In February 2009 an outbreak of subcutaneous abscesses due to Mycobacterium abscessus was detected in Spain which affected healthy women who had undergone mesotherapy procedures in an aesthetic clinic.
MethodsEpidemiological research, health inspection and microbiological studies were conducted. The patients were given antibiotic treatment (according to susceptibility testing) with clarithromycin, and in some cases, combined with amikacin.
ResultsSeventeen out of 77 patients treated in the clinic were affected. The products used for the injections were homeopathic drugs in multi-dose vials. The environmental samples were negative. The sterile injection equipment and the clinical procedures were evaluated as correct. The storage conditions for the drugs were also correct, and all the samples tested negative for Mycobacteria. However Paenibacillus provencensis was isolated from samples of unused multi-dose vials and the withdrawal of the product from distribution was ordered. Deficiencies were detected in the sterile products process of at the homeopathic drug factory, so the production line was suspended.
ConclusionsThe results of environmental investigation suggest the most likely cause of the outbreak could have been the contamination of the products in the factory, although there was no laboratory confirmation. The widespread use of homeopathic products in invasive procedures requires extreme control during the manufacturing, handling and packaging process. It is important to consider mesotherapy and parenteral use of homeopathic medicines as potential sources of infection and therefore the same precautions in the procedures and quality assurance of products should be applied as with any other drug or medical activity.
En febrero de 2009 se detectó en Baleares un brote de abscesos subcutáneos causados por Mycobacterium abscessus que afectaba mujeres jóvenes y sanas que se habían sometido a procedimientos de mesoterapia en una clínica de estética.
MétodosSe realizaron investigación epidemiológica, inspección sanitaria y estudios microbiológicos clínicos y ambientales. Los pacientes iniciaron tratamiento antibiótico (según antibiograma) con claritromicina y, en los casos más graves, amikacina.
ResultadosAparecieron lesiones en 17 de las 77 personas sometidas a la mesoterapia en el período de riesgo. Los productos inyectados eran fármacos homeopáticos en multivial. Las muestras ambientales fueron negativas. No se evidenciaron deficiencias en los equipos y procedimientos. Los medicamentos estaban correctamente almacenados y todas las muestras fueron negativas para Mycobacteria, aunque se identificó Paenibacillus provencensis de multiviales precintados y el producto fue retirado de la distribución. Se detectaron deficiencias en la producción de estériles en la fábrica, por lo que la línea de producción fue suspendida y el producto retirado.
ConclusionesLos resultados de la investigación ambiental sugieren que la causa más probable del brote habría sido la contaminación del producto en origen, aunque no fue confirmada por laboratorio. La difusión del uso de productos homeopáticos en procedimientos invasivos requiere un control riguroso durante la fabricación, manipulación y envasado. Es importante considerar la mesoterapia y el uso parenteral de productos homeopáticos como fuentes potenciales de infección y por lo tanto extremar las precauciones y la garantía de calidad de los productos y los procedimientos de la misma manera que con cualquier otro producto farmacológico o actividad médica.
It is well known that opportunistic mycobacteria occur in soil and water and some are able to produce lesions if introduced into human tissues.1 Occasionally they may enter human skin through injuries and cause localised infection. Nosocomial outbreaks and pseudo-outbreaks caused by the nontuberculous mycobacteria (NTM) have been recognised for more than 30 years and continue to be a problem. Most of these outbreaks have involved the rapidly growing mycobacterial species Mycobacterium fortuitum and M. abscessus (formerly M. chelonae subspecies abscessus).2 The reservoir for these outbreaks is generally municipal and (often separate) hospital water supplies. These mycobacterial species and others are incredibly hardy; able to grow in tap water and distilled water, thrive at temperatures of 45°C or above (M. xenopi and M. avium complex), and resist the activity of organomercurials, chlorine, 2% concentrations of formaldehyde and alkaline glutaraldehyde, and other commonly used disinfectants.3
Nosocomial disease due to rapidly growing mycobacteria was first reported by Da Costa Cruz in 1938, when he described a patient with a post-injection cutaneous abscess. Post-injection abscesses were also the first disease caused by rapidly growing mycobacteria to be recognised in epidemic form.4,5 Nosocomial outbreaks, traceable to NTM, have been diagnosed since 1975. Most involve wound infections following cardiac surgery or plastic surgical procedures, and post-injection abscesses. Sporadic disease of an identical nature has also been recognised. The first reported NTM plastic surgery-related outbreak occurred in 1974-1975 in Barcelona, Spain, and involved contaminated commercial merbromin, an organomercurial used for pre-surgical antisepsis of varicose veins that were to be excised.6 The outbreaks occurred in two hospitals and involved M. abscessus. Additional isolates were recovered from other hospitals using merbromin, and ultimately from the commercial merbromin bottles themselves, but there were no recorded outbreaks. Later, in 1985, there were more outbreaks in the U.S.A. and the infections were related to a 1% aqueous solution of gentian violet prepared in distilled water, contaminated with M. abscessus. Although surgical wound infection outbreaks are relatively rare after the 1990s, they still occur and are a reminder of the remarkable survival capacities of NTM to the seemingly inhospitable environment surrounding modern-day-surgery.7
The first post-injection abscess outbreaks were documented in 1961 and 1962. Over the next 30-year period around 11 such outbreaks have been reported, and in six outbreaks occurring before 1980 the exact species involved is uncertain, because of limited taxonomic methods. The taxonomy of the causative agent was well established in the most recent outbreaks (all since 1985): three were M. abscessus, one was M. chelonae, and one was M. fortuitum. Two clinical settings were observed in these 11 outbreaks. One was the use of multi-dose vials or contaminated biological agent for injections.8 In two of the more recent outbreaks the same organism has been recovered from the vials. The second setting involved the inadequate sterilization of equipment used for injections or non-injecting needles (two outbreaks), with contaminated water used for cleaning or rinsing the equipment being the likely source of the outbreak.9 In one of these outbreaks, the causative organism was recovered from the distilled water used for disinfection. The re-use of the same needle in individual patients and the use of the reusable injector syringes in multiple patients also increased the risk of patient-to-patient transmission.
Mesotherapy consists of multiple injections of very small amounts of therapeutic mixtures into the mesoderm. Until now, the mechanism of action and efficacy of this technique is unproven. However, its practice has been increasing in recent decades and is being used to treat joint pain syndromes and obesity.10,11 Media pressure on physical beauty has extended the use of this technique. Initially, it was only used by physicians, but in recent years it has also been used by unqualified personnel in beauty salons.12,13 If mesotherapy is not performed with quality controlled substances, this can be a predisposing factor for NTM infection.14,15 Another problem could be due to the inadequate sterilization of equipment, or contamination during handling, as in the last outbreak in Spain. In that outbreak, the probable source of infection was during the injection process, despite being performed by a physician with sterile gloves, but in a beauty salon, where they had been practicing waxing, facials, etc.16 Beauty salons also have been associated with pedicures or foot baths with furunculosis by NTM.17,18
Outbreak DescriptionOn 24 February 2009 the Balearic Surveillance Network detected 2 cases of cutaneous infection; with Mycobacterium being isolated in one of them. Both patients had been receiving weekly mesotherapy treatment since the first week of October 2008. Treatment took place in the same aesthetic medicine consultation at a private clinic. Research was initiated to verify that it was an outbreak and if so, to determine the etiology, infection source and transmission mechanism, as well as controlling its spread.
Epidemiological InvestigationWe obtained a list of all those people possibly exposed to mesotherapy at the same medical centre from 1st October 2008 through 26 February 2009. The investigation was extended to October 1, 2008 as it was the first session of the reported cases. Moreover, the practice of mesotherapy in summer is very unusual. We contacted every possible individual at risk to verify the exposure. The epidemiological survey included personal, clinical, microbiological data, antibiotic therapies and data on the mesotherapy (days of treatment, occurrence of lesions, description, number, location, date of onset) and other possible common exposures considered as risks for skin infections (swimming pool, hydrotherapy, baths, hair removal, liposuction, electromyography, acupuncture, tattoos). The dates and procedures of the mesotherapy treatments were obtained from the doctor responsible for the centre and verified by questionnaire.
Case definitionEach person attending the medical centre and presenting cutaneous infection in an area previously treated with mesotherapy, during the period between 1st October 2008 and 26 February 2009, was considered for the study.
Environmental investigationOn 2nd March 2009 an inspection of the medical centre was conducted and 9 samples collected: surfaces, cotton swabs, antithrombotic cream from an open recipient, 96° alcohol from an open bottle, gauzes from an open pack and sealed ampoules of the drugs used for mesotherapy. The environmental samples were analysed in a local laboratory, while the drugs were sent to the AEMPS, (Spanish Agency for the Medicines and Health Products).
Clinical and microbiological investigationThe incubation period usually varies from 7 to 121 days, often shorter in cases of abscess or cellulitis (8-12 days). In most cases a lesion was detected by the patient or a non-clinic physician within 30 days. Papules, nodules, plaques, ulcers and panniculitis-like lesions are common manifestations. Disseminated infection may occur in immunocompromised patients.19
The possible cases were sent to the Infectious Disease Unit of the reference hospital, where the abscesses would be diagnosed and treated. All patients were asked to have at least one skin biopsy from lesions for microbiological studies.
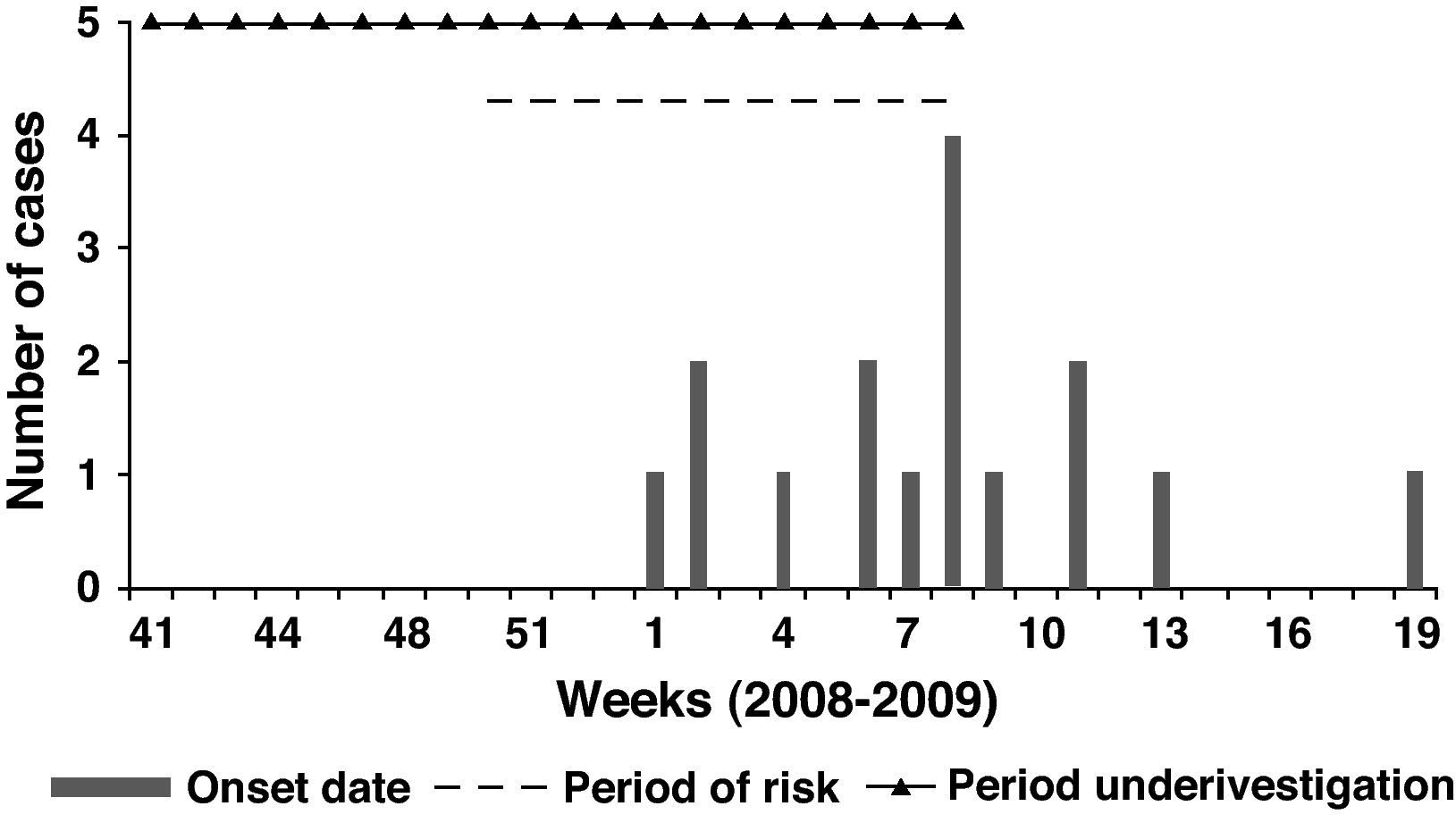
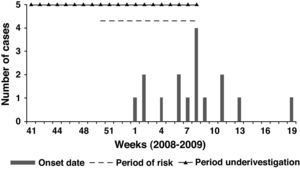
Epidemiological investigation resultsSeventy individuals at risk were found, most of them women (88.6%), with a wide age range, 19 to 67 years. Seventeen cases were identified (attack rate of 23.9%), all of them women between 19 and 65 years. Demographic and personal conditions between the cases and non-cases were similar. The lesions first appeared on 4 January 2009 and the last reported case was on 12 May 2009. Apart from receiving mesotherapy in the first associated medical centre, there was no other common exposure that could have been the infection source. The same physician had performed all the treatments.
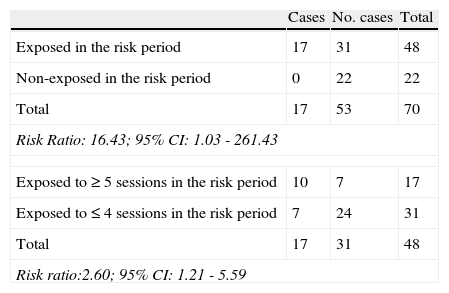
From the analysis of the treatment dates, it was found that no cases had appeared in those people who finished the treatment before 11 December 2008; so the risk period could be adjusted from 11 December 2008 to 26 February 2009 (a 2 month period). To check this hypothesis, we compared the exposed people in the risk period with the non-exposed in this same period. In that cohort of patients, we found 17 cases of infection among 48 exposed in the risk period, and no cases among the 22 non-exposed persons. The Risk Ratio (RR) was 16.4 (95% CI: 1.03 – 261.43). Furthermore, the people who received 5 or more sessions in the risk period had a Risk Ratio 2.6 (95% CI: 1.21 – 5.59) higher than people who received 4 sessions or less during this period (Table 1).
Estimation of the risk of those exposed compared to those not exposed in the risk period, and the risk of having had 5 or more sessions compared with having had less than 5 sessions in the risk period.
| Cases | No. cases | Total | |
| Exposed in the risk period | 17 | 31 | 48 |
| Non-exposed in the risk period | 0 | 22 | 22 |
| Total | 17 | 53 | 70 |
| Risk Ratio: 16.43; 95% CI: 1.03 - 261.43 | |||
| Exposed to ≥ 5 sessions in the risk period | 10 | 7 | 17 |
| Exposed to ≤ 4 sessions in the risk period | 7 | 24 | 31 |
| Total | 17 | 31 | 48 |
| Risk ratio:2.60; 95% CI: 1.21 - 5.59 | |||
Focusing on the risk period, the mean incubation was estimated at 34.7 days (SD 27.8; range 10 – 112; median 23). See the epidemic curve, the risk period and the investigated period in Figure 1.
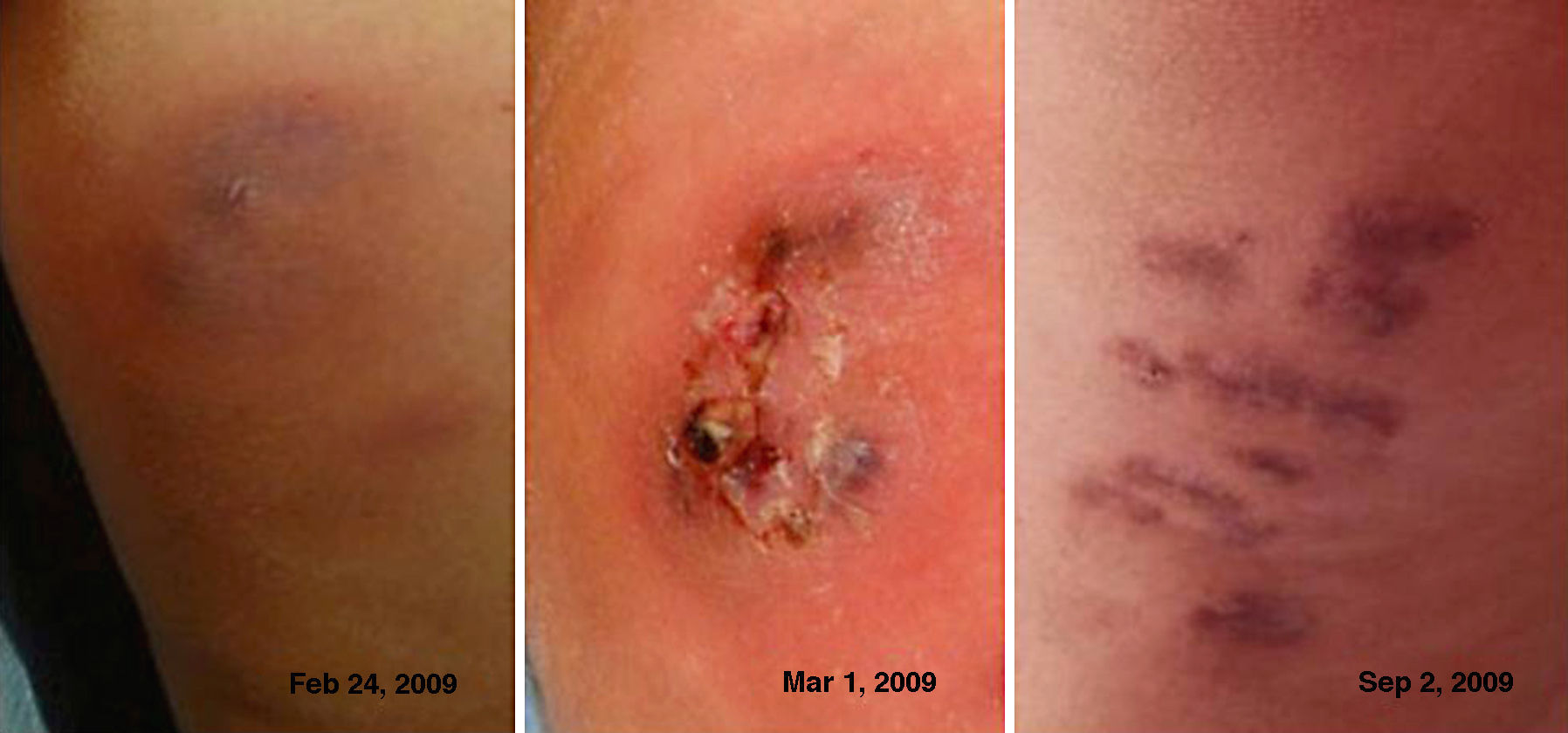
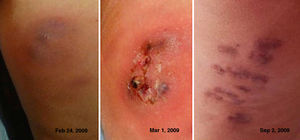
Clinical and microbiological resultsAll subjects developed single or multiple lesions, which at first resembled insect bites. Most of the lesions consisted of indurated erythematous papules and nodules. Some were inflamed, with a purple colour, and some progressed to frank abscess formation with fluctuance, suppuration, fistulisation and scarring (Fig. 2). In all cases the infection areas coincided with the location of mesotherapy injections.
Evidence of mycobacterial infection can be obtained by tissue specimens, culture being the most important for diagnosis. As many mycobacteria only grow on special media and at special temperatures it is crucial that clinical suspicion is raised so correct testing can be performed.20
In 7 out of 17 cases, multidrug-resistant Mycobacterium abscessus was identified. It was only sensitive to clarithromycin and amikacin, with intermediate susceptibility to imipenem and linezolid, and resistant to other antibiotics studied. The 10 remaining cases were classified as epidemiologically confirmed, as they were clinically similar to the other cases, consistent with M. abscessus infection, and no other cause of infections could be identified. Once treatment was begun, the lesions generally regressed.
Treatment is difficult because many NTM are resistant to common antibiotics. Most isolates of M. abscessus are susceptible to clarithromycin, amikacin, and cefoxitin and demonstrate variable susceptibility to erythromycin. Combination chemotherapy with at least two antimicrobial agents to which the isolate is susceptible is advisable because monotherapy has been shown to contribute to the development of resistance. Localized disease usually responds to 2-4 months of therapy in immunocompetent hosts, and disseminated infections can require more than 6 months therapy.
Treatment consisted of clarithromycin (minimum 3 months, maximum 6 months); in 7 patients amikacin was added for 4 weeks. Treatment was well tolerated and supported until the full resolution of lesions. In nine patients residual lesions remained.
Environmental investigation resultsAll environmental samples tested negative for M. Abscessus, although Paenibacillus provencensis – a saprophytic microorganism - was isolated from the non-used vials for mesotherapy. The drugs were 3 homeopathic products, originated from the same laboratory, in multi-dose vials. No deficiencies were found in the storage conditions at the clinic.
Neither the inspection nor the information collected from the physician and the individuals at risk showed deficiencies in the clinic or in the procedures of the treatment. The drugs used were homeopathic products; the storage conditions were correct but there was no register of the batches used. The laboratory did not meet the GMP (Good Manufacturing Practices) sterile standards.
Public Health interventionThe intervention of the Public Health Authority consisted of four measures, as follows: first, the temporary cessation of clinical activity was ordered immediately after inspection and sampling, and a disinfection of the aesthetic medicine clinic by an accredited company was required. After demonstrating the drug contamination, a national public health warning was issued, and the products involved withdrawn. Finally, the sterile production in the homeopathic drugs laboratory was suspended.
Discussion and conclusionsThe proper hygiene of the clinic, the negativity of all environmental samples and the detection of contamination in the products used for mesotherapy suggest that these were the most likely causes of the outbreak, although there was no laboratory confirmation. The use of multivials could be another possible infection mechanism, if the correct sterile manipulation practices had been broken, as was seen in another outbreak with non-tuberculous mycobacteria. Nevertheless, as no deficiencies at the clinic or contaminated environmental samples were found, it seems quite unlikely in this case.
The widespread use of homeopathic products in invasive procedures requires extreme control during the manufacturing, handling and packaging procedures, like all other drugs. It is important to consider mesotherapy and parenteral use of homeopathic medicines as potential sources of infection by rapidly growing mycobacteria. Spanish regulation in homeopathic medicines is in a transitional situation21 and the AEMPS is evaluating the risk benefit ratio to authorise these products, and outbreaks related to them should be taken into account in this evaluation.
In the instance of the poor evolution of an abscess, the clinical suspicion is crucial for correct testing, as the mycobacteria only grow in special media at special temperatures. Clinical doctors should bear in mind this possible diagnosis, and inform the microbiologists in order to find the possible aetiology. Finally, the aesthetic clinics should be aware of the possible adverse effects of any invasive procedure and provide information about the risk to their customers before the treatment is initiated.
Conflict of interestsThe authors declare no conflicts of interest related to this study.
We thank Dr. Javier Gutiérrez de la Peña for helping in the diagnosis and detection of the first cases and Dr. Antoni Campins for contributing to the diagnosis and treatment of the affected persons.