Confocal laser endomicroscopy (CLE) has only recently been added to the gastroenterologist's armamentarium. It allows microscopy of the gastrointestinal mucosa during ongoing endoscopy after the topical or systemic application of fluorescent dyes. CLE has been studied in a multitude of diseases in both the upper and the lower GI tract, and recently even in imaging of the bile duct, the pancreas and the liver. In a translational approach, CLE is a unique tool to study (patho-physiology in vivo, and even enables molecular imaging, thereby widening our understanding of basic and clinical science.
La Endomicroscopía Laser Confocal (ELC) recientemente se ha añadido al arsenal del gastroenterólogo. Permite la microscopía de la mucosa gastrointestinal, durante la endoscopia misma, después de la aplicación tópica o sistémica de los tintes fluorescentes. La ELC se ha estudiado en una multitud de enfermedades, tanto en el tubo digestivo superior como en el tracto gastrointestinal inferior, y recientemente, incluso en imágenes de la vía biliar, el páncreas y el hígado. En un enfoque traslacional, la ELC es una herramienta única para estudiar pato-fisiología in vivo, e incluso permite la proyección de imagen molecular, ampliando así nuestra comprensión de la ciencia básica y clínica.
Introduction
Confocal Laser Endomicroscopy (CLE) is a novel endoscopic technique that was first introduced 2004 into the endoscopist's armamentarium.1 Whereas techniques such as chromoendoscopy and conventional magnification endoscopy try to predict histology from mucosal patterns, CLE actually allows intravital microscopy of the human gastrointestinal mucosa during ongoing endoscopy, enabling real time optical biopsy. A multitude of trials have addressed the use of CLE in neoplastic and inflammatory diseases from the upper, middle and lower gastrointestinal (GI) tract. This has not abrogated the need for histo-pathology. CLE has rather been used to target biopsies or endoscopic interventions to regions of specific interest (smart biopsies) instead of having to rely on untargeted random biopsies, thereby reducing the number of biopsies and at the same time increasing their diagnostic yield.2 A different field of clinical research has been the introduction of CLE to organs not accessible by conventional endoscopy such as bile duct or liver. In a translational scientific approach, CLE has enabled a completely new view onto the GI mucosa. Artifact-free in vivo observation of subcellular mucosal details becomes possible in vivo at real time, within the intact micro-milieu of the organ of interest. Together with the option to perform similar confocal imaging in animal research this has opened a wide array of translational applications in endoscopy and even allows molecular imaging.
Technique of Endomicroscopy and Staining Protocols
Technique of confocal endomicroscopy
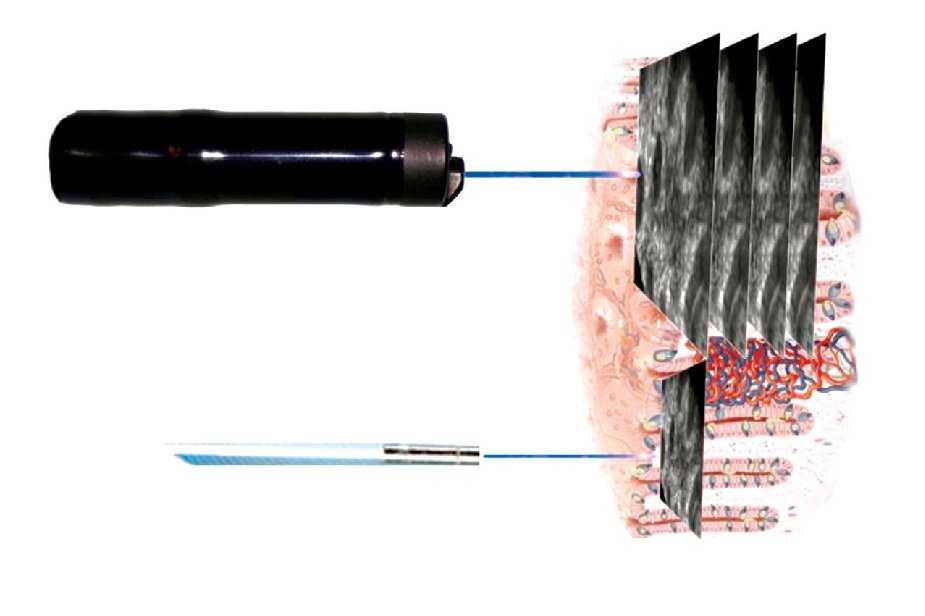
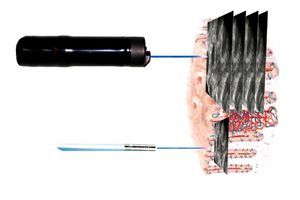
Two endomicroscopy systems are currently available for clinical use. The first human trials of endo-microscopy used a miniaturized laser scanner integrated into the distal tip of a conventional endoscope (Pentax EC-3870CIFK®, Tokyo, Japan). A single optical fiber serves as a pinhole for CLE and delivers a low-energy excitation wavelength of 488 nm. The collection bandwidth is 505 nm to 585 nm. The imaging plane depth can be adjusted from surface to 250 μm. Serial optical sections with a resolution of 1024 x 1024 pixels (lateral resolution of 0.7 μm) are obtained parallel to the tissue surface within a FOV of 475 x 475 μm (Figure 1, upper panel). For animal research, the same scanner was integrated into a handheld probe (FIVE1®, Optiscan, Notting Hill, Australia). The images provided in this review have been captured with endoscope-based CLE (eCLE).
Figure 1. Two systems are available for confocal endomicroscopy: With endoscope-integrated CLE (upper panel), the scanning device is integrated into the distal part of a dedicated endoscope. Imaging plane depth can be adapted and yields serial en face optical sections through the mucosa at high resolution. The CLE probe (lower panel) fits through the working channel of conventional endoscopes. It has lower resolution and fixed imaging plane depth, but faster image acquisition. Resultant images of both systems are parallel to the mucosal surface (90° compared to conventional tissue sections in histopathology).
The second CLE systems uses flexible confocal mini-probes that can be passed through the working channel of most conventional endoscopes and even into the bile duct (probe-based CLE, pCLE®; Cellvizio, Mauna Kea Technology, Paris, France). In pCLE, the imaging plane is fixed. Lateral resolution is defined by the number of the fibers (30 000 pixels). However, image acquisition is fast enough to provide a microscopic video of the mucosa. Probes are available at different FOV and diameters ranging from 0.3 mm to 4.2 mm. For animal research, a longer excitation wavelength (660 nm) is available in addition to excitation at 488 nm (Figure 1, lower panel).
Confocal Imaging in vivo
CLE signal relies on induced fluorescence. Therefore most studies used fluorescein as a fluorescent agent with a favorable safety profile.3 After intravenous injection, fluorescein is rapidly distributed within the tissue. Fluorescein yields good contrast of vessels and tissue architecture. However, it does not stain nuclei. In animal studies with lower regulatory requirements a multitude of staining protocols has been evaluated in vivo,4 and even antibodies have been fluorescently labeled.
As an alternative, topical contrast agents such as acriflavine and cresyl violet have been studied for visualization of nuclei. Acriflavine results in positive nuclear staining. Therefore there may be a theoretical risk of mutagenesis, although this has not been described for acriflavine so far (acriflavine and similar compounds are constituents of many skin disinfectants). Cresyl violet has been studied for simultaneous chromoendoscopy and immediate characterization by eCLE, resulting in indirect nuclear visualization by cytoplasmic enrichment of the dye.5
Just as every advanced endoscopy technique, CLE requires training and thorough knowledge of mucosal pathology. Close collaboration and feedback from an expert GI pathologist helps in difficult cases. From a study on Barrett's esophagus (BE) it has been estimated that after initial training approximately 100 cases are necessary for reliable CLE diagnosis in patients.6 However, for a first basic step to handle the CLE endoscope and to differentiate normal from abnormal tissue, a number of 30 examinations has been deemed sufficient, with online and printed support.7,8
Upper Gastrointestinal Tract
Endomicroscopy in the esophagus
In patients with suspected squamous cell cancer of the esophagus, the complete esophagus should be screened for synchronous lesions (field cancerization). In 21 patients, eCLE was combined with chromoendoscopy with Lugol's solution.9 Of the 43 lesions CLE correctly classified all malignant lesions. Two lesions were falsely classified as neoplastic. In a follow-up trial, irregular cellular arrangement, increased diameter and irregular shape of intrapapillary capillary loops were confirmed as diagnostic patterns in squamous cell cancer.10
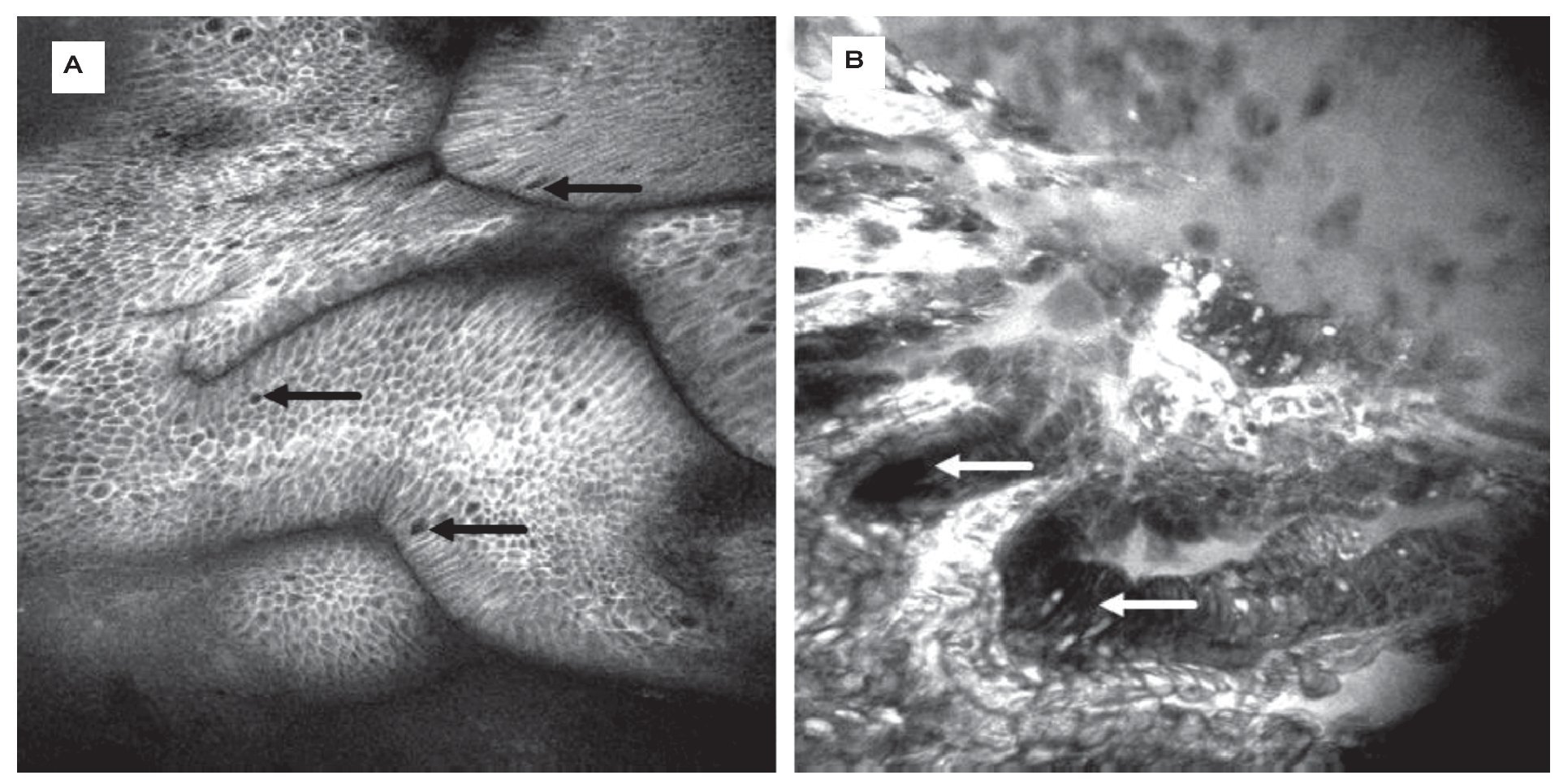
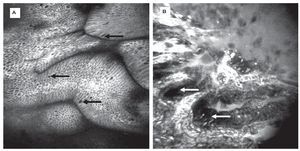
For surveillance of BE, random quadrant biopsies and targeted biopsies from lesions are recommended. Still, detection of neoplasia remains challenging. CLE easily identifies goblet cells as a key structure in BE during endoscopy (Figure 2A). In addition, eCLE correctly predicted the presence of BE (and its differentiation from gastric epithelium) and associated intraepithelial neoplasia (IN) (Figure 2B) with over 90% accuracy using a simple classification.11 This was followed by a cross over trial examining eCLE to target biopsies. CLE improved the diagnostic yield of BE-associated neoplasia compared to the Seattle quadrant biopsy protocol.12 CLE-targeted biopsy almost doubled the diagnostic yield for IN, and two thirds of patients with unsuspicious CLE findings did not need any mucosal biopsies.
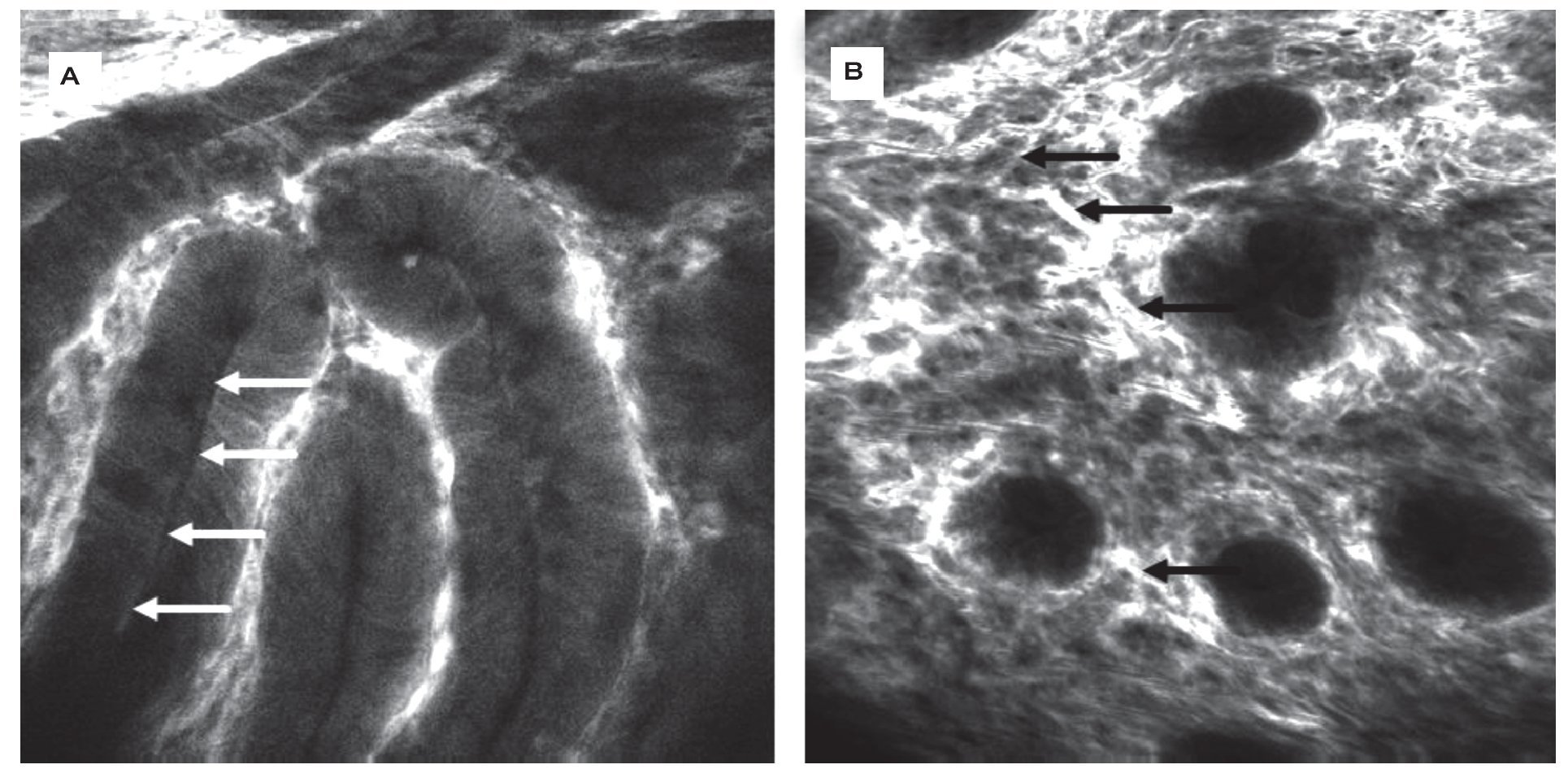
Figure 2.Barrett-Esophagus (BE). CLE is the only endoscopic technique that is able to visualize goblet cells (arrows) by their black mucin intracellular inclusion, defining BE in vivo. Gland structure is regular, no leakage of fluorescein into the (dark) lumen is seen A). In the same patient, another area shows grossly disturbed tissue structure: the epithelial lining of the Barrett's glands is of irregular height (arrows), diffusely dark cells often define neoplasia in the upper GI-tract. The vasculature is irregular, and leakage of fluorescein through the tumor vessels is seen. Immediate endoscopic resection was performed and confirmed the presence of Barrett's associated high grade intraepithelial neoplasia. Edge length 475 μm B).
pCLE diagnosis had negative predictive value (NPV) of 99% and a positive predictive value (PPV) of 44%.13 Similar data was obtained in a trial from three academic centers assessing 68 patients with BE. Compared to the Seattle biopsy protocol, pCLE had a high on-site NPV of 95% to exclude IN however at the price of a low PPV of only 18%.14 A head-to-head comparison of eCLE vs pCLE to explain for differences in PPV and specificity has not been performed. A potential explanation may be higher resolution of eCLE and visualisation of single cells to identify neoplasia whereas the somewhat lower resolution of pCLE is sufficient for pattern recognition of non-neoplastic Barrett's glands. However, this will have to be studied in future trials before random quadrant biopsies as the current gold standard can be abandoned.
Endomicroscopy of gastric pathologies
In the healthy stomach, the mucosa has a cobble-stone pattern with round pits in the corpus and branched pits in the antrum and cardia. Endomicroscopy was able to differentiate hyperplastic from adenomatous lesions with high accuracy.15 In a large follow-up trial, 182 patients were examined to establish criteria for gastric cancer. The following evaluation phase included 1 786 patients, and eCLE was compared to white light endoscopy for real time diagnosis of gastric cancer. CLE showed good accuracy (99%) for the in vivo diagnosis of gastric superficial cancer and high grade IN vs the gold standard histopathology.16
After endoscopic resection of early gastric cancer, R1 at the deep margin is usually an indication for surgery whereas positive lateral margins can be re-treated endoscopically. This mandates exact identification of the tumor residues. Twenty four patients underwent eCLE two weeks after endoscopic resection.17 Of these, CLE identified five patients with indefinite lateral margins that were re-resected under CLE control. Accuracy of CLE to predict incomplete resection was 92% in this study.
Endomicroscopy of the small intestine
The diagnosis of celiac disease is impaired by sampling error. In a first study on celiac disease, 31 patients were examined by Ecle.18 Villous atrophy and crypt hyper-trophy were readily visible whereas quantification of intraepithelial lymphocytes (Marsh I) was difficult to obtain since fluorescein does not stain nuclei. Still, eCLE was able to diagnose celiac disease with high accuracy, and even a trend towards improved diagnostic accuracy for CLE vs histopathology specimens was observed in those patients with established celiac disease, due to a sampling error for real biopsies. Confocal imaging for celiac disease has also been studied in 19 children, again providing good sensitivity and specificity of 100% and 80%, respectively.19
Lower Gastrointestinal Tract
Endomicroscopy for colonic neoplasia
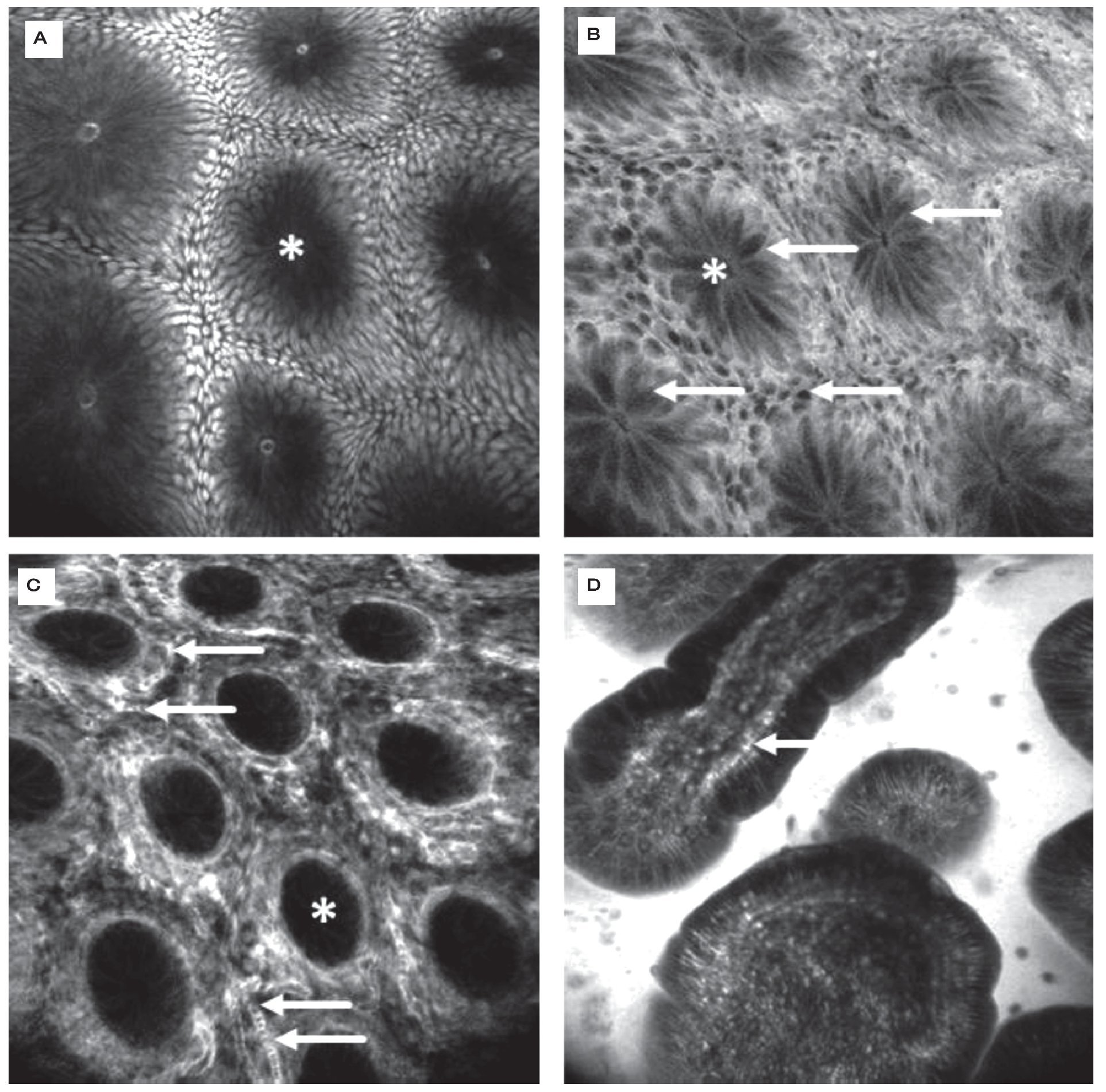
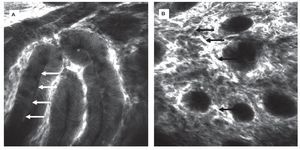
CLE was first evaluated in 2004 in patients with colonic pathologies using fluorescein1. Based on crypt architecture in superficial sections and vessel morphology in deeper mucosal sections (Figures 3 and 4), lesions were classified as normal, regenerative (inflammatory or hyperplastic) or neoplastic. This simple classification allowed differentiation in vivo with an accuracy of 99%. These results were confirmed in a follow-up trial.20 These trials were the first to show that gastroenterologists are able to judge mucosal microarchitecture during ongoing endoscopy. Fluorescein does not stain nuclei, therefore the differentiation of an inflammatory mucosal infiltrate or between high grade and low grade neoplasia cannot be based on nuclear morphology with such a staining protocol. However, by combining systemic fluorescein with topical acriflavine (Figure 3A), such differentiation could be achieved in colonic adenomas.21
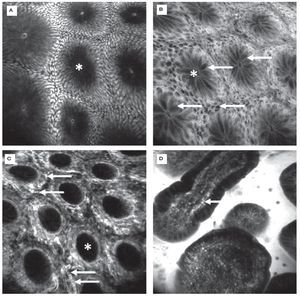
Figure 3.Healthy colon. In the healthy mucosa, round crypt openings (asterisks in A and C) are surrounded by goblet and epithelial cells. After topical acriflavine staining A), the basal nuclei are highlighted. After fluorescein injection B) D), goblet cells (arrows, B) are easily recognized by their black mucin inclusions (Figure 2A). In deeper sections C), the capillary network is seen: the vascular lumen is brightly stained after IV injection of fluorescein (arrows), erythrocytes can be seen as black dots within the lumen. The terminal ileum D) is characterized by intestinal villi with a monolayer of high prismatic enterocytes and hair-pin like vessels directly underneath (arrows) in the lamina propria. Edge length 475 μm.
Figure 4.Colonic neoplasia and inflammation. In colonic adenomas, the crypt lumen is elongated (arrows), and goblet cells are scarce. Increased leakage of fluorescein into the lamina propria, which is then displayed brightly, is a common feature A). In inflammation, crypt distance and size is often irregular. The lamina propria is enlarged by cellular infiltration, increased vasculature (arrows) and leakage of fluorescein, indicating tissue edema B).
Endomicroscopy in colitis
Ulcerative colitis (UC) is a good indication for high-end endoscopic techniques, since patients with longstanding UC are at increased risk for developing neoplasia, but IN is difficult to detect. A pan-endomicros-copy of the entire colon is not feasible. Therefore, CLE has been combined with chromoendoscopy. In this setting, chromoendoscopy helps to unmask suspicious lesions which are subsequently characterized (and biopsies targeted) by CLE. eCLE was able to predict the presence of neoplasia with an accuracy of 98% in a prospective trial.2 Probably as important, if optical biopsies by eCLE suggested normal findings, histology confirmed the presence of normal mucosa in over 99%, thus abrogating the need for random biopsies in these patients.
In microscopic colitis, the inflammation is not visible in conventional endoscopy, and the infiltrate often shows a patchy distribution that may be difficult to pick up with unguided random biopsies. However, eCLE is able to rapidly take multiple optical biopsies and is able to visualize lymphocytic infiltration in lymphocytic colitis and the subepithelial band in collagenous colitis.22-24
Patients with graft vs host-disease (GvHD) after bone marrow transplantation are at immediate need for adequate diagnosis and therapy. In a recent trial, eCLE was able to diagnose and to grade GvHD during ongoing endoscopy with high accuracy based on the presence of inflammation and epithelial apoptosis.25
Endomicroscopy Beyond the Intestinal Lumen
pCLE has been combined with ERCP to provide microscopy of biliary strictures in a pilot trial in 14 patients.26 In this setting, the thin confocal probe is passed through the working channel of the duodenoscope or the cholangioscope and advanced to the region of interest within the biliary system. The presence of irregular vessels at the stenosis site was predictive of biliary neoplasia. pCLE had an accuracy of 86% and showed better sensitivity (83%) than histology (50%). Although a follow-up study found a lower accuracy rate in 14 indeterminate strictures,27 pCLE may represent a good adjunct to assist in the difficult differential diagnosis of biliary strictures.
Recently, probe-based CLE has been combined with endosonography-guided puncture of pancreatic cystic lesion, a procedure termed needle-based CLE (nCLE), in a proof-of-principle approach. Technical feasibility was achieved in 17/18 procedures, and sufficient image quality in 10/17 cases. Although follow up trials are to be awaited, nCLE may prove valuable in the evaluation of such cystic lesions.28
Confocal microscopy has also been combined with mini-laparoscopy to evaluate liver parenchyma. For this, a sterile sheathed rigid confocal probe (containing the scanning device of eCLE) was introduced under laparoscopic guidance. With fluorescein and blue laser light, imaging plane depth was limited to superficial parts of the liver.29 However, combining near-infrared light with indocyanine green as a contrast agent, good imaging of the liver parenchyma was achieved, allowing for diagnosis of steatosis and cirrhosis in vivo.30 Such deeper tissue imaging up to 350 μm may also prove valuable in GI endoscopy in order to determine the infiltration depth of a lesion prior to endoscopic resection. However, indocyanine green does not result in a sufficient submucosal contrast in the gut, and novel contrast agents are still not approved for clinical use.
Outside the field of gastroenterology, CLE has been applied to cancer diagnosis in the oral cavity,31 the urinary bladder32,33 and gynecology34 while its use to define R0 resection during surgery is still awaited and currently tested in neurosurgery.
Endomicroscopy in Clinical and Translational Science
Functional imaging
CLE is unique in its imaging properties: It does not just imitate histology but rather visualizes finest sub-cellular details in their natural surroundings virtually free of artifacts such as by fixation, cutting, or staining. Recent investigations indicated that the healthy gastrointestinal mucosa is punctured by gaps the size of a single cell, probably as a normal process in epithelial regeneration.35 Using eCLE, it could be demonstrated that those gaps can be visualized in vivo in healthy patients, and that these gaps are more numerous in a mouse model of colitis.36 It has been speculated that these gaps occur by apoptotic cell shedding that can be nicely seen by confocal endomicroscopy.25,37 In a first evaluation of such findings in patients with inflammatory bowel diseases (IBD) it was found that gaps are more frequent regardless of type, pattern or therapy of IBD.38 Recently it was determined that ultrastructural changes such as gaps in the epithelial layer may be accompanied by leakage of fluorescein from the lamina propria into the intestinal lumen, or may even result in microscopic erosions. In patients with Crohn's Disease and complete clinical and endoscopic remission (by means of conventional endos-copy), the presence of such functionally relevant gaps (as opposed to gaps without leakage or completely intact epithelium) is associated with a significantly higher rate of relapse.39 These gaps could thus represent a micro-architectural compound of the impaired mucosal barrier function and further define the concept of mucosal healing. In agreement with this, intramucosal bacteria could be visualized more frequently with eCLE in patients with IBD than in healthy controls.40 This again exemplifies the high resolution of CLE for in vivo imaging.
A different aspect of tissue function that is nicely visualized by CLE (and relies on in vivo imaging of intact tissue) is normal and pathologic perfusion.41 Extravasation of fluorescein as an indicator of increased vessel leakiness is part of the pathophysiology in UC42 and colonic neoplasia.1 Alterations in the density of capillaries has helped to identify neoplasia in BE.43
Molecular imaging
In all of the above mentioned studies on the use of CLE, CLE has been combined with nonspecific dyes such as fluorescein, acriflavine or cresyl violet. However, similar to bench top confocal microscopy, exogenous molecular probes such as peptides, antibodies or probes with tumor-specific activation can be labeled with fluorescent agents to provide molecular imaging in live tissue.44,45 Molecular imaging aims at specific detection and characterization of neoplasia or inflammation.
In xenograft animal models of human colorectal cancer, labeled antibodies targeting Epidermal Growth Factor Receptor (EGFR)46 and Vascular Endothelial Growth Factor (VEGF)47 have been studied. In these trials, differentiation of receptor expression was achieved in xenografts in vivo and on resected human tissue with CLE. With a labeled peptide that was sprayed onto the colonic mucosa, even molecular imaging of colorectal adenomas in patients was possible in vivo.48
The aim of such molecular imaging is two-fold: First, it should be studied for enhanced detection (molecular chromoendoscopy) and molecular characterization of lesions. Second, it may even be used to predict response to targeted molecular therapies in cancer and inflammatory disorders by defining expression patterns and uptake behavior.
Conclusion
In conclusion, CLE is a novel tool in the gastro- enterologist's armamentarium that for the first time has allowed not just predicting but actually seeing tissue microscopy during endoscopy. It has been studied in multiple diseases of the upper and lower gastrointestinal tract, but also outside the intestinal lumen. Simplified classification criteria have been established in many disease entities to facilitate diagnosis.
CLE as a tool for translational science has only just started to impact on our understanding of patho-physiology, and first trials suggest that functional imaging may even influence our clinical algorithms. Together with molecular imaging to improve diagnosis and predict response to therapy, CLE bears great potential to further enhance our understanding of gastrointestinal diseases.
Correspondence: Martin Goetz, MD PhD. I.
Medizinische Klinik und Poliklinik. Universitätsmedizin Mainz. Langenbeckstr 1. Z.P. 55131. Mainz, Germany.
Phone: +49 6131 17 1. Fax: +49 6131 17 5552.
E-mail: mgoetz@mail.uni-mainz.de