (SFGR), is primarily transmitted by ixodid tick bites and causes Japanese spotted fever (JSF).2 The classical clinical triad of JSF comprises fever, rash, and eschar.3 However, early manifestations are often nonspecific, necessitating differentiation from other febrile illnesses, particularly severe fever with thrombocytopenia syndrome (SFTS). The first human case of R. japonica infection in China was reported in Anhui Province in 2013, and sporadic cases have since been identified in Zhejiang, Henan, and Hubei Provinces.4–6 Nevertheless, comprehensive epidemiological surveillance data on rickettsial diseases in China remain limited. In this retrospective case series, we analyzed five patients with R. japonica infection confirmed by mNGS in Yichang, western Hubei Province, to delineate clinical features that facilitate early recognition and guide targeted treatment.
MethodsStudy participantsLaboratory-confirmed cases of R. japonica infection were retrospectively identified from the emergency department records of the Xiling Campus, Yichang Central People's Hospital, a tertiary teaching hospital in western Hubei Province. Clinical data from five patients diagnosed between January 2023 and December 2024 were included in the analysis.
Inclusion and diagnostic criteria(1) Suspected case: Defined as a history of outdoor exposure within three weeks before symptom onset, accompanied by at least one of the following: fever (>38.0°C); rash; or lymphadenopathy. (2) Probable case: A suspected case with either the presence of an eschar or an epidemiologically linked exposure during the peak transmission season (May–September).7 (3) Confirmed case: A probable case confirmed by the detection of R. japonica nucleic acid using the metagenomic next-generation sequencing (mNGS). Note: Conventional diagnostic methods, including Weil-Felix test and species-specific PCR, were not available at our institution.
mNGS procedureWhole blood samples were collected and submitted to commercial laboratories for pathogen detection using mNGS. Samples were analyzed using either the Quantitative Metagenomic Next-Generation Sequencing platform (Q-mNGS; Molecular Laboratory of Hangzhou Matridx Biotechnology Co., Ltd.) or the Metagenomic Capture-Based Pathogen Detection with Next-Generation Sequencing platform (MetaCAP-mNGS; KingMed Center for Clinical Laboratory Co., Ltd., Wuhan, China). Genomic DNA was extracted and sequenced on the Illumina MiniSeq platform. After quality control (QC), host-derived sequences were bioinformatically removed, and the remaining reads were aligned against reference databases, including RefSeq, NT, VFDB, and CARD. The output included specific read counts, relative abundance (%), and respective platform-specific identification metrics (Quantitative Index [Q-index] for Q-mNGS and confidence score for MetaCAP-mNGS).
Ethical considerationsThis study was approved by the Institutional Review Board of Yichang Central People's Hospital (approval No. 2025-240-01), with a waiver of informed consent for retrospective data analysis. Written informed consent was obtained from all participants for the publication of anonymized clinical images.
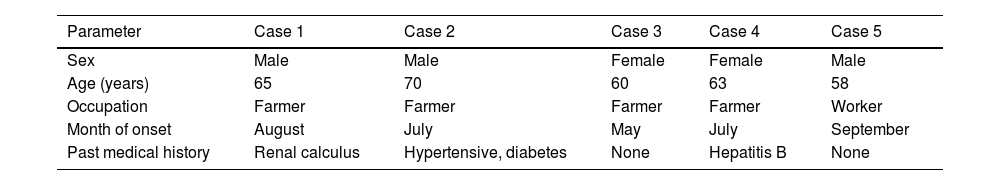
ResultDemographic characteristicsAll five patients were residents of Yichang City, Hubei Province (three males and two females; age range, 58–70 years). Four patients (80%) were farmers residing in mountainous endemic areas. Disease onset occurred exclusively between May and September, and all patients had documented histories of outdoor exposure before illness (Table 1).
Demographic characteristics of patients with JSF.
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Sex | Male | Male | Female | Female | Male |
| Age (years) | 65 | 70 | 60 | 63 | 58 |
| Occupation | Farmer | Farmer | Farmer | Farmer | Worker |
| Month of onset | August | July | May | July | September |
| Past medical history | Renal calculus | Hypertensive, diabetes | None | Hepatitis B | None |
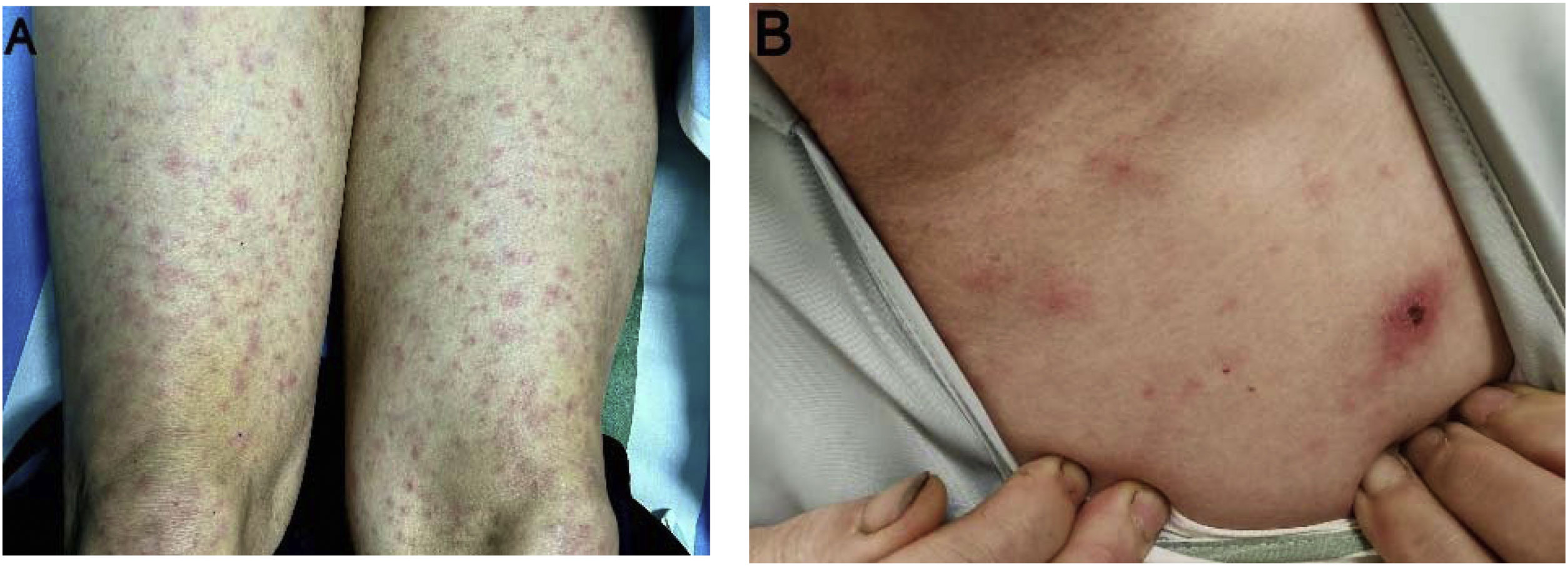
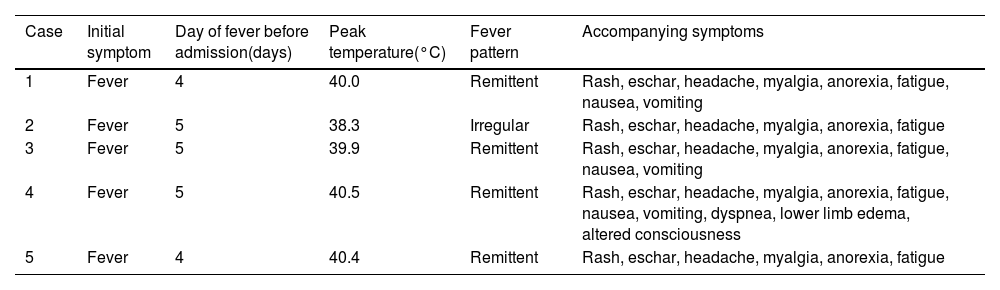
All patients presented with a high-grade fever, with peak temperatures reaching up to 40.5°C. A polymorphic, non-blanching maculopapular rash appeared on the trunk and limbs 2–3 days after fever onset (Fig. 1A), with its color intensity correlating with temperature fluctuations. No desquamation was observed during convalescence period. Eschars were observed in four patients, none of whom recalled arthropod exposure (Fig. 1B). Common accompanying symptoms included headache, myalgia, and fatigue; nausea and vomiting occurred in three patients. No lymphadenopathy, conjunctival injection, hemorrhage, or neurological deficits were observed. One patient with a co-existing hepatitis B virus (HBV) infection developed acute cardiopulmonary failure requiring intensive care support (Table 2).
Clinical manifestations of patients with JSF.
| Case | Initial symptom | Day of fever before admission(days) | Peak temperature(°C) | Fever pattern | Accompanying symptoms |
|---|---|---|---|---|---|
| 1 | Fever | 4 | 40.0 | Remittent | Rash, eschar, headache, myalgia, anorexia, fatigue, nausea, vomiting |
| 2 | Fever | 5 | 38.3 | Irregular | Rash, eschar, headache, myalgia, anorexia, fatigue |
| 3 | Fever | 5 | 39.9 | Remittent | Rash, eschar, headache, myalgia, anorexia, fatigue, nausea, vomiting |
| 4 | Fever | 5 | 40.5 | Remittent | Rash, eschar, headache, myalgia, anorexia, fatigue, nausea, vomiting, dyspnea, lower limb edema, altered consciousness |
| 5 | Fever | 4 | 40.4 | Remittent | Rash, eschar, headache, myalgia, anorexia, fatigue |
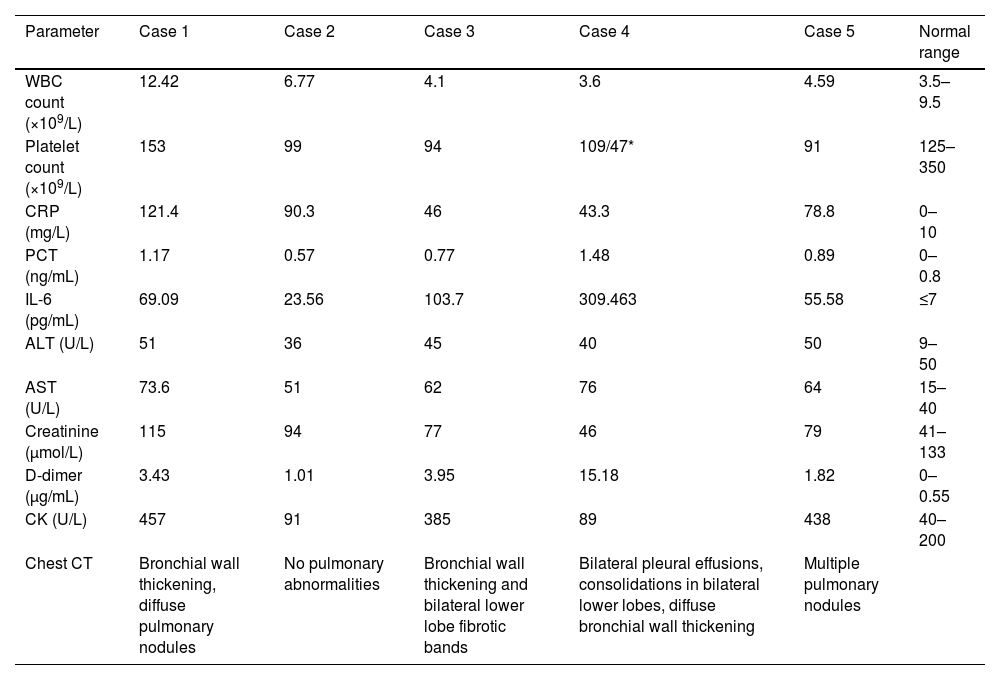
All patients exhibited the following laboratory abnormalities: (1) elevated inflammatory markers (CRP, PCT, and IL-6), (2) coagulopathy (elevated D-dimer), and (3) hepatocellular injury (elevated AST). Elevated creatine kinase levels were observed in three of five patients (60%), and thrombocytopenia in four of five patients (80%). Imaging of the critically ill patient (n=1) revealed bilateral pleural effusions with pulmonary infiltrates (Table 3).
Initial laboratory and imaging findings of patients with JSF.
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Normal range |
|---|---|---|---|---|---|---|
| WBC count (×109/L) | 12.42 | 6.77 | 4.1 | 3.6 | 4.59 | 3.5–9.5 |
| Platelet count (×109/L) | 153 | 99 | 94 | 109/47* | 91 | 125–350 |
| CRP (mg/L) | 121.4 | 90.3 | 46 | 43.3 | 78.8 | 0–10 |
| PCT (ng/mL) | 1.17 | 0.57 | 0.77 | 1.48 | 0.89 | 0–0.8 |
| IL-6 (pg/mL) | 69.09 | 23.56 | 103.7 | 309.463 | 55.58 | ≤7 |
| ALT (U/L) | 51 | 36 | 45 | 40 | 50 | 9–50 |
| AST (U/L) | 73.6 | 51 | 62 | 76 | 64 | 15–40 |
| Creatinine (μmol/L) | 115 | 94 | 77 | 46 | 79 | 41–133 |
| D-dimer (μg/mL) | 3.43 | 1.01 | 3.95 | 15.18 | 1.82 | 0–0.55 |
| CK (U/L) | 457 | 91 | 385 | 89 | 438 | 40–200 |
| Chest CT | Bronchial wall thickening, diffuse pulmonary nodules | No pulmonary abnormalities | Bronchial wall thickening and bilateral lower lobe fibrotic bands | Bilateral pleural effusions, consolidations in bilateral lower lobes, diffuse bronchial wall thickening | Multiple pulmonary nodules |
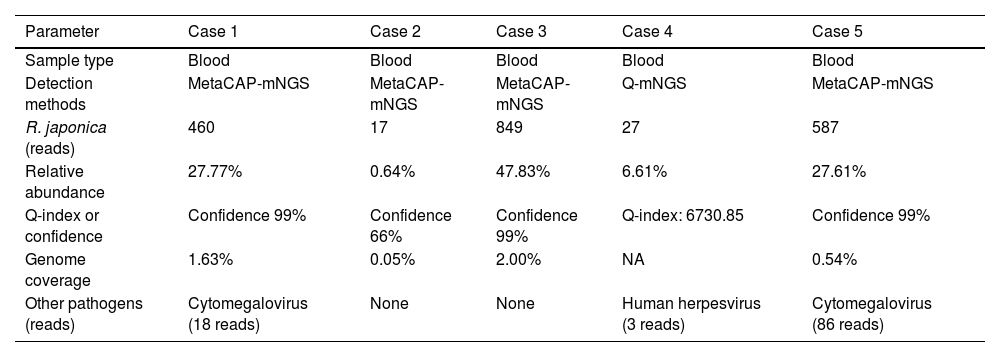
Definitive identification of R. japonica was achieved in all patients using MetaCAP-mNGS (KingMed, n=4) or Q-mNGS (Matridx, n=1). Latent herpesvirus co-infection (EBV/HHV-4 and CMV/HHV-5) was detected at low viral loads in three patients. All samples tested negative for severe fever with thrombocytopenia syndrome virus (SFTSV). Comprehensive quantitative metrics are summarized in Table 4.
Result of mNGS in patients with JSF.
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Sample type | Blood | Blood | Blood | Blood | Blood |
| Detection methods | MetaCAP-mNGS | MetaCAP-mNGS | MetaCAP-mNGS | Q-mNGS | MetaCAP-mNGS |
| R. japonica (reads) | 460 | 17 | 849 | 27 | 587 |
| Relative abundance | 27.77% | 0.64% | 47.83% | 6.61% | 27.61% |
| Q-index or confidence | Confidence 99% | Confidence 66% | Confidence 99% | Q-index: 6730.85 | Confidence 99% |
| Genome coverage | 1.63% | 0.05% | 2.00% | NA | 0.54% |
| Other pathogens (reads) | Cytomegalovirus (18 reads) | None | None | Human herpesvirus (3 reads) | Cytomegalovirus (86 reads) |
Empirical antimicrobial therapy and supportive care were initiated upon admission. Three patients received pre-diagnostic doxycycline for suspected rickettsiosis, whereas the remaining two commenced doxycycline therapy following mNGS confirmation. All patients completed 7–10 days of doxycycline therapy, achieving defervescence at a median of two days (range, 1–7 days). Case 4 exhibited no clinical response to initial amoxicillin–clavulanate. Following mNGS confirmation on day three, therapy was escalated to intravenous cefoperazone–sulbactam combined with doxycycline, alongside supportive interventions including platelet transfusion, non-invasive ventilation (NIV), and thoracentesis. Within 48h, body temperature decreased to 37.3°C, and respiratory distress improved significantly. Rash resolution occurred at 2–4 days after defervescence. All laboratory parameters were normalized before discharge (median, 5 days). All patients achieved full recovery without sequelae.
DiscussionRickettsial infections exhibit summer–autumn seasonality correlating with arthropod vector proliferation, and establishing outdoor exposure as a major risk factor.8 Fever and rash are characteristic clinical features, often accompanied by nonspecific symptoms such as headache and myalgia.9 Previous studies have reported incidence rates of approximately 88%–99% for fever, 94% for rash, and 44%–66% for eschar.3 Although JSF generally has a favorable prognosis, serious complications – including disseminated intravascular coagulation (DIC), acute respiratory distress syndrome (ARDS), and multiple organ failure – have been documented.10 In recent years, sporadic cases of JSF in regions such as Zhejiang, Henan, and Hubei, China, have included severe manifestations such as multiorgan failure.5,6,11
In the present cohort, all cases occurred during the summer–autumn period and were associated with documented outdoor exposure. The universal presence of fever and rash underscores their diagnostic utility in raising early clinical suspicion. Notably, one patient with chronic HBV infection developed multiorgan failure, potentially attributable to: (1) HBV-related immunosuppression (impaired T-cell immunity), (2) diagnostic delay (five days post-symptom onset), and (3) initial β-lactam therapy, which was ineffective against obligate intracellular pathogens.
Rickettsia species primarily invade vascular endothelial cells, resulting in increased capillary permeability, microvascular hemorrhage, and platelet consumption.12 These pathophysiological processes may precipitate severe complications, including acute respiratory distress syndrome (ARDS) and multiorgan failure.13 In this study, several findings supported the pathogenic mechanism of endothelial injury, including elevated D-dimer (indicating microthrombosis), elevated CRP (suggestive of vasculitis), and a non-blanching hemorrhagic rash (characteristic of microvascular damage). Given the potential risk of pulmonary complications, chest CT imaging was performed to evaluate lung involvement.
As an unbiased broad-pathogen detection tool, mNGS plays a critical role in diagnosing JSF.4,14 This study utilized two platforms: (1) Q-mNGS, which employs PCR-free library preparation with spike-in internal standards to calculate pathogen load via the Quantitative Index (e.g., Case 4 had a Q-index of 6730.85), thereby reducing analytical bias in low-biomass microbial samples.15 (2) MetaCAP-mNGS, which applies probe enrichment to enhance sensitivity for detecting low-abundance pathogens.16 Given the substantial symptom overlap between R. japonica, SFTSV, and other infections,17 mNGS provides critical value for differential diagnosis. No SFTSV nucleic acid was detected in this cohort. Integration with clinical data allowed definitive exclusion of SFTSV infection. Notably, the sensitivity of mNGS for detecting obligate intracellular pathogens remains suboptimal, primarily due to the low microbial DNA burden in early-phase plasma/CSF samples compounded by the overwhelming host nucleic acid background.18,19 These technical limitations may consequently result in false-negative interpretations or low-coverage sequencing results. Thus, the presence of at least one species-specific read was considered clinically significant if the following criteria were fulfilled: (1) clinical features were consistent with rickettsial disease, (2) exposure history aligned with endemic regions, and (3) alternative diagnoses were excluded.20 In Cases 2 and 4, despite low mNGS read counts (17 and 27 reads), JSF was diagnosed based on the following criteria: (a) characteristic clinical presentation (fever, rash, and eschar), (b) exposure in endemic areas, (c) pathogen identification by mNGS, and (d) defervescence within 48–72h after the initiation of doxycycline therapy.
Doxycycline inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit and is the first-line therapy for JSF. The recommended regimen is 100mg twice daily for 5–7 days, continued for three days after defervescence.21 In this study, two patients did not respond adequately to initial fluoroquinolones or β-lactams, but all achieved defervescence within five days (median, 72h) after initiation of doxycycline, without adverse events. Therefore, early initiation of doxycycline is strongly recommended in cases of suspected or clinically diagnosed JSF, provided there are no contraindications.
FundingThis study was not supported by any specific research funding or financial grants.
Conflict of interestAll authors declare no competing financial or non-financial interests relevant to this study.