Ceftaroline is a broad-spectrum cephalosporin indicated for the treatment of complicated skin-structure infections and community-acquired pneumonia. Its effect depends on the cumulative percentage of time over the interval by which the free antimicrobial concentration exceeds the MIC.1,2
Plasmapheresis removes abnormal cells or substances in the blood that are associated with or cause a certain disease. Depending on the characteristics of the drug plasmapheresis could affect its clearance rate.3
We report a case of a 72-year-old female (weight 53kg, body mass index: 20.7kg/m2) with no known allergies, and a history of hypertension, dyslipemia and double aortocoronary bypass. In October 2018, was admitted to the emergency department due to dyspnea and cough during the previous 5 days. Upon admission, she presented tachycardia and hypotension. Also blood tests revealed an elevated serum creatinine (SCr) level of 1.97mg/dL and a white blood count of 20.000cells/mm3. Based on the X-ray results, she was diagnosed with a bilateral bronchopneumonia. Her initial clinical condition status was deteriorated and was admitted to the Intensive Care Unit, receiving ceftriaxone 2g q24h and levofloxacin 500mg q12h. An induced sputum culture was obtained, where MRSA was isolated (MIC≤4mcg/mL for linezolid). Then, treatment with levofloxacin was modified to linezolid 600mg q12h and ceftriaxone was maintained.
Clinical evolution declined with respiratory failure, agitation, decreased urinary output, anemia and thrombocytopenia. After respiratory worsening and persistence of anemia and thrombocytopenia, microangiopathic hemolytic anemia secondary to possible severe MRSA pneumonia was suspected. The diagnosis was confirmed with other laboratory data: negative Coombs test, increased lactate dehydrogenase 1115U/L, haptoglobin<2.44mg/dL, presence of 4–5% of schistocytes and ADAMS-13 levels 13%. The patient was diagnosed with thrombotic thrombocytopenic purpura and the antibiotic treatment was changed to ceftaroline. In addition, five sessions of plasmapheresis were prescribed. The patient presented resolution of all signs and symptoms of infection after twelve days of treatment including platelet count and recovery of renal function.
Unlike extracorporeal oxygenation techniques and renal replacement therapy,4,5 there is no information on the PK behavior of ceftaroline in patients during plasmapheresis.6
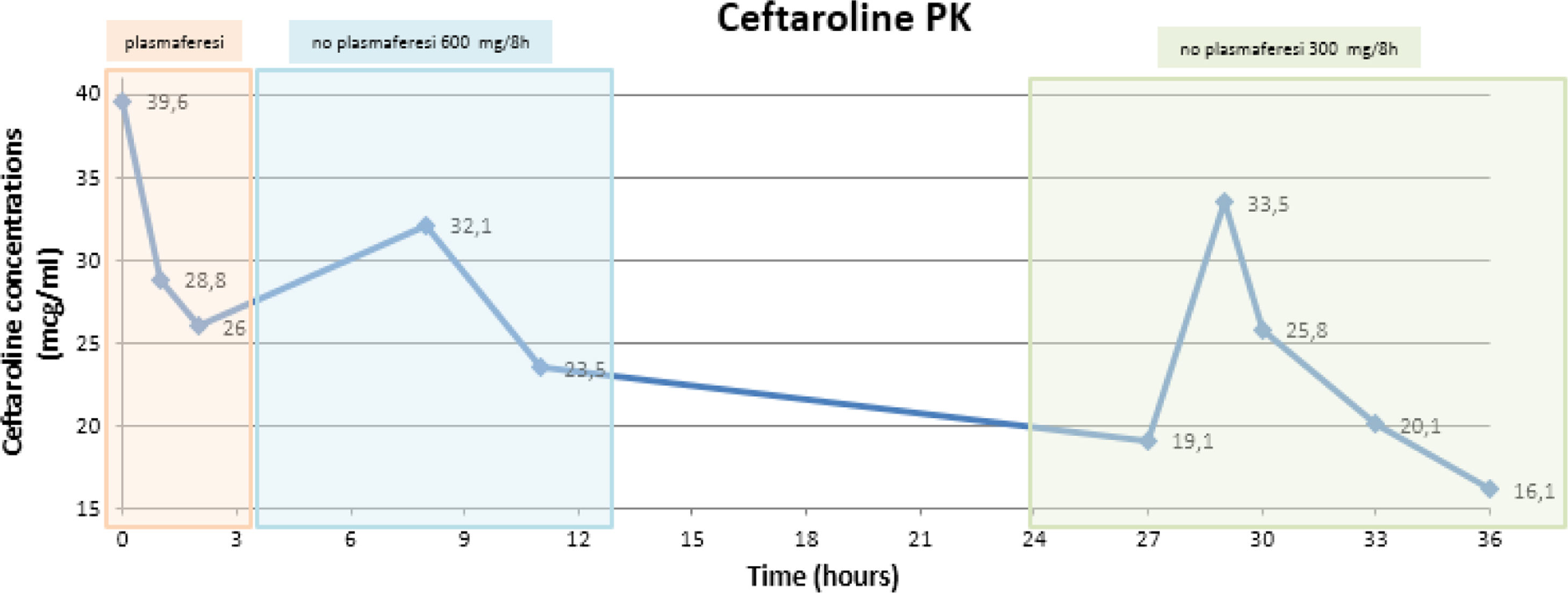
To assess the potential influence of plasmapheresis on ceftaroline PK, consecutive blood samples were taken on day 10 (plasmapheresis session) and 11 (without plasmapheresis). Blood samples were analysed by high performance liquid chromatography. Once ceftaroline plasma concentrations (CPC) were obtained, pharmacokinetic parameters were calculated. Ceftaroline doses were adjusted according to the renal function of the patient and plasmapheresis sessions. CPC described in Fig. 1.
Plasma exchange could indirectly alter drug therapy. Prolonged exchange sessions and a larger volume of plasma collected may be the main reasons for altered drug clearance.3 Some factors that influence drug clearance during plasmapheresis are high plasma protein binding (PPB) (>75%) and low volume of distribution (Vd) (<0.3L/kg).3 Only some information on drug removal during this technique is available from previously published studies, and information on antimicrobials is limited. A review by Kintzel et al.7 concluded that drugs that remain in the intravascular compartment are more susceptible to be removed by plasmapheresis than those with an extensive cellular and tissue distribution.
Another study by Vay et al.8 found no alteration in voriconazole plasma concentrations (Vd 4.6L/kg) after plasmapheresis in a critically ill patient. Ibrahim et al.3 observed that ceftriaxone (PPB=96%; Vd=0.1L/kg) needed to be administered with a difference of 15h from plasmapheresis.
Our results showed that ceftaroline (PPB<20%; Vd=0.3L/kg) clearance was two-fold higher during plasmapheresis (3.79h vs. 6.67h). In fact, ceftaroline half-life has been reported to be approximately 2.5h in healthy subjects.1,2 Taking into account that the patient presented renal impairment during plasmapheresis (SCr=2.31mg/dL, normal range: 0.7–1.2mg/mL), half-life should have risen to higher values, but in our patient, half-life was closer to normal renal function values.
Although ceftaroline clearance rate was higher during plasmapheresis, CPC were still considered therapeutic, according EUCAST clinical breakpoint values for MRSA (MIC=1mcg/mL).9
In conclusion, a significant clearance of CPC during plasmapheresis was observed. Although further pharmacokinetic studies are needed to fully elucidate ceftaroline PK, this antibiotic should be used with caution when using extracorporeal clearance therapies. Therapeutic drug monitoring should be performed to achieve effective antimicrobial plasma concentrations during plasmapheresis.