Thyroglobulin in the needle washout (Tg-FNA) and cytology of fine needle aspiration (cyto-FNA) are recommended for diagnosis of metastatic lymphadenopathies and recurrence of differentiated thyroid cancer. The objective of this study was to assess the value of these procedures in 16 cervical masses from patients with thyroid cancer of the follicular epithelium (TC).
Patients and methodsThe study included six patients with TC and cervical lymphadenopathies evaluated before initial thyroid surgery and 10 patients followed up after TC surgery with cervical lumps discovered. FNA was performed in all 16 masses. Results of cyto-FNA, Tg-FNA and of the combined tests were compared to the final diagnosis of each lesion.
ResultsAmong 10 lesions proven to be malignant at surgery, cyto-FNA, Tg-FNA and the combination of both allowed for adequate diagnosis in 7, 9, and 10 cases respectively. Among 6 lesions considered to be benign, cyto-FNA was able to confirm diagnosis in 4, was non-diagnostic in one, and was falsely negative in the remaining case, while Tg-FNA was below the established cut-off value (to consider malignancy) in all cases.
ConclusionsIn patients with TC and suspect cervical masses, Tg-FNA improved the diagnostic yield of cyto-FNA alone, thus warranting its routine recommendation when FNA is performed. However, universal standardization of the technique and definition of valid cut-off thyroglobulin values (depending on the immunoassay used) above which the lesion should be considered to be malignant are still pending.
La tiroglobulina del lavado de aguja (Tg-PAAF) junto con la citología de la punción-aspiración con aguja fina (cito-PAAF) son procedimientos recomendados para el diagnóstico de metástasis ganglionares cervicales y recurrencias del cáncer diferenciado de tiroides. El objetivo de este estudio fue valorar la utilidad de estas técnicas en 16 lesiones cervicales de pacientes con cáncer de tiroides del epitelio folicular (CT).
Pacientes y métodoSe incluyeron 6 pacientes con CT y adenopatías laterocervicales evaluadas antes de la cirugía tiroidea inicial y 10 pacientes en seguimiento por CT ya operado y lesiones cervicales ecográficamente sospechosas y/o persistentes. Las 16 lesiones fueron sometidas a PAAF. Los resultados de la Tg-PAAF, cito-PAAF y de la combinación de ambas se compararon con el diagnóstico definitivo asignado a cada lesión.
ResultadosEn 10 lesiones comprobadamente malignas tras extirpación quirúrgica (9 metástasis ganglionares y una recurrencia en lecho), la cito-PAAF, la Tg-PAAF y la combinación de ambas técnicas permitieron llegar al diagnóstico correcto en 7, 9 y 10 casos respectivamente. En 6 lesiones consideradas benignas, la cito-PAAF pudo confirmar la benignidad de la lesión en 4, resultó no diagnóstica en una y falsamente positiva en otra, mientras que la Tg-PAAF se halló por debajo del valor de corte establecido (para considerar malignidad) en todos los casos.
ConclusionesEn pacientes con CT y lesiones cervicales sospechosas, la Tg-PAAF mejora la rentabilidad diagnóstica de la cito-PAAF aislada justificándose así su recomendación sistemática al realizar la PAAF. Quedan sin embargo por definir una estandarización universal de la técnica y unos valores de corte válidos de Tg-PAAF (de acuerdo al inmunoanálisis empleado) por encima de los cuales considerar la lesión maligna.
In approximately 5–20% of patients with differentiated thyroid cancer (DTC) locoregional metastases (MTS) are found in the neck during follow-up1,2. In addition, 20–31% have nodal MTS in the neck3,4 in the initial ultrasound examination recommended before thyroidectomy by the various guidelines and consensuses5,6. Many, but not all, studies7–9 suggest a poorer prognosis in subjects with nodal MTS at diagnosis and recurrent disease (as nodal MTS or the recurrence in tumor bed) and advise the resection of such lesions10 by functional lymph node dissection in the event of nodal MTS11,12.
Some diagnostic tools, such as ultrasensitive neck ultrasound and immunoassays for serum thyroglobulin are, due to their technical perfection, mostly responsible for a significant improvement in the identification of recurrent disease. Thus, since the 1990s, the increased sensitivity of immunoassays for serum thyroglobulin has allowed for the earlier diagnosis of recurrent disease (after total thyroidectomy, usually followed by an ablation dose of radioactive iodine) and for the diagnosis of most recurrences thanks to thyroglobulin stimulation by discontinuing levothyroxine or with recombinant TSH13,14. The disadvantages of serum thyroglobulin include its limited value when serum anti-thyroglobulin antibodies (Ab) are positive (in approximately 25% of cases of DTC15) and the lack of information regarding the location of the recurrence. Unlike thyroglobulin measurement, neck ultrasound is an imaging test which is highly sensitive, not only for suspicious lesions during the follow-up of DTC, thus allowing for a potentially curative resection, but also for changing the initial surgery, from total thyroidectomy (with or without central lymphadenectomy) to thyroidectomy associated with lateral neck lymphadenectomy5. Ultrasound examination is also of particular interest in DTC because recurrent disease is located in the neck in most cases16,17. However, the high resolution of ultrasound often leads to the identification of adenopathies or neck lesions other than MTS from thyroid cancer, including MTS from non-thyroid tumors, specific lymphadenitis, or extremely frequent reactive lymphadenitis18–20, and although ultrasonographic criteria for suspected malignancy are avilable21, these frequently lack sufficient specificity for a differential diagnosis between MTS from DTC and adenopathies from other causes22.
In order to increase the diagnostic yield of ultrasound examination, the cytological study of suspicious lesions with fine needle aspiration (FNA) is advised6,23. This procedure markedly improves the diagnosis of MTS from thyroid cancer24, and frequently allows for the ruling out of malignancy in the adenopathies studied. Despite this, cytological examination of FNA leaves undiagnosed a significant incidence of MTS, as evidenced by the high proportion of non-diagnostic or inadequate results (5%-25%)22,25,26 and false negative results (6%-8%)27,28. In these cases, the limitation of FNA is essentially due to the lack of epithelial cell material or to cell degeneration, which prevents recognition of the tumor cells required to document diagnosis. The lack of cell material has been attributed to different factors, including the small size of nodal MTS, which makes the collection of adequate material difficult29, and frequent cystic transformation of the lymph node with MTS from papillary carcinoma, which causes an absence or scarcity of cells in FNA30,31.
Based on the favorable results reported for thyroglobulin measurement in the washout fluid from the needle used to perform FNA from suspicious lesions32–36, both the European Consensus6 and the ATA guidelines5 recommend that the information provided by thyroglobulin from needle washout fluid (Tg-FNA) be combined with that provided by cytology (cyto-FNA).
The aim of this study was to assess the value at our center of Tg-FNA added to cyto-FNA in neck lesions in patients diagnosed with thyroid carcinoma originating in the follicular epithelium.
Patients and methodsPatientsSixteen patients with thyroid carcinoma in the follicular epithelium (one with insular carcinoma, 12 with papillary thyroid carcinoma [PTC], one with PTC of the diffuse sclerosing variant, one with PTC of the columnar cell variant, and one with an unknown variant of PTC) undergoing FNA (for cytological study and thyroglobulin measurement in needle washout fluid) for a suspicious neck lesion were enrolled into the study. All FNAs were performed at Hospital Universitario de Móstoles (Madrid) between July 2010 and July 2012.
In six patients, FNA was performed before total thyroidectomy on adenopathies disclosed by ultrasonography before surgery. Five of these adenopathies located in lateral chains (levels II, III, or IV) met at least one suspicious radiographic criterion, and one adenopathy, at level V, had a reactive appearance. Ultrasonographic features considered to be suspicious included rounded morphology in a cross section (long/short axis ratio less than 2), the loss of echogenic fatty hilum, the presence of cystic changes, internal heterogenicity, and an irregular or peripheral internal vascularization pattern.
In the remaining 10 patients already operated on for thyroidectomy (treated with at least total thyroidectomy and a therapeutic dose of 131I), lesions subject to FNA were located in or near the thyroid bed in two, in lateral neck adenopathies in seven (four ultrasonographically suspicious and three persistent over time), and in the left parotid gland in one patient. Five of these 10 patients had at the time of FNA basal or recombinant TSH-stimulated serum thyroglobulin levels suggesting persistent disease, while the other five patients had both basal and stimulated serum thyroglobulin levels less than 2ng/mL, only one of which was associated with positive anti-thyroglobulin Ab.
Ultrasound-guided fine needle aspiration and thyroglobulin in the needle washoutAll FNAs were performed after informed consent under ultrasonographic guidance, with the neck of the patient hyperextended, using a 21–25 gauge needle coupled to a 10mL syringe. The material collected was extended onto a slide for cytological study by a pathologist. The needle was washed with 1mL of physiological saline using the syringe used for aspiration and repeating washing through the syringe for up to three times in order to collect the maximum amount of material. The fluid thus collected was sent to the laboratory in a dry tube for thyroglobulin measurement. Thyroglobulin was measured in needle washout fluid in all 16 lesions, and also in serum from the 10 patients with a history of thyroidectomy using an electrochemiluminescence immunoassay (Analytics E-170 modular autoanalyzer from Roche Diagnostics) with analytical and functional sensitivity limits of 0.1ng/mL and 1ng/mL respectively. In the 10 patients in whom serum thyroglobulin was measured, anti-thyroglobulin Ab was simultaneously measured in serum using the Immulite 2000 enzyme-labeled immunometric assay (Siemens), with an analytical sensitivity limit of 2.2IU/mL and normal values less than 40IU/mL.
Data analysis and interpretationThe results of cyto-FNA were classified into three groups:
- -
Non-diagnostic cyto-FNA: an absence of epithelial cells, lymphocytes, or plasma cells with the potential presence of blood cells.
- -
Negative cyto-FNA: reactive lymphadenitis (lymphocytes with occasional plasma cells and an absence of epithelial cells) or consistent with the benign lesion of another etiology.
- -
Positive cyto-FNA: consistent with thyroid carcinoma or suspicious due to the presence of atypical epithelial cells or with cytological features of PTC.
Based on the description of detectable thyroglobulin levels in the washout fluid of the FNA needle in non-metastatic adenopathies from thyroid cancer given in multiple studies27,32,37–42, Tg-FNA was considered positive (suggesting MTS from thyroid cancer) with values above 7.4ng/mL, which was the cut-off value used in the Cignarelli et al.37 study (equivalent to the mean plus two standard deviations of the value found in non-metastatic adenopathies from thyroid cancer), and was defined as negative (suggesting no MTS from thyroid cancer) when values were less than 7.4ng/mL.
The results of cyto-FNA and Tg-FNA were compared (separately and in combination) with the final diagnosis assigned to each lesion. A final diagnosis of thyroid malignancy was based on histopathology after surgical resection. A final diagnosis of benign disease was defined based on either the histological study of the surgical specimen or on the course of the lesion over time (ultrasonographic disappearance or a significant decrease in size). To estimate the diagnostic yield of cyto-FNA, all finally malignant lesions not identified with cytological study (non-diagnostic and negative cyto-FNAs) were considered to be falsely negative.
ResultsMalignant lesionsMalignancy was confirmed after surgical resection of the lesions in 10 cases (one recurrence in the bed and nine nodal MTS). These consisted of five adenopathies identified before initial surgery and five lesions found during the follow-up of patients with a history of DTC (Table 1). In four of these five patients, serum thyroglobulin, either basal or stimulated with recombinant TSH, suggested persistent disease (levels higher than 2ng/mL in the absence of anti-thyroglobulin Ab), while the remaining patient had undetectable serum thyroglobulin but an anti-thyroglobulin Ab titer of 2902IU/mL.
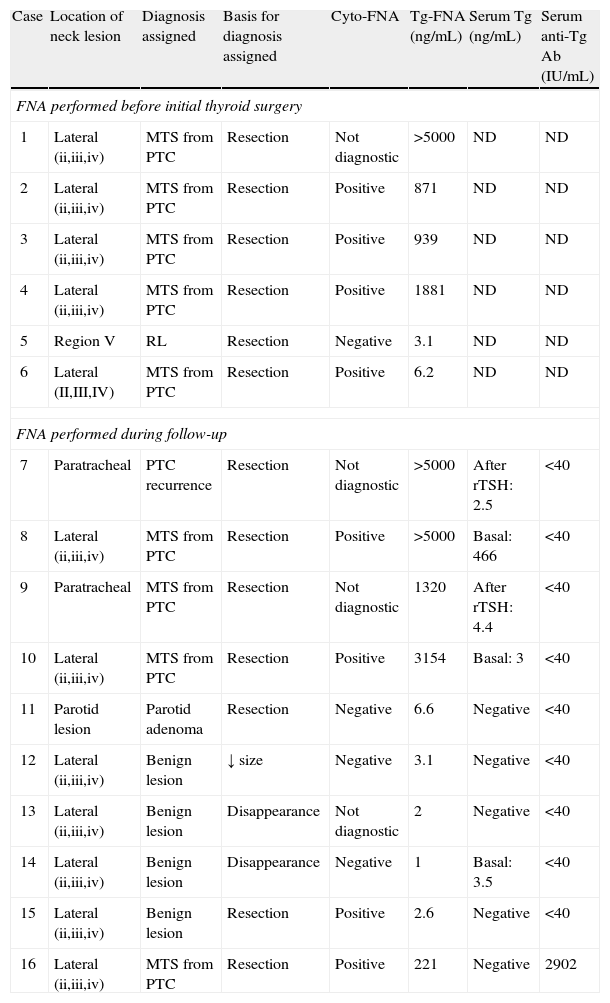
Individual results of cyto-FNA and Tg-FNA and final diagnosis of the 16 neck lesions subject to FNA.
| Case | Location of neck lesion | Diagnosis assigned | Basis for diagnosis assigned | Cyto-FNA | Tg-FNA (ng/mL) | Serum Tg (ng/mL) | Serum anti-Tg Ab (IU/mL) |
| FNA performed before initial thyroid surgery | |||||||
| 1 | Lateral (ii,iii,iv) | MTS from PTC | Resection | Not diagnostic | >5000 | ND | ND |
| 2 | Lateral (ii,iii,iv) | MTS from PTC | Resection | Positive | 871 | ND | ND |
| 3 | Lateral (ii,iii,iv) | MTS from PTC | Resection | Positive | 939 | ND | ND |
| 4 | Lateral (ii,iii,iv) | MTS from PTC | Resection | Positive | 1881 | ND | ND |
| 5 | Region V | RL | Resection | Negative | 3.1 | ND | ND |
| 6 | Lateral (II,III,IV) | MTS from PTC | Resection | Positive | 6.2 | ND | ND |
| FNA performed during follow-up | |||||||
| 7 | Paratracheal | PTC recurrence | Resection | Not diagnostic | >5000 | After rTSH: 2.5 | <40 |
| 8 | Lateral (ii,iii,iv) | MTS from PTC | Resection | Positive | >5000 | Basal: 466 | <40 |
| 9 | Paratracheal | MTS from PTC | Resection | Not diagnostic | 1320 | After rTSH: 4.4 | <40 |
| 10 | Lateral (ii,iii,iv) | MTS from PTC | Resection | Positive | 3154 | Basal: 3 | <40 |
| 11 | Parotid lesion | Parotid adenoma | Resection | Negative | 6.6 | Negative | <40 |
| 12 | Lateral (ii,iii,iv) | Benign lesion | ↓ size | Negative | 3.1 | Negative | <40 |
| 13 | Lateral (ii,iii,iv) | Benign lesion | Disappearance | Not diagnostic | 2 | Negative | <40 |
| 14 | Lateral (ii,iii,iv) | Benign lesion | Disappearance | Negative | 1 | Basal: 3.5 | <40 |
| 15 | Lateral (ii,iii,iv) | Benign lesion | Resection | Positive | 2.6 | Negative | <40 |
| 16 | Lateral (ii,iii,iv) | MTS from PTC | Resection | Positive | 221 | Negative | 2902 |
RL: reactive lymphadenitis; positive cyto-FNA: cytology with malignant or suspected malignant cells; negative cyto-FNA: cytology with a benign result; PTC: capillary thyroid carcinoma; MTS: metastasis; ND: not done; FNA: fine needle aspiration; Tg: thyroglobulin; rTSH: recombinant TSH.
Cyto-FNA was positive in seven of the finally malignant 10 lesions, and non-diagnostic (due to a lack of cell material) in the other three cases (Table 2). Tg-FNA was considered to be positive in 9 out of 10 malignant lesions (Table 2). Values ranged from 221ng/mL to > 5000ng/mL (upper detection limit), including the patient with positive serum anti-thyroglobulin Ab not detected in needle washout fluid. In one patient with nodal MTS from PTC (of an unknown histological variant because thyroid surgery was performed at another center), the Tg-FNA level of 6.2ng/mL was considered to be negative, as it was below the predefined cut-off value (Tables 1 and 2).
Final diagnosis of the 16 lesions and comparison with the FNA result.
| Final diagnosis (n) | Cyto-FNA | Tg-FNA |
| MTS from PTC (10) | Positive: 7Negative: 0 | Positive: 9Negative: 1 |
| Benign lesion (6) | Positive: 1Negative: 4 | Positive: 0Negative: 6 |
| No diagnosis (0) | 4 | – |
Cyto-FNA: cytology of fine needle aspiration; PTC: papillary thyroid carcinoma; MTS: metastasis; Tg-FNA: thyroglobulin measured in washout fluid from the needle used in FNA.
The three malignant lesions not identified by cyto-FNA were identified with Tg-FNA, and in the patient with a false negative in Tg-FNA, cytology revealed malignancy, so the combination of both procedures provided a 100% sensitivity.
Benign lesionsSix lesions were finally diagnosed as benign, and the diagnosis was confirmed after surgical resection in three cases: a parotid pleomorphic adenoma (in a patient followed up for PTC and undetectable basal and stimulated serum thyroglobulin levels) and two reactive lymphadenitis (at level V in a patient with PTC pending surgery at the time of FNA, and at level IIA in a patient followed up for an already operated insular carcinoma and with basal and TSH-stimulated serum thyroglobulin levels less than 2ng/mL). The three lesions for which surgery was not performed were diagnosed as benign based on the ultrasonographic disappearance of radiographically suspicious adenopathies in the patients monitored (n=2, one with undetectable serum thyroglobulin and the other with basal serum thyroglobulin higher than 2ng/mL, both with negative serum Ab) at one year or on a significant (50%) decrease in the size of the adenopathy in an ultrasound examination performed six months after FNA (n=1, with negative serum thyroglobulin and Ab) (Table 1).
Cyto-FNA was negative in four of the six benign lesions (suggesting primary parotid tumor in one patient and reactive lymphadenitis in three), non-diagnostic in one lesion (due to a lack of cell material), and falsely positive (the presence of degenerated cells interpreted as epithelial cells) in a finally resected adenopathy in a patient followed up after surgery for insular carcinoma (83% specificity, Table 2).
In all benign lesions, the Tg-FNA result was negative (range, 1–6.6ng/mL; 100% specificity; Tables 1 and 2).
DiscussionUltrasound examination of the neck plays a relevant role in DTC both before and after initial surgery and during follow-up5,6,23 because of its high sensitivity for the recognition of nodal MTS from thyroid cancer, but it often has no adequate specificity for confirming an etiological diagnosis21,22. Cytological study of the material collected by FNA from ultrasonographically suspicious lesions has become the standard confirmation procedure16, although it leaves undiagnosed a significant number of MTS because of the frequency of inadequate or non-diagnostic results27,32. The measurement of thyroglobulin in the washout fluid from the needle used to perform FNA is an additional and simultaneous procedure which improves sensitivity as compared to cyto-FNA alone, as reported by many studies25–27,33–37,39–44. In our study, nine out of 10 malignant lesions were confirmed with Tg-FNA, as compared to seven with cyto-FNA. This suggests a higher sensitivity of Tg-FNA as compared to cyto-FNA in the small number of lesions studied here, which agrees with the reports from other studies25–32,35–37,39,40,42,43. The high sensitivity of Tg-FNA reported by different authors (81.4% to 100%) is attributed to a high expression of the thyroglobulin gene in even small nodal MTS46, resulting in high levels of the molecule both inside and in the vicinity of metastatic cells. This makes it possible to document the presence of thyroid cells even when cell identification is not possible. The 100% sensitivity of Tg-FNA reported by some authors26,32,35–37,44 has not been confirmed in all studies. The few false negative results have partly been associated with an impaired thyroglobulin producing capacity of some histological variants of thyroid cancer. Thus, in the Baloch et al. study43, eight out of 15 lesions not identified by Tg-FNA corresponded to nodal MTS from a PTC of the tall cell variant, an aggressive variant in which decreased thyroglobulin gene expression has been reported in recurrences47. In another study45, the two metastatic adenopathies with low Tg-FNA values were found in MTS from oncocytic carcinomas whose immunohistochemical study after resection revealed a very weakly positive staining for thyroglobulin. The four false negative results in the Boi et al. study34 and two of the four false negative results in the Bournaud et al. study25 occurred in nodal MTS from anaplastic or poorly differentiated carcinomas, known to have a lower thyroglobulin secreting capacity.
In another study37, however, Tg-FNA was positive in MTS both from DTC and poorly differentiated thyroid carcinomas, although the mean value was clearly higher in the former (16,225ng/FNA versus 503ng/FNA; MTS from DTC versus MTS from poorly differentiated carcinomas), reflecting the persistent, although decreased, thyroglobulin secretion reported for anaplastic carcinomas48. The false negative result in Tg-FNA found in our series corresponded to a nodal from a PTC whose variant could not be documented.
Although most, but not all, studies34,38,45 reported a greater sensitivity of Tg-FNA as compared to cyto-FNA, almost all of them concluded that combined information from both procedures improves the diagnostic yield of cyto-FNA alone. As occurred in our series, which had a very small number of cases, in many studies with a larger sample size the combination of Tg-FNA and cyto-FNA showed 100% sensitivity26,32,34–36,38,39, or at least substantially improved the sensitivity of either of the procedures alone,25,27,40,41,45 so that many of the lesions not demonstrated by one of the procedures were detected by the other procedure.
The high specificity of Tg-FNA is another potential attribute of the test. Since only thyroid follicular cells express the thyroglobulin gene49,50, in patients with epithelial thyroid cancer, adequate identification of the molecule in a neck lesion located outside the thyroid gland (except for thyroglossal duct lesions) or in the thyroid bed in subjects with a history of total thyroidectomy confirms MTS or disease recurrence. However, the designation of a cut-off value in Tg-FNA from which MTS should be considered likely stems from the observation of slightly elevated thyroglobulin levels in Tg-FNA for reactive lymphadenitis and nodal MTS from histopathologically documented non-thyroid tumors27,32,34–36,38,39,41,42,45. Two hypotheses attempt to justify these findings: the blood contamination and the matrix effect hypotheses. The blood contamination hypothesis postulated by Frasoldati et al.27 is based on the presence of thyroglobulin inside the vessels of the lymph node subject to FNA, which would account for the higher thyroglobulin levels in Tg-FNA of nodes from subjects with a non-resected thyroid gland (and presumably higher blood thyroglobulin levels) as compared to Tg-FNA of nodes from patients undergoing thyroidectomy (with low or undetectable serum thyroglobulin levels). Based on this hypothesis, some authors propose a cut-off value in Tg-FNA from which metastatic lesion should be considered, which will be different depending on whether the thyroid gland is present (39.3ng/mL27 and 36ng/mL34) or absent (1.1ng/mL27 and 1.7ng/mL34). According to this approach, other authors have proposed as discriminant a value in Tg-FNA higher than that of simultaneously measured serum thyroglobulin40–42. Other researchers have refuted the blood contamination hypothesis25,45,51 after finding similar Tg-FNA values in non-metastatic adenopathies from thyroid carcinoma irrespective of the presence or absence of thyroid gland and after calculating the small relative contribution of blood thyroglobulin to the thyroglobulin level found in needle washout fluid45. These authors postulated that the slightly positive value of Tg-FNA in reactive lymphadenitis or in lymph nodes with MTD from non-thyroid tumors is due to interference caused by the change in the medium in which thyroglobulin is measured, the so-called matrix effect52. According to this hypothesis, the solution used to wash the needle (physiological saline or thyroglobulin-free solution provided with the kit, depending on the study) does not contain thyroglobulin, but is characterized by physicochemical properties (pH, polarity, particles of the solid lesion swept away in FNA) able to induce non-specific interactions with Ab from some tests designed to measure serum thyroglobulin, thus inducing detectable basal luminosity or radioactivity25. This matrix effect would thus explain the slightly positive thyroglobulin levels in media such as physiological saline39 (values up to 5ng/mL) or in abdominal lipoma and quadriceps muscle (values of 4.7 and 3.9ng/FNA respectively). In the Snozek et al. study35, in the same adenopathies, the value found with Tg-FNA when washing was done with saline was 25% higher as compared to that found when thyroglobulin-free was used for washing, thus confirming interference by a change in the washout medium. The reporting of detectable thyroglobulin levels in Tg-FNA of adenopathies or lesions with no thyroid tissue in these studies requires the definition of a threshold value from which metastatic lesion or thyroid cancer recurrence may be considered. No consistent criterion exists about the best cut-off value to be used. Many consider as positive values those above the mean plus two standard deviations of values found in washout fluid from reactive adenopathies and MTS from non-thyroid tumors (with absolute values ranging from 1.1 to 39ng/mL depending on the fluid used for needle washing, on fluid volume, and on the immunoassay used to measure thyroglobulin27,32,37–39,41). Others consider as indicative of MTS of thyroid origin values in Tg-FNA higher than the maximum value found in reactive lymphadenitis24.
Despite the probable existence of a matrix effect in some studies, the most advanced immunoassays for serum thyroglobulin do not appear to be affected by this interference. For this reason, Tg-FNA levels in more recent studies are similar regardless of the use of saline or thyroglobulin-free solution for needle washing27,45. The absence of interference in these studies is also demonstrated by values in Tg-FNA from lymph nodes with no MTS from thyroid cancer lower than the functional sensitivity limit for thyroglobulin defined in blood36,45. The functional sensitivity limit for serum thyroglobulin is then used as a cut-off value in some studies35,36.
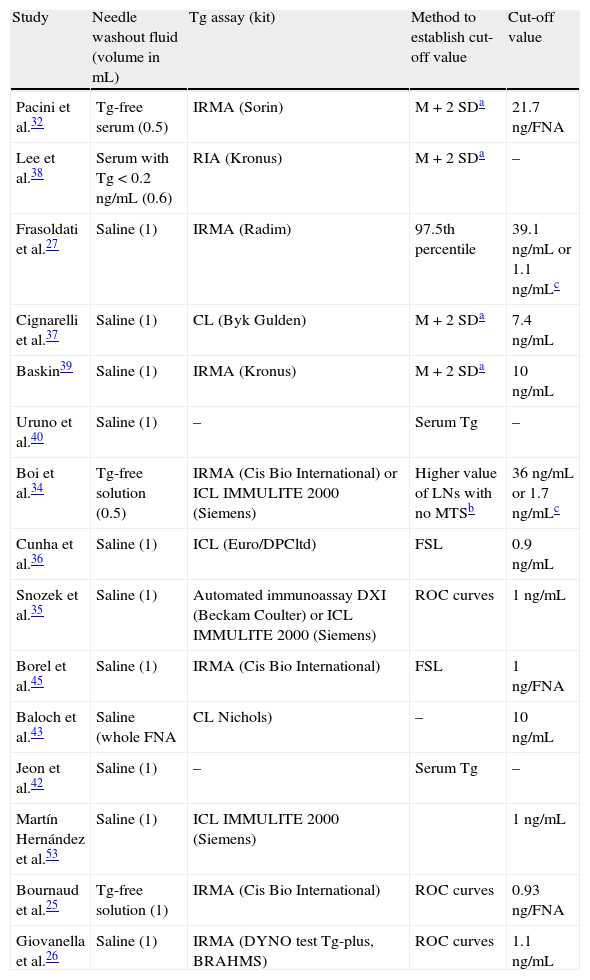
The thyroglobulin threshold value in Tg-FNA which is possibly the most accurate comes from the most recent studies, where each center, depending on the immunoassay and the washing method used, establishes the thyroglobulin value that provides the highest sensitivity and specificity through receiver operating curves (ROC curves) based on the Tg-FNA values of histologically confirmed malignant or benign lesions25,26,41. Table 3 lists different studies on Tg-FNA, stating the method used and the cut-off value of thyroglobulin considered as positive in each of them.
Summary of some reported Tg-FNA methods.
| Study | Needle washout fluid (volume in mL) | Tg assay (kit) | Method to establish cut-off value | Cut-off value |
| Pacini et al.32 | Tg-free serum (0.5) | IRMA (Sorin) | M + 2 SDa | 21.7ng/FNA |
| Lee et al.38 | Serum with Tg < 0.2ng/mL (0.6) | RIA (Kronus) | M + 2 SDa | – |
| Frasoldati et al.27 | Saline (1) | IRMA (Radim) | 97.5th percentile | 39.1ng/mL or 1.1ng/mLc |
| Cignarelli et al.37 | Saline (1) | CL (Byk Gulden) | M + 2 SDa | 7.4ng/mL |
| Baskin39 | Saline (1) | IRMA (Kronus) | M + 2 SDa | 10ng/mL |
| Uruno et al.40 | Saline (1) | – | Serum Tg | – |
| Boi et al.34 | Tg-free solution (0.5) | IRMA (Cis Bio International) or ICL IMMULITE 2000 (Siemens) | Higher value of LNs with no MTSb | 36ng/mL or 1.7ng/mLc |
| Cunha et al.36 | Saline (1) | ICL (Euro/DPCltd) | FSL | 0.9ng/mL |
| Snozek et al.35 | Saline (1) | Automated immunoassay DXI (Beckam Coulter) or ICL IMMULITE 2000 (Siemens) | ROC curves | 1ng/mL |
| Borel et al.45 | Saline (1) | IRMA (Cis Bio International) | FSL | 1ng/FNA |
| Baloch et al.43 | Saline (whole FNA | CL Nichols) | – | 10ng/mL |
| Jeon et al.42 | Saline (1) | – | Serum Tg | – |
| Martín Hernández et al.53 | Saline (1) | ICL IMMULITE 2000 (Siemens) | 1ng/mL | |
| Bournaud et al.25 | Tg-free solution (1) | IRMA (Cis Bio International) | ROC curves | 0.93ng/FNA |
| Giovanella et al.26 | Saline (1) | IRMA (DYNO test Tg-plus, BRAHMS) | ROC curves | 1.1ng/mL |
ICL: immunochemiluminescence; IRMA: immunoradiometric; FSL: functional sensitivity limit; CL: chemiluminescence; RIA: radioimmunoassay; Tg: thyroglobulin.
Tg-FNA possesses another additional advantage. It does not only facilitate localization diagnosis in patients with persistent disease suggested by high serum thyroglobulin levels, but also in patients with positive serum anti-thyroglobulin Ab. In our series, Tg-FNA was able to confirm the presence of nodal MTS in a patient with undetectable serum thyroglobulin and positive serum Ab based on a clearly positive value (221ng/mL), possibly due to the lack of interference of Ab, which were undetectable in needle washout fluid. In the Boi et al. study34 in six patients monitored for thyroid cancer and positive serum Ab, Tg-FNA also gave a positive result in all nodal MTS from PTC. As occurred in our patient, no Ab were identified in this study in needle washout fluid from four out of six adenopathies, nor in any of the 17 adenopathies of the Martín Hernández et al.53 study, conducted on patients monitored for PTC with positive serum Ab. In the two nodal MTS with positive anti-thyroglobulin Ab in needle washout fluid, Boi et al.34 attributed the positive Tg-FNA to massive local thyroglobulin secretion in the lymph node saturating the Ab binding sites.
ConclusionTg-FNA is an easy to perform, rapid, and inexpensive procedure whose information, added to the information provided by cyto-FNA, improves the diagnostic yield of FNA in patients with DTC. The reported advantages warrant the recommendation of Tg-FNA for the identification of MTS or its recurrence by the various consensuses and guidelines5,6, although standardization of the procedure and the availability of reference cut-off values in Tg-FNA depending on the immunoassay used at each center is desirable.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Familiar Casado C, Antón Bravo T, Moraga Guerrero I, Ramos Carrasco A, García García C, Villanueva Curto S. Utilidad de la tiroglobulina en lavado de aguja del aspirado de 16 lesiones cervicales en pacientes con cáncer de tiroides. Endocrinol Nutr. 2013;60:495–503.