Staphylococcus lugdunensis is a coagulase-negative staphylococcus (CNS) whose pathogenicity varies from colonisation of the skin in healthy subjects to the production of potentially fatal invasive infections, such as endocarditis, prosthetic infections or peritonitis related to peritoneal dialysis (PD). Its virulence factors and antimicrobial sensitivity patterns differentiate it from the other CNS, with it being more similar to Staphylococcus aureus due to its pyogenic nature.1 We present here a case of acute peritonitis due to methicillin-resistant S. lugdunensis in a patient with cirrhosis.
This was a 68-year-old male with a history of type 2 DM, COPD and liver cirrhosis who was admitted to the intensive care unit (ICU) for septic shock due to spontaneous bacterial peritonitis. He received treatment with meropenem (1&#¿;g/8&#¿;h) for 10 days. However, his condition worsened as he developed acute respiratory distress syndrome requiring invasive mechanical ventilation and methylprednisolone (40&#¿;mg/12&#¿;h), and acute kidney injury (AKIN II), and he had several episodes of recurrent ascites requiring paracentesis on multiple occasions. While in ICU the patient's condition deteriorated, with fever of 38.5°C, distributive shock and ascites, suggesting an underlying infection. Blood tests showed C-reactive protein (CRP) 41&#¿;mg/dl, leucocytes 4,580/μl (71.6% neutrophils), lymphocytes 440/μl and procalcitonin 0.31&#¿;ng/mL; in ascitic fluid, pH 7.55, leucocytes 65/mm3 and proteins 1&#¿;g/dl.
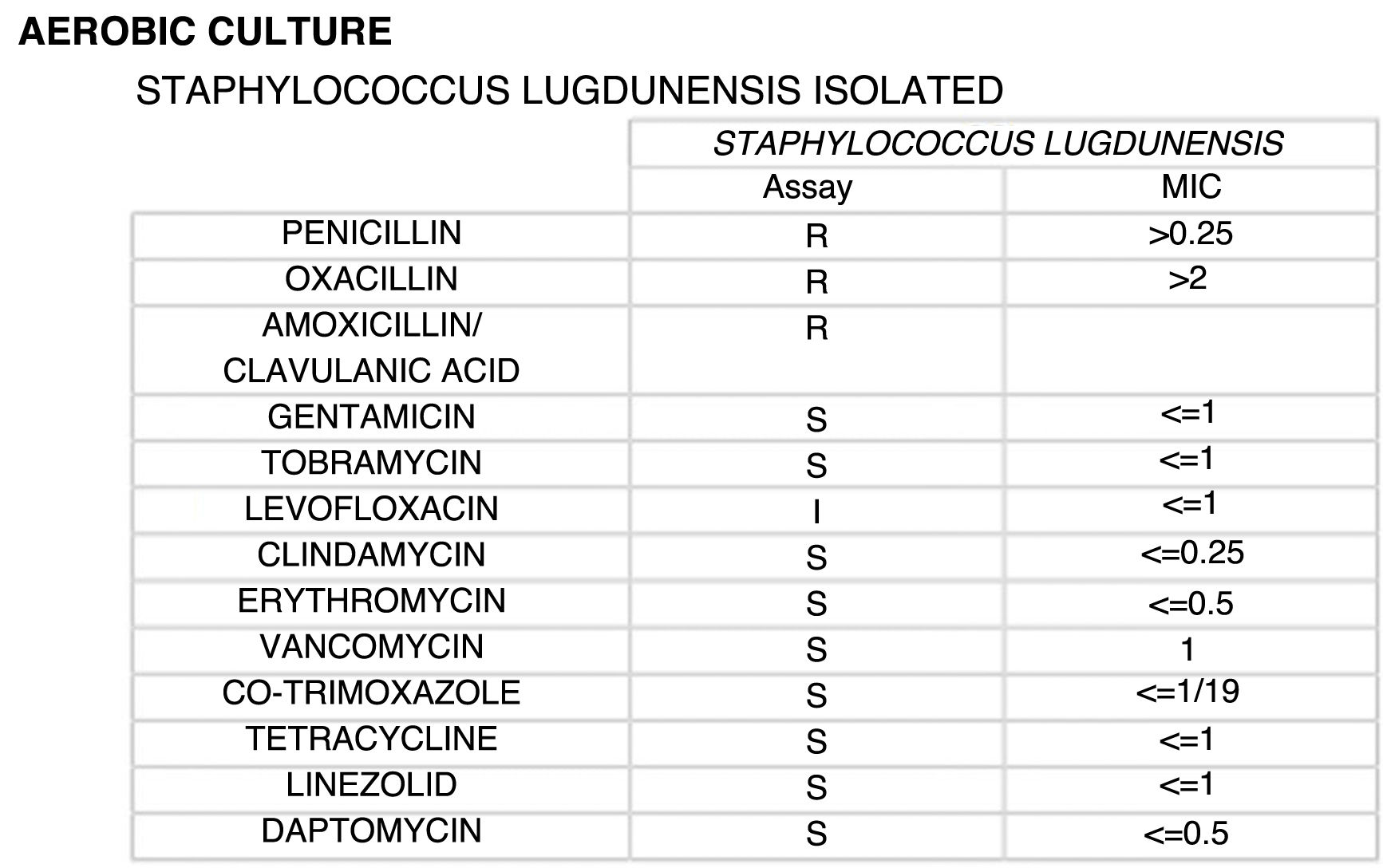
In this context, a further paracentesis was performed, releasing 5,000&#¿;ml of purulent fluid. Ascitic fluid samples cultured on CNA agar and chocolate agar showed growth of S. lugdunensis identified by mass spectrometry (MALDI-TOF, Bruker). The sensitivity study performed by microdilution (Microscan, Beckman Coulter) showed a minimum inhibitory concentration (MIC) for oxacillin greater than 2 μg/mL. Although molecular testing for methicillin resistance was not performed, screening for cefoxitin resistance was positive, with an MIC greater than 4 μg/mL, with this criterion being a reliable predictor of methicillin resistance mediated by the mecA or mecC genes. These genes encode the penicillin-binding protein (PBP) PBP2a, which confers resistance to all beta-lactams (Fig. 1).2 With these results, in accordance with the criteria of the International Society for Peritoneal Dialysis,3 the patient was diagnosed with acute bacterial peritonitis due to methicillin-resistant S. lugdunensis. The patient was treated with linezolid (600&#¿;mg/12&#¿;h intravenously for 10 days), due to its adequate pharmacokinetic profile in critical patients with kidney injury. The patient made a satisfactory recovery and was discharged on treatment with norfloxacin.
S. lugdunensis was described by Freney et al.4 in 1988, and the first reference recording its identification in ascitic fluid is from 1989, by Ludlam and Phillips5 in a group of patients on PD. Since then, literature references relating it to infection of ascitic fluid are limited to case reports6 or case series of patients on PD.1 In these patients, the communication of the peritoneal cavity with the outside through the dialysis catheter facilitates the entry of microorganisms that colonise the skin.3 Similarly, in the case presented here, the successive paracentesis procedures acted as a gateway for the microorganism to enter the peritoneal cavity.
Unlike the other CNS, S. lugdunensis is susceptible to most antibiotics and the prevalence of methicillin-resistant strains is rare (0–8.3%).7 Spanish series, such as by Mateo et al.,8 report 12% of penicillinase-producing strains, without finding resistance to methicillin. In more recent series, 44.6% of strains were resistant to penicillin, and only 1.8% were resistant to methicillin.9 In contrast, in Southeast Asia, strains resistant to penicillin and methicillin are as high as 87% and 20%, respectively, highlighting an emerging problem.10
In conclusion, acute bacterial peritonitis due to S. lugdunensis is a disease poorly documented outside the spectrum of patients on PD. In the case described here, the performance of successive paracentesis procedures in the intensive care setting was a significant risk factor for acquiring the infection. Furthermore, the pressure imposed by the antibiotics administered contributed to the selection of a strain of S. lugdunensis resistant to beta-lactams, a very rare circumstance in our setting.
FundingThe authors declare that they received no funding to conduct this study.