To evaluate dietary calcium intake (DCI) and vitamin D serum concentrations in patients with prostate cancer.
MethodsWe conducted a cross-sectional study including 91 subjects with prostate cancer. We determined DCI by a questionnaire, 25 OH vitamin D levels and bone mineral density (BMD) by dual energy X-ray absorptiometry (DXA).
ResultsAccording to current guidelines (1000mg/day), calcium intake was low in patients with prostate cancer (394±201mg/day). Twenty-two percent (20) of patients had adequate levels of vitamin D, whereas 29.7% (27) of patients were vitamin D deficient and 48.3% (44) were classified as vitamin D insufficiency. Vitamin D levels were not different in patients with or without androgen-deprivation therapy. There were no correlation between DCI, 25 OH vitamin and BMD.
ConclusionsIn summary, in our group of prostate cancer patients DCI was low and vitamin D deficiency is highly prevalent. Although this is a common condition in other populations, in this group of patients especially prone to osteoporosis could have more relevance. Additional research is needed to establish the consequences of low calcium intake and vitamin D deficiency in prostate cancer patients.
Evaluar la ingesta dietética de calcio (DCI) y las concentraciones de vitamina D en pacientes con cáncer de próstata.
MétodosEstudio transversal que incluyó a 91 pacientes con cáncer de próstata. Se determinó la ingesta dietética de calcio a través de un cuestionario, las concentraciones séricas de 25 OH vitamina D y la densidad mineral ósea por dual energy X-ray absorptiometry (DXA).
ResultadosSegún las recomendaciones actuales (1000mg/día) la ingesta dietética de calcio fue baja en pacientes con cáncer de próstata (394±201mg/día). 22% (20) de los pacientes presentaron concentraciones adecuadas de vitamina D, mientras que el 29,7% (27) fueron deficitarios y el 48.3% (44) se clasificó como insuficiencia de vitamina D. Las concentraciones de vitamina D no mostraron diferencias de acuerdo al tratamiento o no con terapia de deprivación androgénica. No existió correlación entre la ingesta diaria de calcio, las concentraciones de vitamina D o la densidad mineral ósea.
ConclusionesEn nuestro grupo de pacientes con cáncer de próstata la DCI es baja y el déficit de vitamina D es muy prevalente. Aunque esta es una situación frecuente en otros grupos de población, en este grupo de pacientes especialmente predispuestos al desarrollo de osteoporosis podría tener una mayor relevancia. Son necesarios estudios adicionales para establecer las consecuencias de estas dos situaciones en pacientes con cáncer de próstata.
Vitamin D deficiency remains a common condition. Serum vitamin D is not only a predictor of bone health but is also an independent predictor of risk for cancer1,2 and other chronic diseases.3 There are several data supporting the relationship between vitamin D deficiency and cancer prognosis4,5 and numerous studies suggest that vitamin D deficiency is associated with an increased risk of medical complications to which patients with cancer are predisposed, i.e. infection, falls and immune disfunction.6–8
The effect of vitamin D in cancer processes has been demonstrated in experimental studies9 and may influence cancer incidence through mechanisms affecting cancer development and progression.4 Moreover, vitamin D deficiency has been proposed to be a risk factor for prostate cancer10 although increased risk of aggressive disease with higher circulating 25 OH vitamin D concentrations11,12 or no association13,14 had also been reported.
The mainstay of treatment for men with metastatic disease is androgen-deprivation therapy (ADT). Currently, ADT is increasingly prescribed to men with no evidence of metastatic disease. Osteoporosis is the main complication of ADT, and the rate of osteoporosis is directly related to ADT duration.15 Numerous publications indicate the importance of calcium and vitamin D intake as risk factors for developing osteoporosis in men.16 In this population, especially prone to the development of osteoporosis, adequate calcium and vitamin D intake may be especially relevant. There are scarce data about calcium intake in prostate cancer. Previous studies had reported a calcium intake below the NIH recommendation, 1000mg/day, in 90% of prostate cancer patients,17,18 and calcium intake was an independent factor of osteoporosis.17 There are also few studies reporting the frequency of vitamin D deficiency among patients with prostate cancer. There have been reported a 17% of vitamin D levels below 15ng/ml17 and 75–80% of vitamin D deficiency (<30ng/ml) among patients with either clinically localized or recurrent prostate cancer.19,20
Our aim was to evaluate calcium intake and vitamin D levels in patients with prostate cancer, and to determine the relationship between dietary calcium intake (DCI), 25 OH vitamin D and bone mineral density (BMD).
Material and methodsOur cross-sectional study included 100 subjects with a diagnosis of prostate cancer who have been referred to the Bone Metabolic Unit of Hospital Universitario San Cecilio in Granada, Spain (latitude 37.11 N) from Urology Department. From November 2006 to January 2009, 100 consecutive patients were approached. Only 91 met eligibility criteria and were included in the study. Inclusion criteria were the following: males with prostate cancer, older than 50 years, ambulatory and that provided written informed consent. All were Caucasians, had normal values of serum calcium and phosphorus, and had neither renal, hepatic, gastrointestinal or thyroid diseases nor other secondary causes for low BMD except secondary hypogonadism. None of them had been treated with calcium supplements, vitamin D preparations, antiresorptive therapy, thiazides, steroids, or other medications that might affect bone mass. After that, we considered two groups: group 1 (n=42): patients who have never received hormonal therapy; group 2 (n=49): patients receiving androgen deprivation therapy (ADT) with GnRH agonists: triptorelin, goserelin, leuprorelin or antiandrogens: bicalutamide, flutamide. The mean duration of ADT was 27 months (range 3–96 months).
Dietary calcium intake was estimated using a standard questionnaire. The Short Calcium Questionnaire (SCQ) is a 1-page, 25-item FFQ developed from the Calcium Questionnaire, reflecting major dietary sources of calcium.21 For each food item listed, subjects write in the number of servings eaten in a typical week. A reference serving size is included. SCQ has demonstrated to perform well at estimating dietary calcium intake compared to food records and compared to other questionnaires.21
The study was conducted with the approval of the ethical committee of the San Cecilio University Hospital and conform the relevant ethical guidelines for human and animal research. Written informed consent was obtained from all subjects.
Bone mineral density measurementsDXA (Dual Energy X-Ray Absorptiometry) was performed in all patients at lumbar spine (L2–L4) and femoral regions (FN: femoral neck and TH: total hip). The BMD was determined by Dual Energy X-Ray Absorptiometry (DXA; Hologic QDR 4500, Whatman, MA; variation coefficient <1%). We used the World Health Organization criteria for osteopenia and osteoporosis.
Biochemical measurementsSamples of venous blood were taken in the morning after fasting overnight. Samples were centrifuged immediately after collection at 4000×g for 8min. Biochemical parameters were measured by standard biochemical methods.
25 OH vitamin D was measured using a competitive radioimmunoassay (RIA) (Diasorin, Stillwater, MN, USA). The RIA is a combined measure of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 which have similar biological activities. The total analytical coefficient of variation was 10%, and interassay CV was 8.6%. Vitamin D status was defined as follows: vitamin D deficiency was defined as a 25 OH vitamin D level <15ng/ml, and insufficiency 15–30ng/ml. Patients with 25 OH vitamin D levels above 31ng/ml were considered as vitamin D sufficiency.22
PTH-i was measured by ELISA (Roche Diagnostics SL, Barcelona, Spain). The normal values are 15–65pg/ml. The analytical and inter assay coefficient of variation were 3%.
Other parametersHeight and weight were measured at baseline according to standard procedures. Weight was measured to the nearest 100g using digital electronic scales. Height was measured to the nearest 1mm using a stadiometer and a metal anthropometric tape, respectively. Body mass index (BMI) in Kg/m2 was calculated as weight divided by the square of height in meters.
Statistical analysisData were recorded and analyzed with SPSS version 15.0 (SPSS Inc., Chicago, IL). Descriptive statistics, including means, frequencies and percentages, were used to describe the study population and look at differences between groups. Data were expressed as mean±standard deviation (SD). A p value <0.05 was considered to be significant. The normal distribution of variables was determined by Kolmogorov–Smirnov test.
Mean values in groups were compared by parametric statistics (Student's t-test, ANOVA) or nonparametric statistics (Mann–Whitney, Kruskal–Wallis) depending on the distribution of the variable of interest. Correlations between the variables were evaluated using Pearson's simple and partial correlation coefficient. The association among qualitative variables was realized by means of the Chi-square test or Fisher's exact test in case the conditions of the first one were not fulfilled.
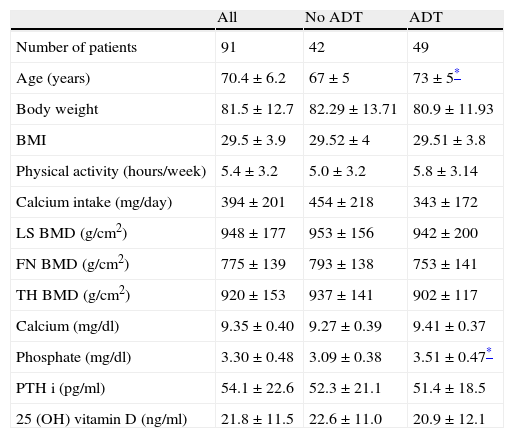
ResultsThe baseline characteristics of study subjects are shown in Table 1. Patients in ADT group were older and had higher serum phosphate levels than no-ADT patients. There were no differences in other baseline characteristics according to the presence of bone metastases or ADT treatment.
Baseline characteristics of study subjects. Data expressed as n, n (%), or mean±SD.
| All | No ADT | ADT | |
| Number of patients | 91 | 42 | 49 |
| Age (years) | 70.4±6.2 | 67±5 | 73±5* |
| Body weight | 81.5±12.7 | 82.29±13.71 | 80.9±11.93 |
| BMI | 29.5±3.9 | 29.52±4 | 29.51±3.8 |
| Physical activity (hours/week) | 5.4±3.2 | 5.0±3.2 | 5.8±3.14 |
| Calcium intake (mg/day) | 394±201 | 454±218 | 343±172 |
| LS BMD (g/cm2) | 948±177 | 953±156 | 942±200 |
| FN BMD (g/cm2) | 775±139 | 793±138 | 753±141 |
| TH BMD (g/cm2) | 920±153 | 937±141 | 902±117 |
| Calcium (mg/dl) | 9.35±0.40 | 9.27±0.39 | 9.41±0.37 |
| Phosphate (mg/dl) | 3.30±0.48 | 3.09±0.38 | 3.51±0.47* |
| PTH i (pg/ml) | 54.1±22.6 | 52.3±21.1 | 51.4±18.5 |
| 25 (OH) vitamin D (ng/ml) | 21.8±11.5 | 22.6±11.0 | 20.9±12.1 |
BMD, bone mineral density; LS, lumbar spine; FN, femoral neck; TH, total hip; ADT, androgen-deprivation therapy; BMI, body mass index.
PTH and 25 OH vitamin D showed an inverse correlation, but it did not reached statistical significance (r −0.181, p=0.09). i-PTH levels and serum calcium were negatively correlated (r −0.210, p<0.05). There was no correlation between i-PTH or 25 OH vitamin D and other analyzed variables (age, weight, renal function, BMI, BMD, calcium intake, prostatic specific antigen).
DCI was low in prostate cancer patients (394±201mg) according to current recommendations. Only two patients (2.2%) had a DCI of 1000mg/day. There was no difference in calcium intake according to ADT treatment (ADT 343±172mg/day vs. no ADT 454±218mg/day, p=0.08) (Table 1). Medium vitamin D levels showed no differences in patients with or without ADT (ADT 20.92±12.1ng/dl vs. no ADT 22.58±10.96ng/dl, p=0.51) (Table 1). There was no correlation between DCI, 25 OH vitamin D levels and BMD.
Vitamin D deficiency and insufficiency were highly prevalent. In the entire cohort, only 22% (20 patients) of patients had adequate levels of vitamin D, whereas 29.7% (27) of patients were vitamin D deficient and 48.3% (44) were classified as vitamin D insufficiency.
DiscussionIn our study a high proportion of prostate cancer patients (98%) had a DCI below the NIH recommendations (more than 1000mg/day). Two previous studies in prostate cancer patients had showed a high proportion of patients (about 90%) with a low DCI,17,18 but our data show an insufficient DCI in almost all patients. In this population, especially prone to the development of osteoporosis because of age and ADT treatment, dietary counselling and probably treatment with calcium supplements are warranted.
In our study, vitamin D deficiency was highly prevalent in patients with prostate cancer. Our data showed a higher percentage of patients with vitamin D deficiency and insufficiency than previous reports in prostate cancer patients.18–20 There have been proposed many factors contributing to low vitamin D levels: the infrequent use of vitamin D as a part of treatment, the use of inadequate doses as a result of the conservative level of vitamin D supplementation usually recommended, the limited availability of vitamin D in foods, and the use of sun screens and limited outside activity. The known consequences of vitamin D deficiency in prostate cancer, i.e. increased risk of medical complications,6–8 a possible effect in cancer progression and prognosis,4,5 and the importance of vitamin D in the development of osteoporosis16 confirms the relevancy of this condition.
We did not found differences in vitamin D deficiency levels according to ADT. Our results are in agreement with previous reports evaluating vitamin D levels in patients with prostate cancer that showed similar levels of vitamin D deficiency and insufficiency independently of stage of disease.19,20 However, the cross-sectional design and the low number of patients with bone metastasis do not allow to establish definite conclusions.
The determination of serum 25 OH vitamin D levels and the use of vitamin D supplements in patients with prostate cancer is low despite of the likehood that vitamin D deficiency would predispose to osteoporosis and may impair bone quality in this group of patients predisposed to bone loss. The present study shows a high frequency of low vitamin D levels among patients with prostate cancer that warrants attention to vitamin D levels determination and careful repletion in this group of patients.
This study has some limitations. The cross-sectional design does not allow to determinate a relationship between serum vitamin D levels and the progression of disease. Strengths of the study are the well characterized cohort of prostate cancer patients, the evaluation of DCI by a validate questionnaire, the determination of 25 OH vitamin D levels in all patients, and the evaluation of BMD in all patients by DXA.
In summary, in prostate cancer patients dietary calcium intake is low according to current recommendations and vitamin D deficiency is highly prevalent. Additional research is needed to establish the consequences of low calcium intake and vitamin D deficiency in prostate cancer patients. Dietary counselling and adequate treatment with calcium and vitamin D supplements could improve bone health in this group of patients.
Conflict of interestThe authors declare that they have no conflict of interest.