It is a usual practice to discontinue thyroxine treatment for four weeks before 131I ablation. Symptoms of hypothyroidism usually occur during this time. Use of rhTSH is a helpful alternative in some cases, but problems of availability of this agent during 2012 will limit its use.
Patients and methodsPlasma TSH and FT4 levels were measured on days 7, 14, 21, and 28 after total thyroidectomy (12 patients) or discontinuation of thyroxine treatment (20 patients). A Mann–Whitney U-test was used to compare quantitative variables, and a Chi-square test was used for nominal variables.
ResultsOn day 14, TSH levels were 30μIU/mL of higher in 71% of patients (66% in the thyroidectomy group and 75% in the group discontinued thyroxine treatment). On day 21, almost all patients from both groups (91% in the thyroidectomy group and 100% in the group discontinued thyroxine treatment) had TSH levels of 30μIU/mL or higher. On day 14, most patients in both groups had FT4 levels below the normal range.
ConclusionsDiscontinuation of thyroxine treatment for four weeks is not required. Fourteen days is an adequate time in most patients, and 21 days are sufficient in virtually all patients.
Es frecuente que la suspensión de aporte de tiroxina como preparación para un rastreo-ablación se prolongue durante 4 semanas, en las que es habitual la aparición de clínica de hipotiroidismo. Una alternativa útil en algunos casos es utilizar TSHhr, pero sus problemas de disponibilidad durante el año 2012 limitarán su uso.
Pacientes y métodosSe realizó un análisis de la concentración de TSH y T4 libre en los días 7, 14, 21 y 28 desde el momento de la realización de una tiroidectomía total (12 pacientes) o desde la suspensión del tratamiento con tiroxina (20 pacientes). Se usó el test de Mann Wittney para analizar las comparaciones de variables cuantitativas y el Chi-cuadrado para las nominales.
ResultadosEn el día 14, la concentración plasmática de TSH fue igual o superior a 30μUI/mL en el 71% de los pacientes (66% de los del grupo de tiroidectomía y en el 75% de los del grupo de suspensión de tratamiento). En el día 21 esta condición la cumplía la práctica totalidad de los pacientes (el 91% en el grupo de tiroidectomía y el 100% en el grupo de suspensión de tratamiento). En el día 14, la mayoría de los pacientes de ambos grupos tenían un hipotiroidismo franco, con una concentración de T4L inferior a lo normal.
ConclusionesNo es necesario prolongar el tiempo sin aporte de tiroxina durante 4 semanas. Catorce días es suficiente en la mayoría de los pacientes, y 21 días lo es en prácticamente todos.
Radioiodine (131I) is the main adjuvant to surgery for the treatment of differentiated thyroid cancer. It is used for two purposes, scintigraphic localization of tissue able to take up the isotope and tissue ablation, irrespective of whether it is a healthy thyroid remnant or tumor tissue.1
Scintigraphy is performed 48–72h after the administration of 2–5mCi of 131I. For ablation, 30–150mCi of the isotope are used for healthy thyroid remnants,2,3 and 150–200mCi for residual tumor or metastatic tissue.4 Ablation doses of 131I make it possible to perform whole body scintigraphy 2–8 days later, and identify uptake sites not visible with lower doses in up to 20% of cases.5,6 This scintigraphy–ablation–scintigraphy sequence (generically called scan-ablation) may be repeated every 6–12 months if the persistence of tissue taking up isotope is shown despite prior ablations, and recent recommendations suggest that the first scintigraphy should only be performed in patients in whom the actual extent of thyroidectomy is not known from the surgical or ultrasound reports.7
The efficacy of 131I as a tracer and ablation agent depends on four factors: tumor histology, tissue location, dosage of 131I administered, and patient preparation.
Tumor histology: 131I is only useful in well-differentiated thyroid tumors of a follicular origin (which excludes its use in medullary, anaplastic carcinoma and thyroid lymphoma). Papillary and follicular carcinomas have a similar capacity for taking up 131I,8 although certain subtypes such as oxiphilic follicular carcinoma (commonly called Hürthle cell carcinoma), tall cell papillary carcinoma, and poorly differentiated carcinoma show a less avid uptake.
Tissue location: healthy thyroid tissue remaining after thyroidectomy takes up the tracer more readily than metastases.9 Lung metastases take up 131I more strongly than bone metastases.10,11
Dose of 131I: as noted above, the amount of 131I required for a scan is significantly higher than the amount needed for a scintigraphy, and the dose of 131I required for ablation is different depending on whether the involved tissue is a healthy thyroid remnant12 or tumor tissue.
Patient preparation: a low-iodine diet and high plasma TSH levels promote isotope uptake. Several studies suggest that optimum 131I levels in tissue are achieved with plasma TSH levels of 30mIU/L or higher.13
There are two equally effective ways14 of achieving an adequate increase in TSH level, the discontinuation of thyroxine treatment (which causes endogenous TSH elevation) and the administration of recombinant human TSH (rhTSH). Although the latter has the advantage that it prevents the clinical signs associated with hypothyroidism, it has not fully replaced the discontinuation of thyroxine treatment for two reasons:
- 1.
Although, several studies suggest that the short-term course of patients is independent of the approach used,15,16 few long-term studies are available.17 For this reason, the discontinuation of thyroxine treatment is recommended as a preparation for scan-ablation in high-risk patients (those with residual tumor after thyroidectomy and those with metastases).1
- 2.
Price of rhTSH: the dose required for TSH to reach an adequate concentration costs 807.29 €.
No agreement exists as to the time during which thyroxine treatment should be discontinued in order to reach the required TSH level. This is a relevant issue because the resulting hypothyroidism will be more marked (analytically and clinically) the longer the time. Patients are usually discontinued treatment for 4 weeks,19 although some publications suggest that shorter treatment discontinuations are equally effective.20,21
This study analyzes change over time in plasma TSH and FT4 levels in two patient groups scheduled to undergo scan-ablation. One of the groups consisted of patients seen after total thyroidectomy, and the other of patients undergoing thyroidectomy on thyroxine replacement therapy, which was discontinued as a prior step to performing the above test. We postulated that a plasma TSH level of 30mU/L would be reached before four weeks had elapsed from thyroidectomy or the discontinuation of thyroxine treatment.
Patients and methodsA consecutive sampling of all patients scheduled for scan-ablation at our hospital was performed for six months. The resulting sample consisted of 12 patients who received the isotope just after surgery (this was called the thyroidectomy group) and 20 patients undergoing thyroidectomy in whom thyroxine replacement therapy was discontinued before the test (treatment discontinuation group). All thyroidectomies were performed by a single surgeon widely experienced in thyroid surgery.
Our hospital protocol was followed. This provides for at least 28 days on a low-iodine diet from the time of total thyroidectomy or from discontinuation of thyroxine treatment to scan-ablation.
Plasma TSH and FT4 levels were tested in all patients 7, 14, 21, and 28 days after thyroidectomy or thyroxine discontinuation. Plasma TSH levels were measured using the Cobas 6000® chemiluminescent immunometric method (Roche Diagnostics. Mannheim) with interassay coefficients of variation of 1.90%, 2.33%, and 2.69% at concentrations of 0.48, 6.08, and 34.91μIU/mL. FT4 levels were measured using the Cobas 6000® chemiluminescent competitive analog immunoassay (Roche Diagnostics. Mannheim) with interassay coefficients of variation of 1.82%, 1.81%, and 2.64% at concentrations of 1.06, 2.71, and 6.59ng/dL.
Data are given as mean±standard deviation. Measurements of quantitative variables were compared between the groups using a Mann–Whitney test, and nominal variables were compared using a Chi-square test.
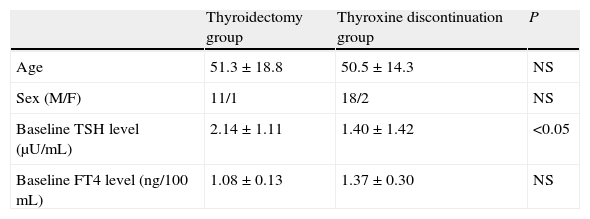
ResultsTable 1 shows the clinical characteristics of the study patients. Three patients were male (one in the thyroidectomy group and two in the treatment discontinuation group). At study entry, plasma TSH levels were lower (p<0.05) and plasma FT4 levels were higher (not significant) in the thyroxine discontinuation group as compared to the thyroidectomy group.
Baseline characteristics of the two patient groups undergoing ablation with 131I.
| Thyroidectomy group | Thyroxine discontinuation group | P | |
| Age | 51.3±18.8 | 50.5±14.3 | NS |
| Sex (M/F) | 11/1 | 18/2 | NS |
| Baseline TSH level (μU/mL) | 2.14±1.11 | 1.40±1.42 | <0.05 |
| Baseline FT4 level (ng/100mL) | 1.08±0.13 | 1.37±0.30 | NS |
Sex figures are absolute values. Mean±standard deviation for all other variables.
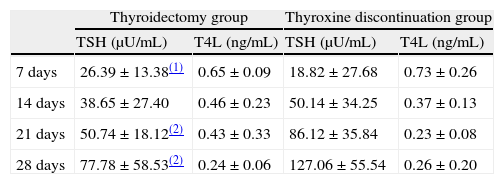
Mean plasma TSH levels at 7, 14, 21, and 28 days without thyroxine administration were 20.78, 46.06, 75.23, and 112.28μIU/mL, respectively, in the whole sample tested. Table 2 shows changes over time in each of the two groups of our sample. The plasma TSH level at 7 days was significantly higher (p<0.05) in the thyroidectomy versus the treatment discontinuation group. At 14, 21, and 28 days, plasma TSH levels were higher in the treatment discontinuation group (with differences at 21 and 28 days being significant at p<0.01).
Change over time in plasma TSH and FT4 levels (mean±standard deviation) in both groups of patients undergoing 131I ablation.
| Thyroidectomy group | Thyroxine discontinuation group | |||
| TSH (μU/mL) | T4L (ng/mL) | TSH (μU/mL) | T4L (ng/mL) | |
| 7 days | 26.39±13.38(1) | 0.65±0.09 | 18.82±27.68 | 0.73±0.26 |
| 14 days | 38.65±27.40 | 0.46±0.23 | 50.14±34.25 | 0.37±0.13 |
| 21 days | 50.74±18.12(2) | 0.43±0.33 | 86.12±35.84 | 0.23±0.08 |
| 28 days | 77.78±58.53(2) | 0.24±0.06 | 127.06±55.54 | 0.26±0.20 |
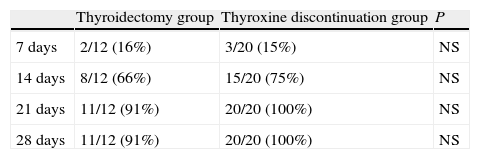
Table 3 shows the cumulative number and percentage of patients achieving plasma TSH levels of 30μIU/mL of higher. Fourteen days after thyroxine discontinuation, 66% of patients in the thyroidectomy group and 75% of those in the treatment discontinuation group had reached that level. At 21 days, 91% and 100% of patients in the thyroidectomy and treatment discontinuation groups, respectively, had met that condition. A female patient in the thyroidectomy group did not achieve the level of 30μIU/mL, even at 28 days.
Cumulative number (and percentage) of patients achieving TSH levels higher than 30μU/mL at 7, 14, 21, and 28 days in the thyroidectomy and thyroxine discontinuation patient groups.
| Thyroidectomy group | Thyroxine discontinuation group | P | |
| 7 days | 2/12 (16%) | 3/20 (15%) | NS |
| 14 days | 8/12 (66%) | 15/20 (75%) | NS |
| 21 days | 11/12 (91%) | 20/20 (100%) | NS |
| 28 days | 11/12 (91%) | 20/20 (100%) | NS |
NS: not significant.
Mean plasma FT4 levels at 7, 14, 21, and 28 days without thyroxine administration were 0.71, 0.40, 0.29, and 0.25ng/mL, respectively, in the whole sample tested. Table 2 shows changes over time in FT4 levels in each of the two study groups. Differences in FT4 levels between the two groups did not reach statistical significance.
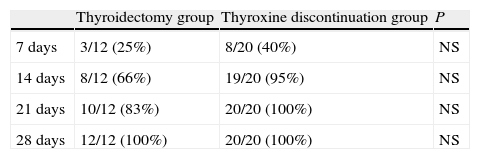
Table 4 shows the cumulative number and percentage of patients who achieved plasma FT4 levels of 0.7ng/100mL or less (the lower limit of normal at our laboratory). At 14 days, 66% of patients in the thyroidectomy group and 95% of those in the thyroxine discontinuation group had FT4 levels within those limits. At 28 days, all patients met this condition.
Cumulative number (and percentage) of patients achieving FT4 levels less than 0.7ng/100mL at 7, 14, 21, and 28 days in the thyroidectomy and thyroxine discontinuation patient groups.
| Thyroidectomy group | Thyroxine discontinuation group | P | |
| 7 days | 3/12 (25%) | 8/20 (40%) | NS |
| 14 days | 8/12 (66%) | 19/20 (95%) | NS |
| 21 days | 10/12 (83%) | 20/20 (100%) | NS |
| 28 days | 12/12 (100%) | 20/20 (100%) | NS |
NS: not significant.
The incidence of differentiated thyroid carcinoma is higher in females as compared to males in a 4:1 ratio.22 In our sample, a significantly different 10:1 ratio was found. Although such a disproportion may appear to bias the results achieved, we think that this is unlikely because no significant differences have been reported between males and females in thyroxine catabolism and the response of thyrotropic cells to decreased FT4 levels.
Plasma TSH levels below the lower limit of normal improve the post-thyroidectomy prognosis of patients with thyroid carcinoma of a follicular origin.23 This explains the lower TSH level and the higher FT4 level in the group of patients who discontinued thyroxine treatment. We attribute the lack of statistical significance of the difference in FT4 to sample size.
According to prior publication data,24 we expected plasma TSH levels measured in the different tests to be lower in the thyroxine discontinuation group. However, the differences between the groups were already significant at 14 days, and TSH levels at 21 and 28 days were significantly higher in the thyroxine discontinuation group as compared to the thyroidectomy group. This was a surprising evolution for which we have no explanation.
Overall, more than 71% of patients had TSH levels of 30μIU/mL or higher 14 days after surgery or thyroxine treatment discontinuation, and all patients but one met this condition at 21 days. Our results suggest that the time period without thyroxine administration may substantially be decreased from the usual 28 days to 21 days, and that scan-ablation could be performed in 14 days in a great majority of patients. We recommend that this be done.
A female patient did not reach a TSH level of 30μIU/mL even at 28 days, despite the marked decrease in T4 level (unpublished data). In this patient, plasma thyroglobulin level on the day of the scan was 6.5mg/mL, antithyroglobulin antibodies were undetectable, and the scan showed the presence of a small remnant taking up tracer in the thyroid bed. A study of pituitary function and MRI of the sella turcica showed no functional or anatomical changes. An extension of time off treatment to 28 days does not appear to be the optimum approach even in patients such as this one.
A reduction in the time off treatment is not a clinically minor issue: in tests performed at 14 days, 84% of patients had plasma FT4 levels under the lower limit of normal, which makes it likely that symptoms of hypothyroidism will occur and that they will probably increase over time.
In years gone by, thyroxine treatment was often discontinued six weeks before scan-ablation.25,26 While the currently required off-treatment time is usually four weeks, patients commonly experience marked analytical and clinical hypothyroidism. The data reported in this paper suggest that optimum TSH levels for performing scan-ablation may be achieved with a waiting time substantially shorter than usual: 14 days are sufficient in most patients, and 21 days are adequate in virtually all of them, including both those with recent thyroidectomy and those in whom thyroxine has been discontinued. Clinical signs of hypothyroidism may be expected to occur in most patients from 14 days.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Luna R, et al. ¿Es necesario suspender durante 4 semanas el tratamiento con tiroxina antes de la realización de un rastreo-ablación? Endocrinol Nutr. 2012;59:227–31.