Transsphenoidal surgery (TSS) is the treatment of choice for Cushing's disease (CD). However, the best treatment option when hypercortisolism persists or recurs remains unknown. The aim of this study was to analyze the short and long-term outcome of repeat TSS in this situation and to search for response predictors.
Patients and methodsData from 26 patients with persistent (n=11) or recurrent (n=15) hypercortisolism who underwent repeat surgery by a single neurosurgeon between 1982 and 2009 were retrospectively analyzed. Remission was defined as normalization of urinary free cortisol (UFC) levels, and recurrence as presence of elevated UFC levels after having achieved remission. The following potential outcome predictors were analyzed: adrenal status (persistence or recurrence) after initial TSS, tumor identification in imaging tests, degree of hypercortisolism before repeat TSS, same/different surgeon in both TSS, and time to repeat surgery.
ResultsImmediate postoperative remission was achieved in 12 patients (46.2%). Five of the 10 patients with available follow-up data relapsed after surgery (median time to recurrence, 13 months). New hormone deficiencies were seen in seven patients (37%), and two patients had cerebrospinal fluid leakage. No other major complications occurred. None of the preoperative factors analyzed was predictive of surgical outcome.
ConclusionsWhen compared to initial surgery, repeat TSS for CD is associated to a lower remission rate and a higher risk of recurrence and complications. Further studies are needed to define outcome predictors.
La cirugía transesfenoidal (TE) es el tratamiento de elección en primera línea en la enfermedad de Cushing (EC). Sin embargo, se desconoce cuál es el tratamiento más adecuado cuando el hipercortisolismo persiste o recidiva. El objetivo del estudio es analizar el resultado a corto y largo plazo de la reintervención TE e identificar factores predictores de respuesta.
Pacientes y métodosSe revisaron retrospectivamente los datos de 26 pacientes con hipercortisolismo persistente (n=11) o recidivado (n=15) reintervenidos por un mismo cirujano entre 1982 y 2009. Se consideró remisión a la normalización del cortisol libre urinario (CLU) y recidiva a la presencia de CLU elevado después de una remisión. Como potenciales predictores de respuesta se analizaron los siguientes factores: función adrenal tras la cirugía inicial (persistencia o recidiva), visibilidad del tumor en las pruebas de imagen, grado de hipercortisolismo antes de la reintervención, mismo/diferente cirujano en ambas cirugías y tiempo hasta la reintervención.
ResultadosDoce pacientes remitieron inmediatamente tras la reintervención (46,2%). De los 10 con seguimiento a largo plazo recidivaron 5 (mediana de tiempo hasta la recidiva: 13 meses). Se indujeron nuevos déficits hormonales en 7 pacientes (37%) y fístula de líquido cefalorraquídeo en 2. No se observaron otras complicaciones. Ninguno de los factores estudiados se asoció con la respuesta.
ConclusionesComparada con la cirugía inicial, la reintervención TE en la EC se asocia con una menor tasa de remisión y un riesgo mayor de recidivas y complicaciones. Son necesarios más estudios para definir factores predictores de respuesta.
Transsphenoidal surgery (TSS) is the treatment of choice in Cushing's disease (CD) since it enables complete resection of the tumor while preserving the rest of the pituitary function with a low morbidity rate. This approach leads to immediate remission of hypercortisolism in 70–85% of the patients when carried out by an experienced neurosurgeon.1 However, about 10–25% of these patients will recur within the following 10 years.1–5
When hypercortisolism persists or recurs after initial TSS, several treatment options have to be considered, including repeated TSS, pituitary radiotherapy, medical treatment or bilateral adrenalectomy. Until now, few reports on the results of a repeated TSS in CD have been published, and even less have described the long term outcome, so little is known about recurrence rates.
At our institution the second line treatment, after initial TSS failure, is pituitary irradiation in around 70% of patients. A second TSS is considered when initial TSS has been performed by a non-experienced neurosurgeon or when a visible and accessible tumor remnant is located with magnetic resonance imaging (MRI).
The present study describes the results of repeated TSS at our hospital in patients with persistent or recurrent hypercortisolism.
Patients and methodsBetween July 1982 and April 2009, 26 patients with CD underwent repeated TSS by a single neurosurgeon (JG-U) due to the presence of persistent (11 patients) or recurrent (15 patients) hypercortisolism after initial TSS. Persistent hypercortisolism was considered when the patient had an increased urinary free cortisol (UFC) in the immediate postsurgical evaluation and recurrent hypercortisolism when an elevated UFC was detected following a period of adrenal insufficiency or normocortisolism.
All the patients had been diagnosed with CD before the first surgery based on the following features: absence of a circadian rhythm in serum cortisol, increased UFC excretion, elevated or inappropriately normal ACTH plasma levels, characteristic serum cortisol responses to the administration of dexamethasone (failure to suppress serum cortisol below 1.8mcg/dl at 8 am following a single dose of 1mg dexamethasone at 23h the night before (1mg DST); and decrease of UFC levels greater than 50% following a 2mg dexamethasone dose every 6h for 48h) and imaging of the sella turcica. Inferior petrosal sinus sampling was performed in 6 patients in whom prior tests were inconclusive.
Before repeated TSS, all patients had clinical features of hypercortisolism which was biochemically confirmed by elevated UFC measurement in every case. At this time, serum cortisol at 8 am (24 patients), serum cortisol at 11 pm (18 patients), ACTH plasma levels (17 patients) and response to the 1mg DST (18 patients) were also evaluated. In all cases, the results obtained supported the diagnosis. New imaging of the sella turcica with computed tomography (3 patients) or magnetic resonance (MRI) (23 patients) was obtained in every case. Imaging studies did not reveal any lesion in 12 patients; a microadenoma was identified in 10 and remaining tissue of a macroadenoma was evident in 4. Pituitary function, including IGF1 levels but not GH after stimulatory test, was evaluated before reoperation in 22 patients. It was normal in 21 while the other one had an isolated gonadotropin deficiency.
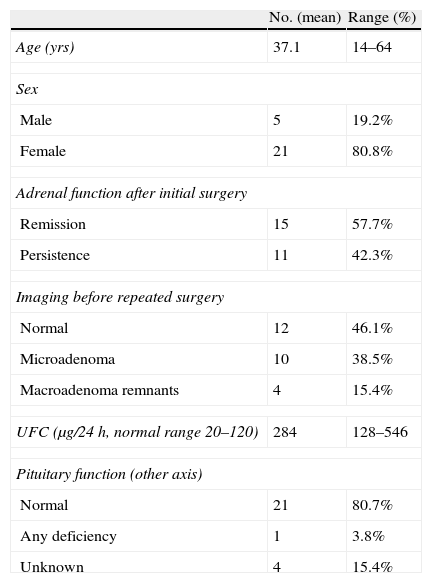
Initial surgery was performed at a different hospital by a different neurosurgeon in 12 patients who were referred to our institution for reoperation (one of them had a macroadenoma and was referred after two surgical failures to perform a third TSS; for the rest it was the second procedure). The median time interval between both procedures was 20 months (mean 48 months, range 2–295). The clinical and biochemical characteristics before the repeated surgery are summarized in Table 1.
Clinical and biochemical characteristics of 26 patients with persistent or recurrent Cushing's disease at the time of the repeated transsphenoidal surgery.
| No. (mean) | Range (%) | |
| Age (yrs) | 37.1 | 14–64 |
| Sex | ||
| Male | 5 | 19.2% |
| Female | 21 | 80.8% |
| Adrenal function after initial surgery | ||
| Remission | 15 | 57.7% |
| Persistence | 11 | 42.3% |
| Imaging before repeated surgery | ||
| Normal | 12 | 46.1% |
| Microadenoma | 10 | 38.5% |
| Macroadenoma remnants | 4 | 15.4% |
| UFC (μg/24h, normal range 20–120) | 284 | 128–546 |
| Pituitary function (other axis) | ||
| Normal | 21 | 80.7% |
| Any deficiency | 1 | 3.8% |
| Unknown | 4 | 15.4% |
UFC: urinary free cortisol.
A sublabial transsphenoidal microsurgical approach was used at the time of reexploration in every patient. On the day of the operation, a steroid cover of 300mg hydrocortisone was given, then tapered and discontinued 24h before any hormonal determination. All patients underwent an evaluation 8–12 days after surgery that included: 4 consecutive measurements of serum cortisol at 8h intervals starting at 8 am, a basal plasma ACTH level on 2 consecutive days and a UFC measurement. Patients were then classified into two groups. Group I included patients with remission of hypercortisolism, defined as adrenal insufficiency (mean serum cortisol <5μg/dl) or normal serum and UFC measurements; group II included patients with persistent hypercortisolism (UFC greater than 120μg/dl). After discharge, 7 patients were lost for follow up; long term follow up data were available for the remaining 19 patients.
Patients with postoperative adrenal insufficiency were discharged with hydrocortisone replacement treatment (30mg/day) and reevaluated every 3 months with a determination of serum cortisol obtained between 8 am and 9 am, 24h after the last hydrocortisone dose. Treatment was tapered and discontinued once serum cortisol levels were higher than 10μg/dl. Afterwards, the adrenal axis was assessed measuring UFC and serum cortisol at 8 am, 11 pm and after the 1mg DST. Subsequently, they underwent annual evaluation with the 1mg DST. Patients with normal serum cortisol and UFC levels after surgery were discharged without corticosteroid replacement therapy and evaluated 3 months after discharge. They were subsequently reevaluated every 6 months with the 1mg DST, determination of UFC and serum cortisol at 8 am and 11 pm. Serum tiroxine, TSH, IGF1, gonadotropins and testosterone or estradiol levels were measured 3 months after surgery.
Recurrence was diagnosed when patients met all of the following criteria: signs and symptoms of hypercortisolism, lack of cortisol suppression in the 1mg DST and UFC persistently high after a period of adrenal insufficiency or normocortisolism.
Hormone assaysPlasma ACTH was measured by radioimmunoassay (RIA) before 1989 (Immuno Nuclear Corporation, Stillwear, USA), by immunoradiometric assay (IRMA) until 2000 (Nichols Institute Diagnostics, San Juan Capistrano, USA) and thereafter by a chemiluminescent immunometric assay (CLIA) (Immulite 2000, Siemens Healthcare Diagnostics, Erlangen, Germany). Serum cortisol was measured by RIA until 1992 (ICN Biomedicals, Inc., Costa Mesa, USA; and Immunotech International, Marseille, France), by time-resolved fluorescence immunoassay (FIA) until 2000 (Delfia System, Wallac, Inc., Oy, Turku, Finland) and thereafter by CLIA (Immulite 2000, Siemens Healthcare Diagnostics, Erlangen, Germany; and ADVIA Centaur, Siemens Healthcare Diagnostics). UFC was measured in unextracted urine at low pH by RIA (Diagnostic Systems Laboratories, Inc., Los Angeles, USA; ICN Biomedicals, Inc.; and Immunotech International, Marseille, France).
Serum TSH was measured by IRMA until 2000 (Kodak Amerlite TSH-30 ultrasensitive assay; Amersham International, Buckinghamshire, United Kingdom) and thereafter by CLIA (Immulite 2000, Siemens Healthcare Diagnostics, Erlangen, Germany; and ADVIA Centaur, Siemens Healthcare Diagnostics). Serum free T4 was measured by RIA until 1998 (LIA-mat; Byk Sangtec Diagnostica GmbH, Frankfurt, Germany) and thereafter by CLIA (Architect i4000, Abbot Diagnostics; and ADVIA Centaur Siemens Healthcare Diagnostics). Plasma E2 and T, serum LH, FSH, and PRL were measured by FIA until 2000 (Delfia System, Wallac, Inc., Oy, Turku, Finland) and thereafter by CLIA (Immulite 2000, Siemens Healthcare Diagnostics, Erlangen, Germany; and ADVIA Centaur, Siemens Healthcare Diagnostics). Serum GH was measured by RIA until 2000 (Nichols Institute Diagnostics, San Juan Capistrano, USA until 1988; and Immunotech International, Marseille, France thereafter). Since 2000 GH was measured by CLIA (Immulite 2000, Siemens Healthcare Diagnostics, Erlangen, Germany). IGF1 was measured by RIA (Immunotech International, Marseille, France) until 2000 and thereafter by CLIA (Immulite 2000, Siemens Healthcare Diagnostics, Erlangen, Germany).
Statistical analysisThe Mann–Whitney test was used to compare continuous variables and χ2 test with Yates correction for categorical variables. For all analyses, an alpha level of 0.05 and two-tailed testing were used. All statistical analyses were performed using SPSS v14.0.
ResultsSurgical and pathological findingsTissue thought to be an adenoma was identified and selectively resected in 20 patients (complete macroscopic resection in 14 and incomplete in the other 6 due to extension or invasiveness of the tumor). In one patient a completely empty sella was found and no resection could be carried out and in the other 5 patients, in which the tumor could not be found, the surgeon removed two thirds of the pituitary gland.
The pathological study confirmed the presence of an adenoma in only 6 patients. In 5 of them (all of which had been selectively resected) ACTH immunohistochemical staining was positive, while it was negative in the other one (in which a subtotal hypophysectomy had been performed). The excised pituitary tissue was apparently normal in 8 patients and was not adequate for evaluation in the remaining 12.
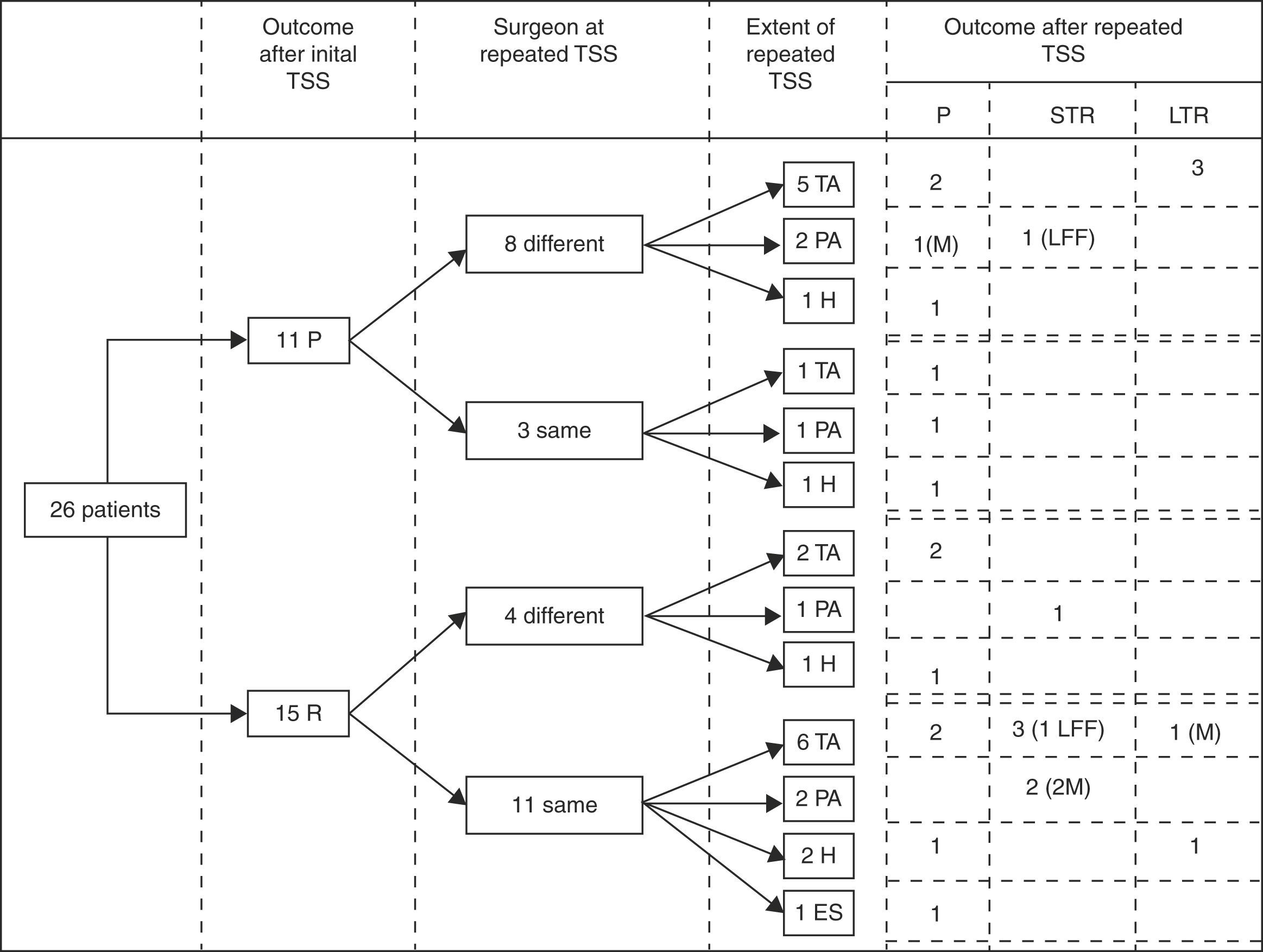
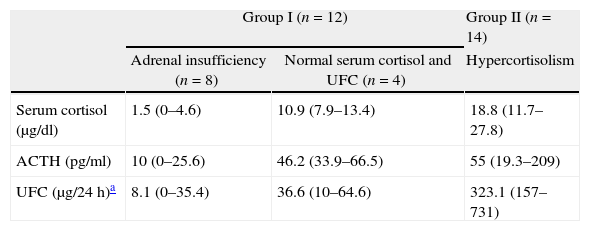
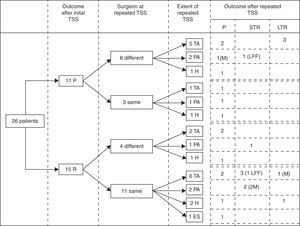
Immediate remission rateA treatment and outcome flow chart is shown in Fig. 1. After repeated TSS, 12 patients (46.2%; IC 95%: 25.6–66.7) were included in the remission group (Group I): 8 presented adrenal insufficiency and 4 attained normal serum cortisol and UFC levels (mean serum cortisol 4.6±4.9μg/dl, UFC 17.6±21.2μg/24h, ACTH 23.6±22.4pg/ml). The remaining 14 patients (53.8%) were included in the persistence group (Group II) (mean serum cortisol 18.8±8.1μg/dl, UFC 323.1±169.1μg/24h, ACTH 55±59.3pg/ml) (Table 2).
Treatment and outcome flow chart. TSS: Transsphenoidal surgery; P: Persistence; R: Remission; TA: Total adenomectomy; PA: Partial adenomectomy; H: Subtotal hypophysectomy; ES: Empty sella, no resection performed. STR: Short term remission (initial remission followed by recurrence), LTR: Long term remission; LFF: lost for follow up; M: macroadenoma.
Classification according to adrenal status after repeated transsphenoidal surgery.
| Group I (n=12) | Group II (n=14) | ||
| Adrenal insufficiency (n=8) | Normal serum cortisol and UFC (n=4) | Hypercortisolism | |
| Serum cortisol (μg/dl) | 1.5 (0–4.6) | 10.9 (7.9–13.4) | 18.8 (11.7–27.8) |
| ACTH (pg/ml) | 10 (0–25.6) | 46.2 (33.9–66.5) | 55 (19.3–209) |
| UFC (μg/24h)a | 8.1 (0–35.4) | 36.6 (10–64.6) | 323.1 (157–731) |
Data given in mean and range; UFC: urinary free cortisol.
When analyzing the results, considering the extent of the surgery, postoperative remission was achieved in 7 of the 14 patients (50%) with a complete macroscopic resection of the adenoma, in 4 of the 6 patients (66%) with an incomplete macroscopic resection of the adenoma and only in 1 of the 5 patients (20%) with a subtotal hypophysectomy.
ComplicationsNine patients had transient diabetes insipidus (DI) while no permanent DI occurred. Two of the 26 patients had postoperative cerebrospinal fluid leakage (CSF leak) that was successfully treated with an external lumbar drain. One of them was complicated with meningitis that resolved after antibiotic treatment. No other major complications were observed.
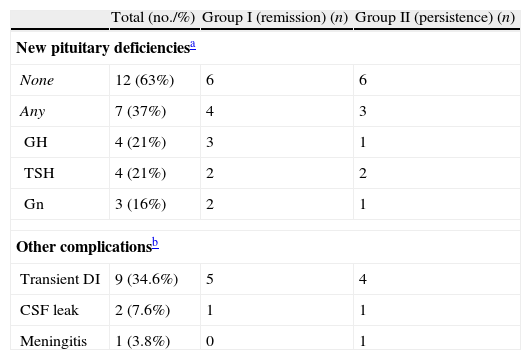
Pituitary function was evaluated 3 months after surgery in the 19 patients with follow up data. New pituitary deficiencies were observed in 7 patients (37%) but a complete hypopituitarism occurred in only one of them. Complications are summarized in Table 3.
Complications of repeated transsphenoidal surgery.
| Total (no./%) | Group I (remission) (n) | Group II (persistence) (n) | |
| New pituitary deficienciesa | |||
| None | 12 (63%) | 6 | 6 |
| Any | 7 (37%) | 4 | 3 |
| GH | 4 (21%) | 3 | 1 |
| TSH | 4 (21%) | 2 | 2 |
| Gn | 3 (16%) | 2 | 1 |
| Other complicationsb | |||
| Transient DI | 9 (34.6%) | 5 | 4 |
| CSF leak | 2 (7.6%) | 1 | 1 |
| Meningitis | 1 (3.8%) | 0 | 1 |
Gn: gonadotropins; DI: diabetes insipidus; CSF: cerebrospinal fluid.
Of the 19 patients with follow up data, 10 were in the remission group (group I): 6 with adrenal insufficiency and 4 with normal serum cortisol and UFC levels. These 10 patients were followed up for a mean period of 139 months (range 62–184). During this time 5 recurrences (50%) were registered: 2 of the 6 patients with adrenal insufficiency recurred 10 and 14 months after the surgery; and 3 of the 4 patients with normal serum cortisol and UFC recurred at 2, 13 and 18 months after the surgery. Four of the 5 patients in sustained remission were adrenal insufficient for 13, 26, 30 and 54 months after surgery and have not recurred after 140, 170, 106 and 144 months of follow up respectively. The other patient in sustained remission had normal serum cortisol and UFC in the postsurgical evaluation and has not recurred after 184 months of follow up.
We also analyzed the long term outcomes of the patients with immediate remission according to the extent of the surgery. Follow up data were available for 6 of the 7 patients in which a complete macroscopic resection had been performed. Recurrence occurred in 2 of them (at 10 and 18 months) and the other 4 patients are in sustained remission after 151 months of mean follow up (range 106–184 months). Of the 4 patients in which an incomplete macroscopic resection had been performed, 3 had available follow up data and all of them recurred (at 2, 13 and 14 months); the only patient with a subtotal hypophysectomy and immediate remission, has not recurred yet after 140 months of follow up.
When specifically analyzing the outcomes of the macroadenomas in our series, 3 out of 4 achieved immediate remission. One developed adrenal insufficiency following a selective and complete macroscopic resection and has not recurred after 144 months of follow up. The other 2 attained normal serum cortisol and UFC levels following a selective but incomplete macroscopic resection, however both eventually recurred (at 2 and 13 months).
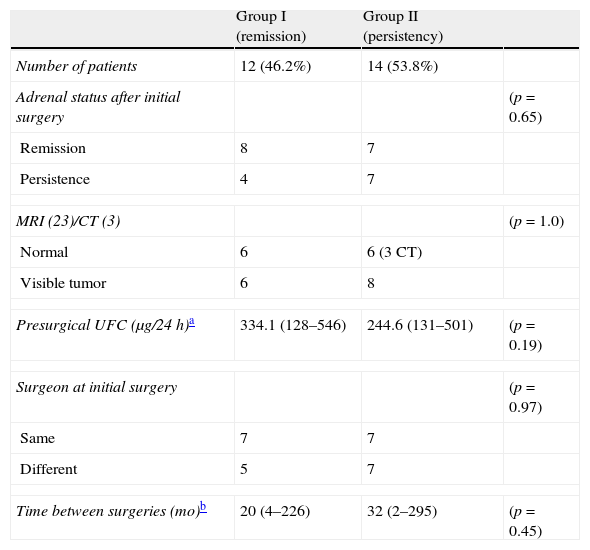
Predictive factorsThere were no statistical differences between groups when compared by any of the analyzed presurgical characteristics: adrenal status – persistence or remission – after initial TSS, tumor identification in imaging studies and degree of hypercortisolism prior to repeated TSS, same/different surgeon in both TSS and time between surgeries (Table 4).
Comparison of presurgical characteristics between groups.
| Group I (remission) | Group II (persistency) | ||
| Number of patients | 12 (46.2%) | 14 (53.8%) | |
| Adrenal status after initial surgery | (p=0.65) | ||
| Remission | 8 | 7 | |
| Persistence | 4 | 7 | |
| MRI (23)/CT (3) | (p=1.0) | ||
| Normal | 6 | 6 (3 CT) | |
| Visible tumor | 6 | 8 | |
| Presurgical UFC (μg/24h)a | 334.1 (128–546) | 244.6 (131–501) | (p=0.19) |
| Surgeon at initial surgery | (p=0.97) | ||
| Same | 7 | 7 | |
| Different | 5 | 7 | |
| Time between surgeries (mo)b | 20 (4–226) | 32 (2–295) | (p=0.45) |
CT: computed tomography.
After an initial TSS, further transsphenoidal approaches are technically more complex mainly because of scar tissue formation and loss of the anatomical references used by the neurosurgeon.1 Thus, a lower remission rate is expected after a repeated surgery. Most series have described a remission rate of 22–75% in this setting, either with a microscopic4–26 or an endoscopic approach.27 However, the outcomes of a repeated TSS in published studies are not easily comparable due to lack of standardized remission criteria in CD and to patient heterogeneity. In some studies, patients without a complete exploration of the sella turcica during the initial operation were included and this fact could have improved their outcomes.6,7 Higher remission rates have been published in two series: 87% in one with a small number of patients28 and 100% in another one with a selected group of non-invasive microadenomas identified clearly during surgery.29 In the present study, immediate remission of hypercortisolism was achieved in 46% of the patients, a similar rate to most series and clearly lower than after the first TSS (83.4% at our institution),30 a phenomenon described as well in other series.8,20
Identification of the tumor in the presurgical MRI or during the initial surgery, an histologically confirmed adenoma after the initial surgery or inadequate exposure of the pituitary gland in the first procedure have been associated with better outcomes after repeated TSS. On the other hand, macroadenomas, non-selective resection and exhaustive exploration of the pituitary gland during initial surgery have been associated with worse outcomes.6,29 In our series, none of the analyzed presurgical characteristics were associated with a higher remission rate after repeated TSS, probably due to the small number of patients included.
A non-selective resection during repeated TSS has been associated with a worse outcome in some studies as well.6,16,20 This fact is probably a consequence of the inability of the neurosurgeon to locate the tumor. In our series we also observed this association: only 20% of patients with a subtotal hypophysectomy achieved remission in contrast with 55% with a selective resection.
A selective but incomplete macroscopic resection of the adenoma is also expected to be associated with disease persistence. In our series the immediate remission rate among the 6 patients in this situation was surprisingly high (4 out of 6, 66%). However, recurrence occurred in all 3 patients with available follow up data. So, in the end, sustained remission in this group is probably very low if any.
Most published series have focused on the immediate postsurgical outcome. Follow up has not been described in many of them and if so, only for a short period of time (11–36 months).6,12–15,27 Therefore, published relapse rates (0–25%) could represent an underestimation. Our study showed a much higher rate of relapses (50%) that could be due either to a longer follow up (139 months) or to a random error because of the small number of patients. Moreover, our general strategy of irradiating most patients after initial TSS failure might have skewed the results. It is possible that if repeated TSS had been offered to a more extensive (and non-selective) group of patients, the outcomes would have been worse, with higher rates of persistence and long term recurrence.
Since the resection of all tumor cells must be followed by an undetectable serum cortisol measurement,31,32 some authors have proposed an early reoperation (days or weeks after the initial surgery) on patients who do not attain this objective to avoid the difficulties associated with a delayed repeated TSS due to fibrosis and scar tissue formation.7,9–13,29 However, in our experience some of the patients who do not achieve postsurgical undetectable serum cortisol will remain in remission for many years.31 Furthermore, delayed remission has been described in as many as 10% of patients initially thought to have persistent disease.20,33 For these reasons, immediate postoperative adrenal status is not accurate enough to support an early reoperation strategy.
Regarding the adverse effects of repeated TSS, we observed a low rate of major complications (Table 3) similar to the one described after initial surgery (6–15%)5,20 and better than after repeated TSS in most series. CSF leaks occurred in 15–20%7,9,13,27 of patients in most series and in nearly 50% of the cases in another.17 However, in our study only 2 patients (7.7%) suffered this complication. Besides, no permanent DI occurred, whereas in other series it was present in 10–45% of the patients after repeated TSS.7,14,27 Both facts could be explained by our lower rate of non-selective resections (subtotal hypophysectomies) in comparison with others.9,10,13 New hormone deficiencies were diagnosed in 7 (37%) of the 19 patients in whom pituitary function was evaluated after repeated surgery, which is similar to the rates described by others (around 40%, ranging from 0 to 100%).6,19,21,25,27 GH deficiency could be underestimated since it was not evaluated under stimulation in every case.
To our knowledge, no studies have compared outcomes of repeated TSS vs. pituitary irradiation in CD patients with persistent or recurrent hypercortisolism after the first TSS. At our institution, when initial surgery fails or hypercortisolism recurs, we achieve better remission rates with pituitary irradiation (83% after a median follow up of 42 months)34 than with repeated TSS (46%). Furthermore, relapses after reoperation are far more frequent (up to 50%) than after external beam radiotherapy (exceptional cases).34,35 In our experience, few major complications occurred with both treatments, with a moderately higher rate of pituitary deficiencies in irradiated patients (57 vs. 37%).34
From our point of view, when hypercortisolism persists or recurs after initial TSS, repeated intervention should only be considered in very well selected patients in whom the tumor is visible with MRI, is located in an accessible place for the neurosurgeon and does not show evident invasion of the adjacent structures that could limit its total excision. Furthermore, reintervention could be considered in fertile women who wish to get pregnant because it seems to have a lower rate of hypopituitarism than radiotherapy. However, results after repeated TSS are expected to be modest (immediate remission rate around 50%) with a relapse rate of nearly 50%, leaving a long term remission rate close to 25%. Until larger series are analyzed and remission predictive factors for repeated TSS are well defined, we consider that pituitary irradiation is the treatment of choice for the majority of patients with persistent or recurrent hypercortisolism after the initial TSS.
Conflict of interestThe authors declare that there is no conflict of interest.
We are indebted to Isabel Millán for the statistical analysis and to Antonio Ojeda, Ignacio Bernabéu, Guillermo Serra, Itziar Aznar and Lourdes Benítez for collecting the follow up data of the 4 patients who were followed at their institutions.