There are studies showing a strong association between thyroid dysfunction and increased cardiovascular risk due to lipid profile changes.
The purpose of this study was to assess the degree of association and predictive power of thyroid-stimulating hormone (TSH) levels in relation to lipid profile changes, identifying the TSH cut-off point beyond which lipid changes occur.
Patients and methodsA cross-sectional, retrospective study in Quito (Ecuador) was conducted from January 2004 to December 2008 on patients first attending the endocrinology department.
ResultsA total of 278 histories were analyzed, and a 36.3% prevalence of subclinical hypothyroidism was found.
No association was found between sex and cholesterol or between sex and low density lipoprotein (LDL). However, associations were found between sex and dyslipidemia, sex and body mass index (BMI), and sex and TSH.
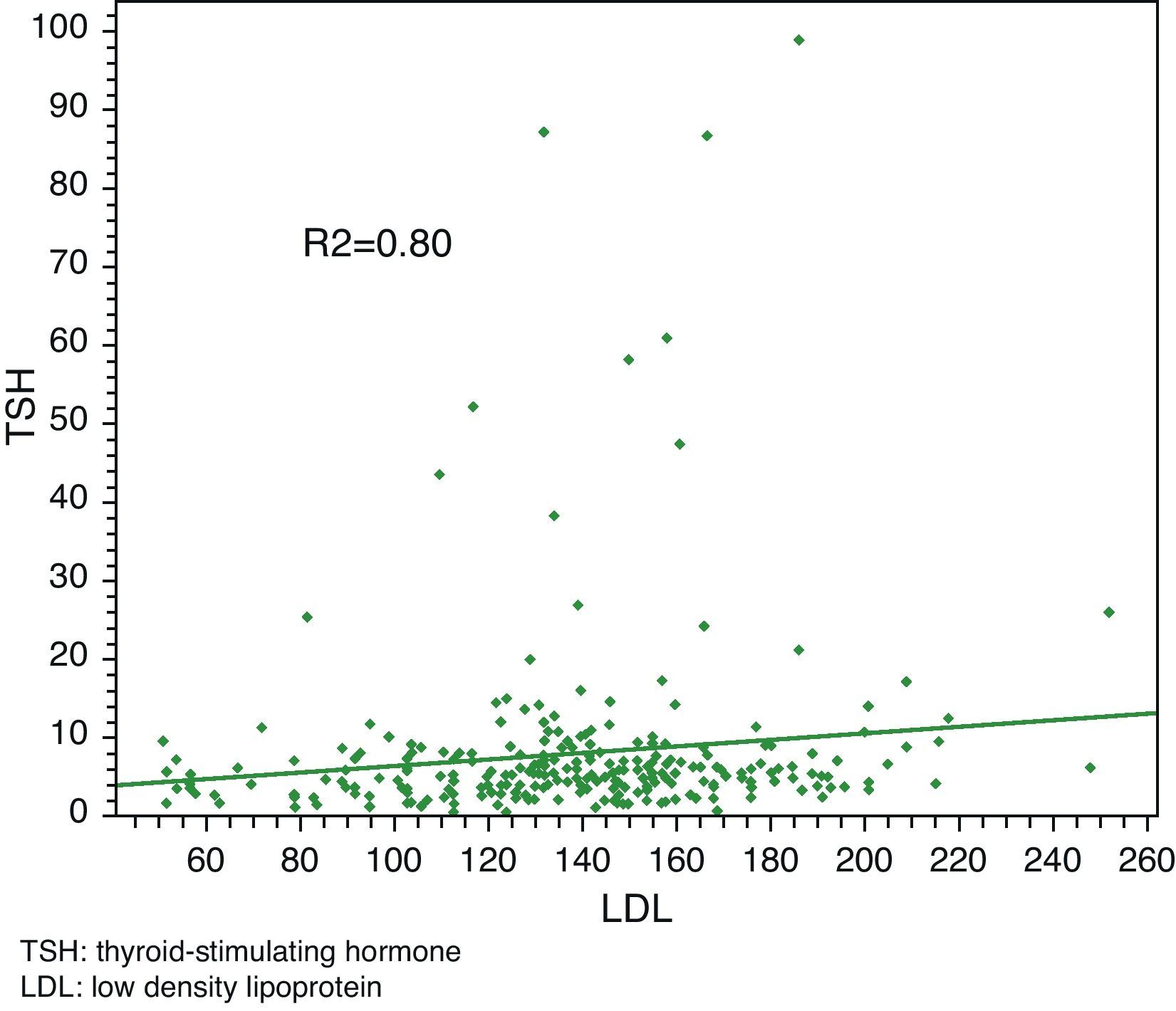
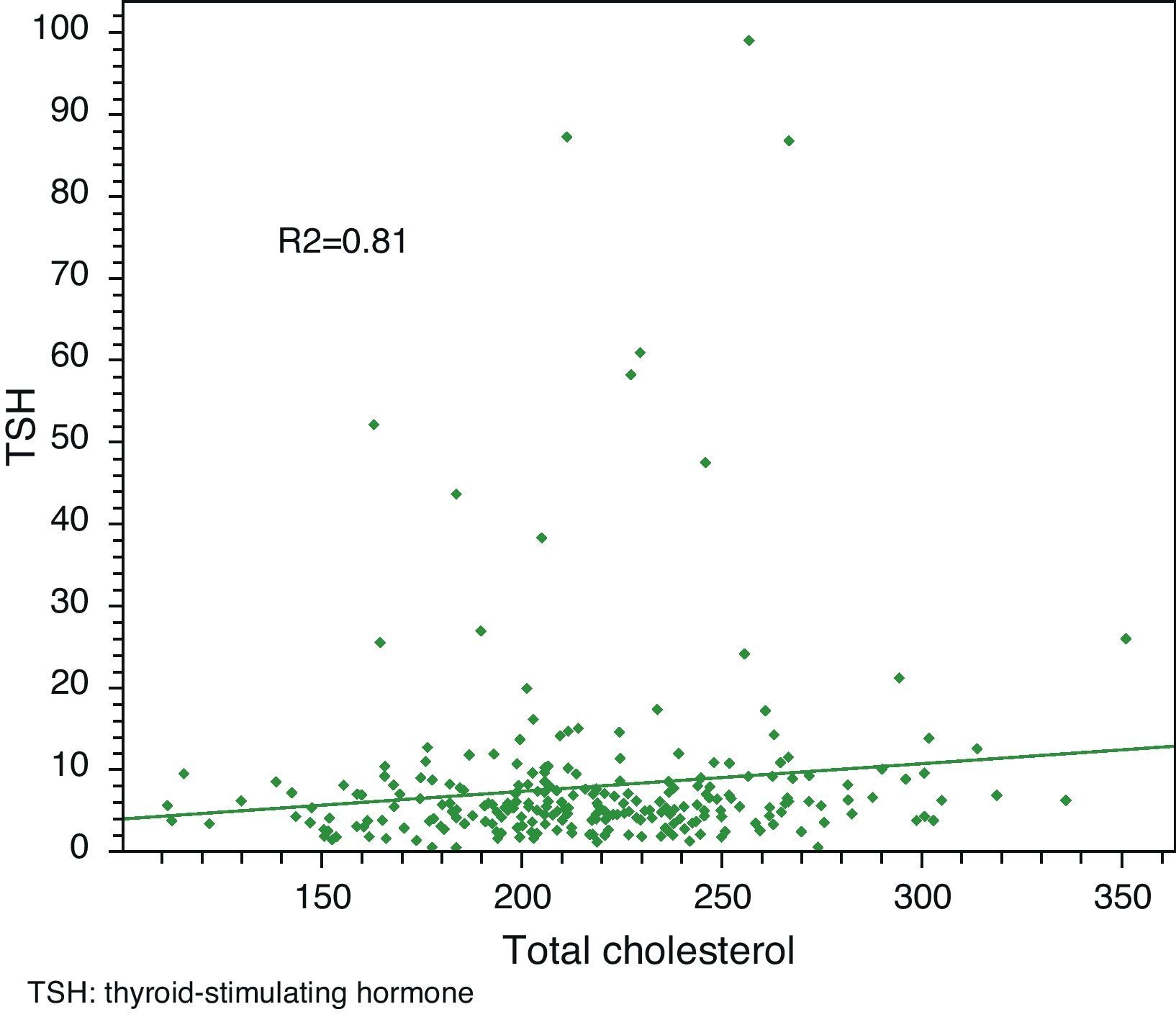
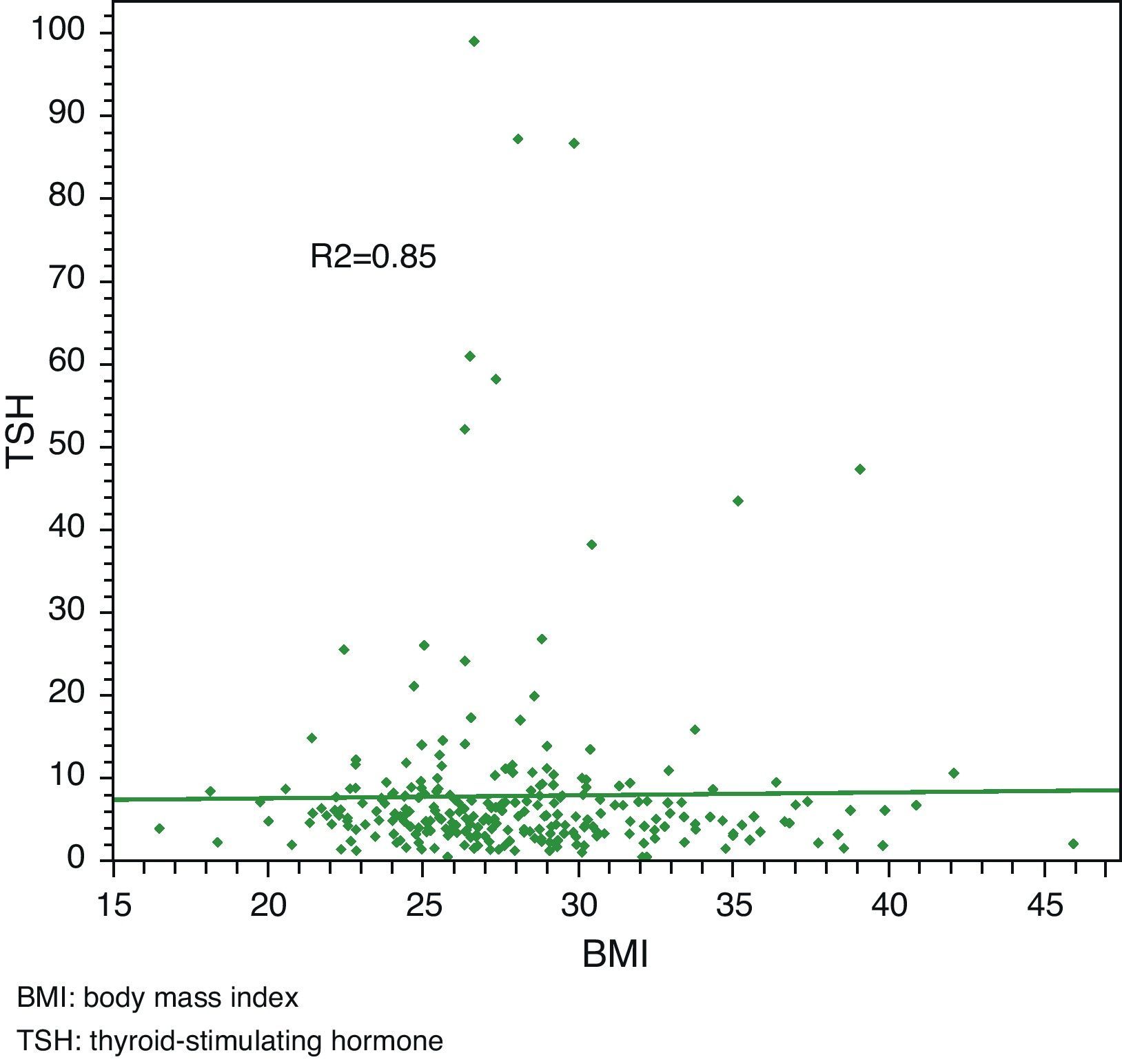
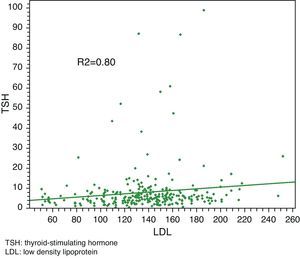
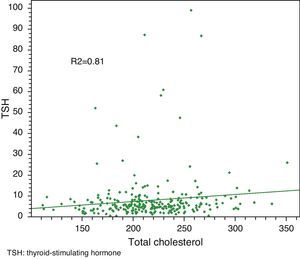
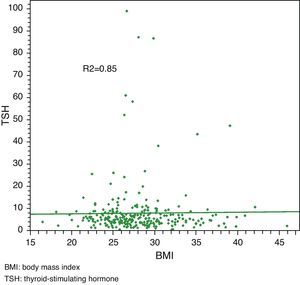
Linear regression analysis between LDL and TSH, cholesterol and TSH, and BMI and TSH showed significant associations in all cases, with Pearson R2 coefficients of 0.80, 0.81, and 0.85 respectively.
ConclusionsTSH levels show a statistically significant association to total cholesterol and LDL levels, but are not a good clinical predictor in this process. A cut-off point beyond which replacement therapy should be started to prevent occurrence of dyslipidemia cannot therefore be established.
Hay estudios que demuestran una gran asociación entre disfunción tiroidea e incremento del riesgo cardiovascular por alteraciones del perfil lipídico.
El objetivo de este estudio es determinar el grado de asociación y poder predictor de las concentraciones de tirotropina (TSH) en relación con alteraciones del perfil lipídico, identificando el punto de corte de TSH tras el cual se producen alteraciones lipídicas.
Material y métodoEstudio trasversal retrospectivo en Quito (Ecuador) en el periodo enero 2004-diciembre 2008, en pacientes de primera consulta al servicio de Endocrinología.
ResultadosSe analizaron 278 historias reportando una prevalencia de hipotiroidismo subclínico del 36,3%.
No se encontró asociación entre sexo y colesterol ni entre sexo y lipoproteínas de baja densidad (LDL); sin embargo, sí hubo asociación entre sexo y dislipidemia, así como entre sexo e índice de masa corporal (IMC) y entre sexo y TSH.
En el análisis de regresión lineal entre LDL y TSH, colesterol y TSH e IMC y TSH se encontró una asociación significativa para cada una de ellas con un coeficiente R2 de Pearson de 0,80, 0,81 y 0,85 respectivamente.
ConclusionesLas concentraciones de TSH presentan una asociación estadística en relación con los valores de colesterol total y LDL, pero no constituye un buen predictor clínico de este proceso, razón por la que no se puede establecer un punto de corte tras el cual iniciar tratamiento sustitutivo para así prevenir el aparecimiento de dislipidemias.
Thyroid hormone deficiency may occur as clinical or subclinical hypothyroidism. Clinical hypothyroidism is characterized by increased thyroid-stimulating hormone (TSH) levels, low triiodothyronine (T3) and thyroxine (T4) levels, and associated clinical signs, while in its subclinical, asymptomatic form (which is known to affect 6–17% of the population), elevated TSH levels and normal free T4 levels are found and its etiology is sometimes unclear.1–4
The role of thyroid hormones on the cardiovascular system is crucial, and a strong association has been found between thyroid hormones and both lipid profile and atherosclerotic disease as predictors of cardiovascular risk.5,6
Studies conducted in hypothyroid patients have reported high levels of total and low density lipoprotein (LDL) cholesterol and decreased levels of high density lipoprotein (HDL) cholesterol as compared to euthyroid controls.1,7–10
The pathophysiological mechanism accounting for the atherogenic process consists of a decreased affinity of LDL for its receptors, decreased biliary excretion of cholesterol, and decreased lipoprotein lipase activity, resulting in the prolongation of the half-lives of total and LDL cholesterol.1,11,12
It should be borne in mind that cardiovascular disease is the leading cause of disability and early death worldwide, and makes a substantial contribution to the high costs of health care. Atherosclerosis is the main condition, related to coronary artery disease and a significant increase in morbidity and mortality.13–15
Dyslipidemia is one of the five significant risk factors for the development of cardiovascular diseases,16,17 and hypothyroidism is the second leading endocrinological disease causing dyslipidemia after diabetes mellitus. Thyroid function screening studies in populations with hypercholesterolemia have found clinical and subclinical hypothyroidism in 2–9% of patients.14,18
It should also be noted that a study where patients were distributed into groups based on their severity of dyslipidemia found the greatest proportion of patients with subclinical hypothyroidism in the group with the highest serum cholesterol levels, which confirmed that subclinical hypothyroidism is a risk factor for atherosclerosis and myocardial infarction.11,19–25
The Third U.S. National Health and Nutrition Examination Survey (NHANES III) showed higher cholesterol and LDL levels in patients with subclinical hypothyroidism as compared to euthyroid patients, but after adjustment for variables such as sex, race, age, and the concomitant use of oral lipid lowering drugs, hypothyroidism was not related to an abnormal lipid profile.1,8,26
The main controversy in the vast majority of studies on the adequate time to start hypothyroid treatment is focused on the TSH cut-off value below which hormone replacement therapy should be started in order to normalize lipid levels. The presence or absence of antibodies and the risk-benefit of treatment should also be considered.11,27–30
The purpose of this study was to establish the degree of clinical and statistical association of TSH levels and lipid profile, what the contribution of replacement therapy as an intervention to prevent the occurrence of dyslipidemia would be, and the ideal cut-off point for starting such therapy.
Subjects and methodsThis was a retrospective, cross-sectional study enrolling all patients attending the endocrinology department of the General Army Hospital No. 1 (also known as HG-1 and Quito Military Hospital) with suspected subclinical hypothyroidism during the period 2004–2008. Thyroid hormone and lipid profile tests of all patients were requested during their first visit, and clinical histories from patients who had complete results for thyroid function, anthropometrics, LDL cholesterol, and total cholesterol were also selected for the research.
Patients attending the endocrinology outpatient clinic whose clinical history included all variables proposed in the case report form were considered to be eligible, while patients having a concurrent disease as the causative factor of lipid changes, pregnant women, and patients already receiving lipid lowering treatment before being diagnosed with hypothyroidism were excluded from the study.
Data were collected by reviewing clinical histories. These mainly consisted of progress notes of the first visit and results obtained at the laboratory of the Military Hospital.
In the first stage, a univariate analysis was performed. Continuous variables were summarized with measures of central tendency and dispersion. Categorical variables were summarized using frequencies and their respective 95% confidence intervals.
In the second stage, a bivariate analysis was performed to determine the degree of association of TSH with LDL cholesterol, cholesterol, and body mass index (BMI). Linear regression with Pearson's R2 coefficient was used for this purpose.
The receiver operating characteristics (ROC) curve was used to measure the clinical predictive power of the association between TSH and dyslipidemia.
In addition, variables that included the age groups of patients over and under 50 years of age and a variable including cholesterol levels higher than 200mg/dL and/or LDL cholesterol levels higher than 130mg/dL as indicative of dyslipidemia were used.
ResultsAll clinical histories from patients attending the endocrinology department of the Military Hospital from January 2005 to December 2008 were reviewed, and TSH tests were requested. There were 805 clinical histories that met this criterion. Of these, 130 clinical histories not including the results of lipid and thyroid profiles, height or weight were excluded, leaving 675 histories. Exclusion criteria, as reported in the methodology, were met by 397 clinical histories. A total of 278 clinical histories were therefore used for the final analysis.
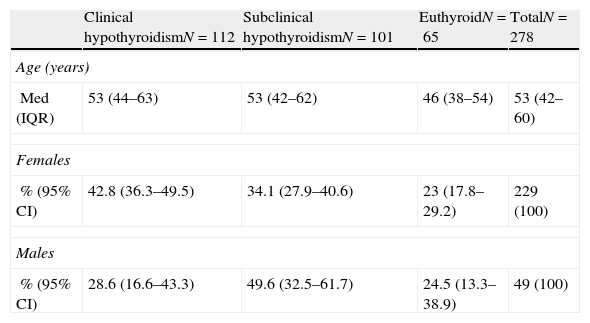
The median age of the 278 patients was 51 years (IQR [second interquartile]: 42–60), and female/male ratio was 4.67–1. One hundred and twelve subjects (40.3% 95% CI [95% confidence interval]: 34.5–46.3%) had a diagnosis of clinical hypothyroidism; 101 (36.3% [95% CI: 30.7–42.3%]) had a diagnosis of subclinical hypothyroidism; and 65 (23.4% [95% CI 18.5–28.8%]) patients were euthyroid. Median age was similar across diagnoses (Table 1).
Diagnosis in relation to age and sex.
| Clinical hypothyroidismN=112 | Subclinical hypothyroidismN=101 | EuthyroidN=65 | TotalN=278 | |
| Age (years) | ||||
| Med (IQR) | 53 (44–63) | 53 (42–62) | 46 (38–54) | 53 (42–60) |
| Females | ||||
| % (95% CI) | 42.8 (36.3–49.5) | 34.1 (27.9–40.6) | 23 (17.8–29.2) | 229 (100) |
| Males | ||||
| % (95% CI) | 28.6 (16.6–43.3) | 49.6 (32.5–61.7) | 24.5 (13.3–38.9) | 49 (100) |
CI: confidence interval; IQR. second interquartile; Med: median.
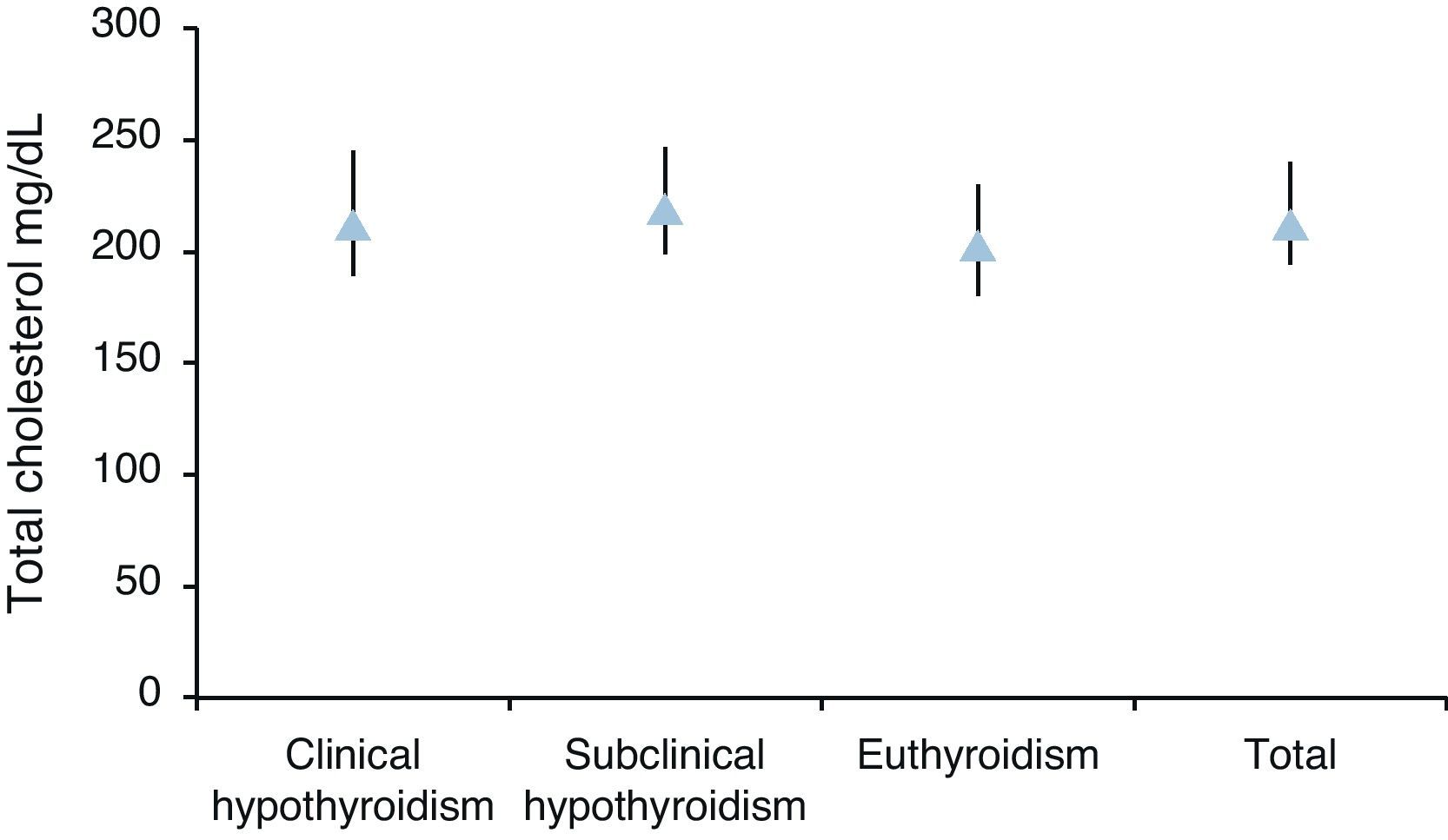
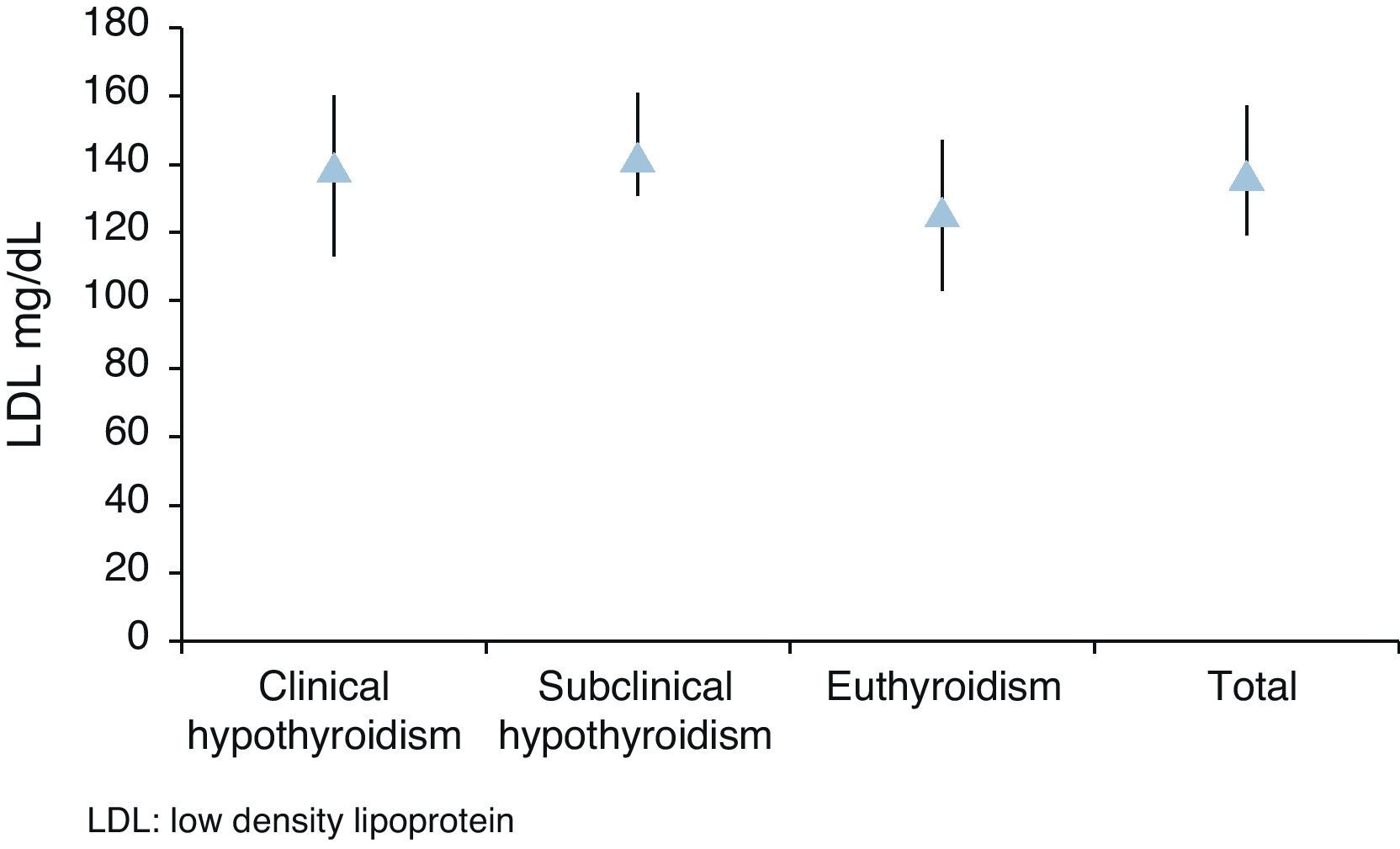
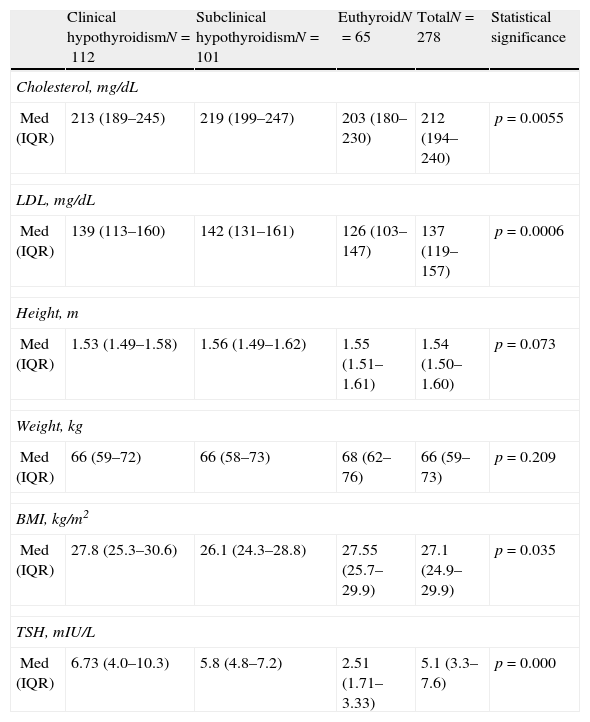
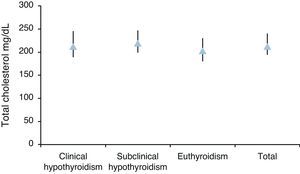
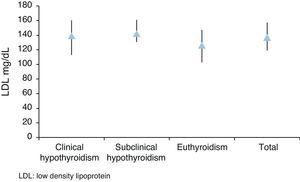
Median total cholesterol level in the 178 patients was 212mg/dL (IQR: 194–240), and median LDL cholesterol level was 137mg/dL (IQR: 119–157). As regards anthropometric data, median height was 154cm (IQR: 150–160) and median weight was 66kg (IQR: 59–73), with a resulting median BMI of 27.1kg/m2 (IQR: 24.9–29.9) (Table 2, Figs. 1 and 2).
Diagnosis in relation to lipid changes and anthropometric values.
| Clinical hypothyroidismN=112 | Subclinical hypothyroidismN=101 | EuthyroidN=65 | TotalN=278 | Statistical significance | |
| Cholesterol, mg/dL | |||||
| Med (IQR) | 213 (189–245) | 219 (199–247) | 203 (180–230) | 212 (194–240) | p=0.0055 |
| LDL, mg/dL | |||||
| Med (IQR) | 139 (113–160) | 142 (131–161) | 126 (103–147) | 137 (119–157) | p=0.0006 |
| Height, m | |||||
| Med (IQR) | 1.53 (1.49–1.58) | 1.56 (1.49–1.62) | 1.55 (1.51–1.61) | 1.54 (1.50–1.60) | p=0.073 |
| Weight, kg | |||||
| Med (IQR) | 66 (59–72) | 66 (58–73) | 68 (62–76) | 66 (59–73) | p=0.209 |
| BMI, kg/m2 | |||||
| Med (IQR) | 27.8 (25.3–30.6) | 26.1 (24.3–28.8) | 27.55 (25.7–29.9) | 27.1 (24.9–29.9) | p=0.035 |
| TSH, mIU/L | |||||
| Med (IQR) | 6.73 (4.0–10.3) | 5.8 (4.8–7.2) | 2.51 (1.71–3.33) | 5.1 (3.3–7.6) | p=0.000 |
IQR: second interquartile; BMI: body mass index; Med: median; TSH: thyroid-stimulating hormone.
Dyslipidemia (according to the criteria given in the subjects and methods section) was found in 211 patients (75.9% [95% CI; 70.4–80.8]), with a mean TSH level of 5.8mU/L (IQR [4.8–7.2]) in patients with subclinical hypothyroidism.
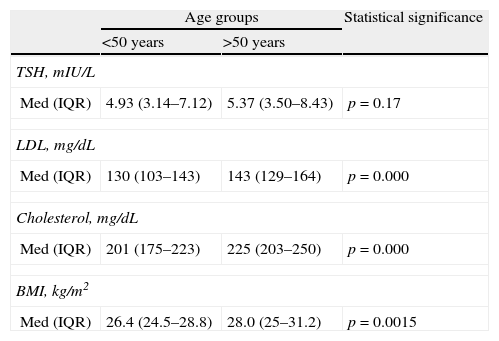
A 1.2–1 ratio was seen between subjects over and under 50 years of age. No significant difference was found in sex distribution in these age groups (OR: 0.87 [95% CI: 0.47–1.62]). No significant difference was seen either in TSH levels between both age groups. A significant difference was however seen in total and LDL cholesterol levels and BMI, with higher values found in subjects older than 50 years (Table 3).
TSH levels, dyslipidemia, and body mass index by age group.
| Age groups | Statistical significance | ||
| <50 years | >50 years | ||
| TSH, mIU/L | |||
| Med (IQR) | 4.93 (3.14–7.12) | 5.37 (3.50–8.43) | p=0.17 |
| LDL, mg/dL | |||
| Med (IQR) | 130 (103–143) | 143 (129–164) | p=0.000 |
| Cholesterol, mg/dL | |||
| Med (IQR) | 201 (175–223) | 225 (203–250) | p=0.000 |
| BMI, kg/m2 | |||
| Med (IQR) | 26.4 (24.5–28.8) | 28.0 (25–31.2) | p=0.0015 |
IQR: second interquartile; BMI: body mass index; LDL: low density lipoprotein; Med: median; TSH: thyroid-stimulating hormone.
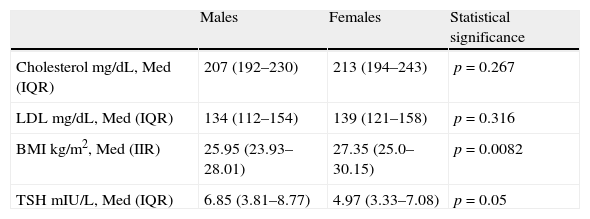
No association was found between sex and total cholesterol or between sex and LDL cholesterol. However, associations were seen between sex and dyslipidemia, and between sex and BMI and sex and TSH (Table 4).
Total cholesterol, LDL, and TSH levels and BMI by sex.
| Males | Females | Statistical significance | |
| Cholesterol mg/dL, Med (IQR) | 207 (192–230) | 213 (194–243) | p=0.267 |
| LDL mg/dL, Med (IQR) | 134 (112–154) | 139 (121–158) | p=0.316 |
| BMI kg/m2, Med (IIR) | 25.95 (23.93–28.01) | 27.35 (25.0–30.15) | p=0.0082 |
| TSH mIU/L, Med (IQR) | 6.85 (3.81–8.77) | 4.97 (3.33–7.08) | p=0.05 |
IQR: second interquartile; BMI: body mass index; LDL: low density lipoprotein; Med: median; TSH: thyroid-stimulating hormone.
Linear regression analysis between LDL and TSH, cholesterol and TSH, and BMI and TSH showed significant associations for all of them, with Pearson's R2 coefficients of 0.80, 0.81, and 0.85 respectively (Figs. 3–5).
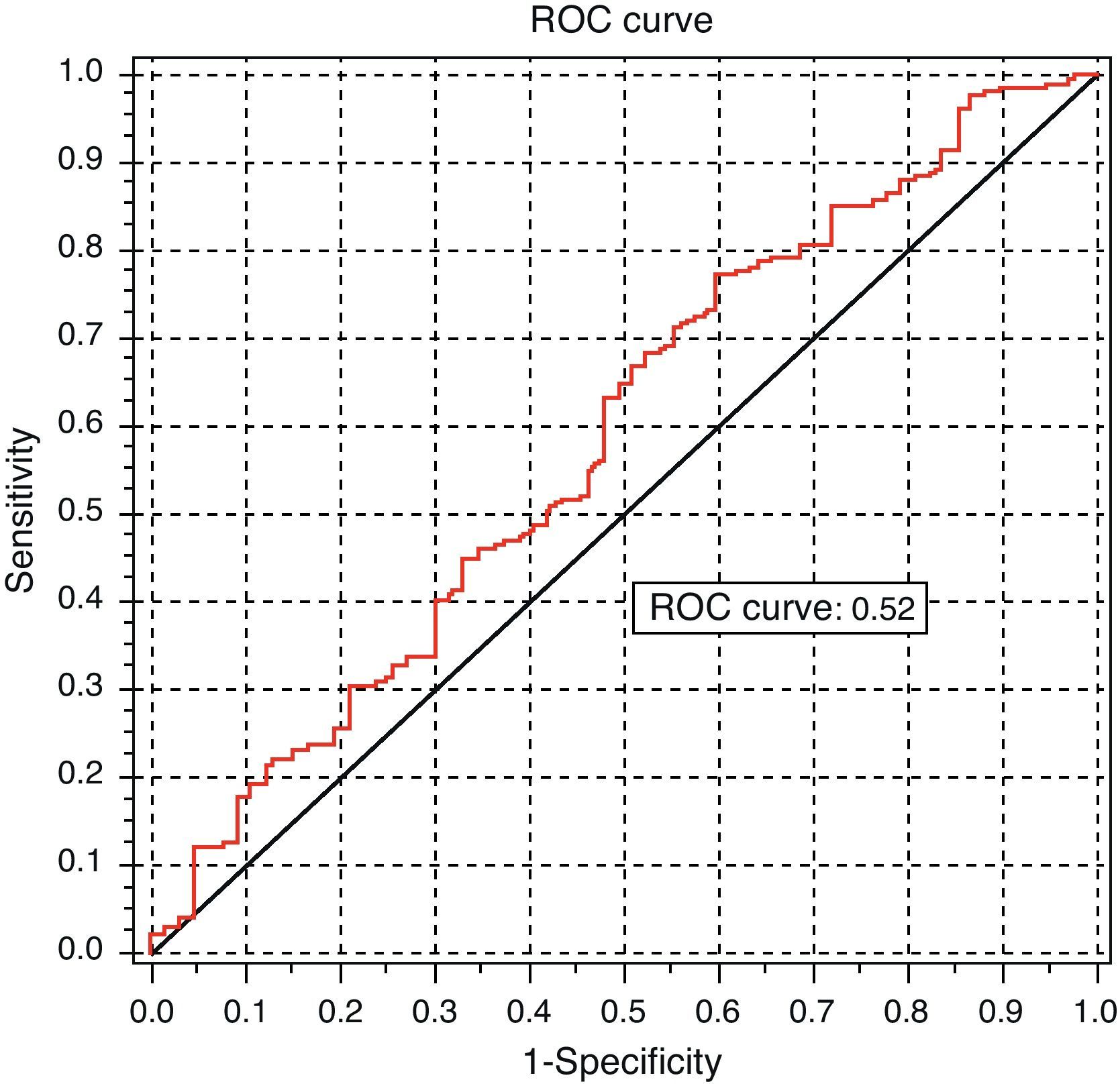
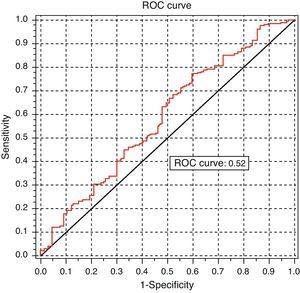
Analysis of the ROC curve, intended to test the discriminant power of TSH measurement for predicting dyslipidemia, provided a non-significant area under the curve (AUC=0.52 [95% CI: 0.5121–0.6632]) (Fig. 6).
Since a clinical association was not found between TSH levels and lipid profile changes, the next planned step, namely the determination of the cut-off point for TSH beyond which treatment would be started, was not warranted.
DiscussionThis study showed a statistical association between TSH levels and lipid profile changes. It may therefore be stated that TSH level is a risk factor for the development of lipid changes, but is not a good clinical predictor of such changes. However, this association has no clinical implications for prediction, as it is shown by the analysis of the ROC curve.
In studies on clinical predictors, it is very important to distinguish between the existence of an association between two variables and the strength of such an association with its attendant clinical implications. In fact, it has been shown that in many studies where a statistical association has been found between two variables, the association loses predictive power when it is converted into confirmatory and exclusionary powers.
This is precisely what happened in this study: a statistical association was indeed shown between dyslipidemia and altered TSH levels. However, the strength of this association was very weak, and it had therefore no value as a clinical predictor. This was the reason why assessment of the cut-off point made no sense.
It should be noted that this does not mean that TSH is no longer significant as a risk factor for dyslipidemia. However, it should be used for primary prevention, not for taking treatment decisions.
It is significant that 8 out of every 10 histories reviewed were of female patients, which agrees with the results of previous studies. It should be stressed that the study was conducted at the Army Hospital, where the majority of patients are male. This confirms the predominance of thyroid disease in females. The increasing prevalence of subclinical hypothyroidism was also confirmed by the higher prevalence rates reported in patients between the fifth and sixth decades of life.
A predominance of patients with clinical hypothyroidism was also found, but it should be noted that the highest total cholesterol and LDL cholesterol levels were seen in patients with subclinical thyroid disease with highly significant p values, in whom a TSH cut-off value of 5.8mU/L was found. These figures agree with the results of other studies and suggest that, despite the low predictive power for dyslipidemia of TSH discussed in prior paragraphs, lipid profile should be tested in patients with TSH levels higher than the cut-off point found.
Mention should be made of the worldwide controversy about the relationship between subclinical hypothyroidism and dyslipidemia. Prevalence rates of hypothyroidism were shown to be 7.5% in females and 2.8% in males, ranging from 3% to 15% in the adult population.
Dyslipidemia is one of the five risk factors for the development of cardiovascular disease,13 and hypothyroidism is the second leading endocrinological disease causing dyslipidemia after diabetes mellitus. Thyroid function screening studies in populations with hypercholesterolemia found clinical and subclinical hypothyroidism in 2–9% of patients.14
Different views also exist regarding screening in the general population. The American Thyroid Association recommends TSH measurements from the age of 35 years and every five years thereafter in asymptomatic adults, while the U.S. Preventive Services Task Force questions screening, particularly in men, who have a much lower incidence of subclinical hypothyroidism as compared to older women.
Their main argument, however, is that studies do not lead to clear conclusions as to whether early treatment does or does not decrease morbidity and mortality or improves quality of life in these cases. This agrees with the findings reported in our environment.4
Among consensuses reached in Latin American countries, it should be noted that the Cuban Consensus on Subclinical Hypothyroidism requires a TSH level higher than 3.5mU/L and normal free or total T4 levels for diagnosis of the condition, and the identification of positive peroxidase antibodies before treatment is started.4
Increased levels of total and LDL cholesterol and apolipoprotein A, a highly atherogenic LDL variant, like apo B, have also been reported. Studies in patients with hypothyroidism showed prolongation of the half-life of LDL cholesterol due to decreased catabolism, an effect which is reversible upon the administration of hormone treatment.5,7
Additional data from human fibroblasts confirm that T3 induces an increased degradation of LDL cholesterol, which is a direct mediator of the increase in the number of LDL receptors with no change in LDL affinity for these receptors.1,8
Studies have shown that each mmol/L reduction in LDL cholesterol is associated with a 19% decrease in coronary morbidity and mortality. This results in a 23% reduction in myocardial infarctions and coronary deaths, a 24% reduction in the coronary revascularizations required, and a 17% reduction in fatal and nonfatal stroke, with a 21% overall reduction in cardiovascular events.15
The NHANES III survey of 17,353 US patients showed high TSH levels consistent with hypothyroidism in 4.6%, and noted that such levels were more common in women with low birthweight and low BMI in childhood,26 data which should be investigated in future studies in our country.
Research on patients with hypothyroidism showed an increase in LDL cholesterol half-life secondary to decreased catabolism, an effect that is reversible with replacement therapy. It needs therefore to be confirmed whether or not LDL cholesterol levels decrease when replacement therapy is started with levothyroxine despite the absence of lipid lowering treatment.14
Levothyroxine sodium should also be prescribed at the doses required to normalize TSH in the presence of positive antibodies, dyslipidemia, or TSH levels higher than 10mU/L or gradually increasing.4
This study is the first retrospective research conducted in our country to assess the association between subclinical hypothyroidism and lipid profile. It provides data about TSH levels that cause lipid changes in our environment, although it should be borne in mind that they are not the best predictor for the occurrence of such changes. It is also expected to be the basis for future prospective studies.
One of the limitations of this study was its retrospective nature. In addition, data for the clinical histories were recorded by other people. It should be stressed that many medical charts were excluded from the study because they did not include the data required for the analysis. A significant disadvantage that should be mentioned is the lack of lipid profile results in patients diagnosed with subclinical hypothyroidism, probably because of a lack of awareness about the correlation between the disease investigated and dyslipidemia.
A bias that should be taken into account is that a majority of the study population from the Military Hospital belonged to the middle and upper middle classes, and the study results can therefore only be extrapolated to populations with the same socioeconomic characteristics.
This research, which was conducted in order to find an association between TSH levels and lipid profile changes, will serve as the basis for future prospective research where long-term monitoring of patients may allow for analyzing parameters that could not be studied in a retrospective study such as this.
It will also be appropriate to consider studies that allow for establishing the prevalence of cardiovascular risk factors in relation to subclinical hypothyroidism, with regard to blood pressure levels, cardiac muscle hypertrophy, the size of atheromatous plaque at the aortic level, the development of peripheral neuropathy, and the influence of associated factors such as smoking and diabetes mellitus, taking into account that subclinical hypothyroidism is a marker of nephropathy and coronary artery disease in diabetic patients.
The clinical implications of this study are very important, because it helps us to understand the true value of TSH measurements in patients with suspected subclinical hypothyroidism. In fact, based on the results obtained, there would appear to be no point in using TSH levels when making the decision whether or not to start replacement therapy to prevent dyslipidemia.
FundingThe study was fully financed by the authors.
Conflicts of interestThe authors state that they have no conflicts of interest.
MA and VS are specialists in internal medicine (Instituto Superior de Postgrado-Facultad de Ciencias Médicas-Universidad Central del Ecuador [ISP-FCM-UCE]). This research was conducted in the setting of activities related to their degree dissertations.
We thank doctors Rodrigo Rovayo P. and Juan Moreira for their cooperation in the scientific and methodological conduct of the study. We also thank the General Army Hospital No. 1.
Please cite this article as: Sarzosa Terán V, Astudillo Calle MA. Concentraciones de tirotropina con relación al desarrollo de dislipidemia y determinación de punto de corte ideal para el inicio de tratamiento sustitutivo. Endocrinol Nutr. 2012;59:575–82.