Because of the physiological changes which occur during pregnancy, pregnant women have reference thyroid hormone levels different from those of the general population.1 Both the Spanish Society of Endocrinology and Nutrition (SEEN)2 and North American and European scientific bodies3,4 recommend that thyroid hormone levels be assessed according to the reference values for each trimester and population using adequate laboratory procedures. This study aimed at establishing the reference values of TSH, free T4 and free T3 during the first trimester of pregnancy, the prevalence of autoimmune thyroid disease, and the current degree of implementation of universal thyroid screening in our healthcare area. This is the only study published to date that provides two reference ranges for the first trimester of pregnancy (<11 weeks and 11–13 weeks).
A prospective study was conducted enrolling all pregnant women who attended the clinical laboratory of our hospital complex for prenatal screening for aneuploidy (weeks 11 to 13) during June–July 2004. In addition to the sample for detecting fetal chromosome diseases, a blood sample was taken for measuring TSH, free T4, free T3, thyroid peroxidase antibodies, and thyroglobulin antibodies. All laboratory parameters were tested using a two-step chemiluminescent microparticle immunoassay with Chemiflex® protocols in an Architect® i2000sr analyzer from Abbott-Diagnostics (USA). For each variable, the confidence interval of the 2.5th and 97.5th percentiles, corresponding to the lower and upper limits of reference values, were calculated following the recommendations of the International Federation of Clinical Chemistry.5
The sample size was 454 pregnant women, i.e. 12% of the 3516 deliveries occurring in our healthcare area in 2014. One hundred and nineteen women (26.2%) were excluded from the calculation of the reference values, 74 (16.3%) for thyroid autoimmunity, 33 (7.3%) for a history of thyroid disease, 4 (0.9%) for prior diabetes, and 5 (1.1%) for twin pregnancy. Finally, three pregnant women were excluded because of a missing laboratory result.
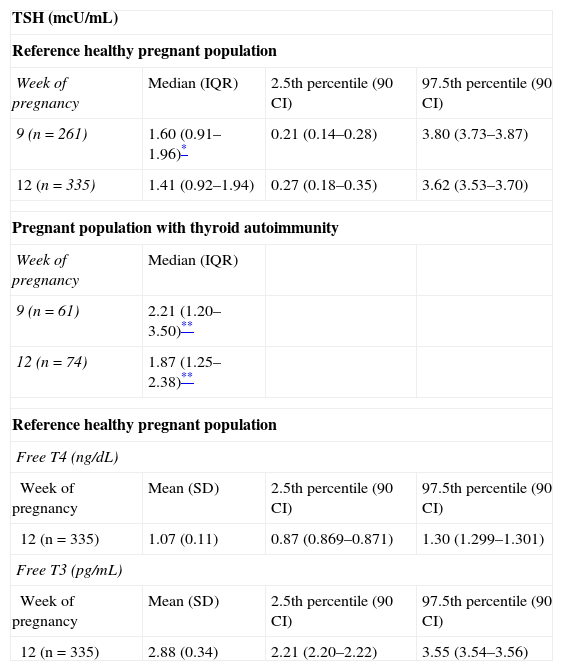
Consequently, reference values were calculated for 335 women taken as representing the healthy pregnant population. Prior laboratory tests including as a minimum TSH measurement in the first part of the first trimester (before week 11) were available for 261 (77.9%) of these women. Table 1 shows the reference values obtained for thyroid hormones and TSH before week 11 (median week 9) and between weeks 11 and 13 (median week 12). The results obtained during the first trimester were statistically different from the reference values in the non-pregnant population; significantly higher TSH levels were found in patients with thyroid autoimmunity.
Reference ranges for thyroid function tests (2.5th–97.5th percentiles) in the healthy pregnant population and thyroid function results in women with thyroid autoimmunity in the two study periods.
| TSH (mcU/mL) | |||
| Reference healthy pregnant population | |||
| Week of pregnancy | Median (IQR) | 2.5th percentile (90 CI) | 97.5th percentile (90 CI) |
| 9 (n=261) | 1.60 (0.91–1.96)* | 0.21 (0.14–0.28) | 3.80 (3.73–3.87) |
| 12 (n=335) | 1.41 (0.92–1.94) | 0.27 (0.18–0.35) | 3.62 (3.53–3.70) |
| Pregnant population with thyroid autoimmunity | |||
| Week of pregnancy | Median (IQR) | ||
| 9 (n=61) | 2.21 (1.20–3.50)** | ||
| 12 (n=74) | 1.87 (1.25–2.38)** | ||
| Reference healthy pregnant population | |||
| Free T4 (ng/dL) | |||
| Week of pregnancy | Mean (SD) | 2.5th percentile (90 CI) | 97.5th percentile (90 CI) |
| 12 (n=335) | 1.07 (0.11) | 0.87 (0.869–0.871) | 1.30 (1.299–1.301) |
| Free T3 (pg/mL) | |||
| Week of pregnancy | Mean (SD) | 2.5th percentile (90 CI) | 97.5th percentile (90 CI) |
| 12 (n=335) | 2.88 (0.34) | 2.21 (2.20–2.22) | 3.55 (3.54–3.56) |
SD: standard deviation; 90 CI: 90% confidence interval; IQR: interquartile range.
These results are not substantially different from those found in the last six years by other Spanish working groups in different geographical areas of Spain with different test methods and in different gestational weeks.6–8 It should be noted that the reference ranges obtained for TSH and thyroid hormones in this and in prior national studies are clearly higher than the reference values recommended by the international guidelines (<2.5mcU/mL)3,4 if no normal ranges for the first trimester are available at the center. An upper normal limit of TSH levels of 2.5mcU/mL in the first trimester would have immediate consequences for thyroid dysfunction management in pregnancy. It would represent an important care and financial overload, and would also lead to the overdiagnosis of hypothyroidism in a non-negligible proportion of pregnancies.
Reference ranges for TSH in two different periods of the first trimester (at weeks 9 and 12) will allow us to more accurately assess thyroid dysfunction in the pregnant population of our region. Screening should ideally be performed before week 10 for the early detection of thyroid dysfunction, given that subclinical hypothyroidism is associated with an increased risk of complications during pregnancy and of neurocognitive deficiencies in the developing fetus. In women who have not undergone the study in the first part of the trimester, the sample for neonatal screening may be used in order to obtain thyroid function data and to interpret them with specific reference values between weeks 11 and 13. Somewhat lower TSH levels in this period of pregnancy may be related to the peak production of chorionic gonadotropin, which occurs in the final part of the first trimester (from week 9 of pregnancy). These results are similar to those reported by Bocos-Terraz et al.,6 who found between weeks 11 and 20 TSH levels somewhat lower than those obtained before week 11.
Having our own reference values allows us to know the true prevalence of thyroid dysfunction in our pregnant population, which is currently unknown, and will also allow us to implement universal screening schemes in our healthcare area. Today, screening is done in only 77.9% of pregnancies, and TSH measurement is the test most commonly requested. In this regard, the SEEN issued a consensus document that recommends universal screening versus a selective search for thyroid dysfunction in pregnancy.2 It should be noted that this advice is not yet included in international guidelines,3,4 most of which recommend risk-based screening.
The prevalence of thyroid autoimmunity in this study is among the highest reported in the different national studies.6–8 Prior analyses conducted by our group9 had already found a high thyroid autoimmunity rate in pregnant women in our area: positive thyroid peroxidase and thyroglobulin antibodies were found in 11.6% and 9.9% respectively. This high prevalence of thyroid autoimmunity supports the results of Jaén et al.,10 who showed in pregnant women from the urban area of Toledo a prevalence of postpartum thyroiditis much higher than that reported in other Spanish regions (15.9%). Women with thyroid autoimmunity have higher TSH levels than the population with no underlying thyroiditis, and should therefore be excluded from the calculation of reference values. They are also a population at risk of developing thyroid dysfunction during pregnancy and after delivery. Interventional studies are lacking. There is therefore no adequate scientific evidence to show the potential benefit of thyroxine treatment in this group of patients, both in the obstetric results and in terms of fetal and infantile neurological development.
In conclusion, the reference ranges of TSH and thyroid hormone levels obtained in this study will allow for an adequate interpretation of thyroid function tests in pregnant women in our healthcare area, so avoiding the misdiagnosis of subclinical hypothyroidism in a significant number of patients or allowing for an early start of treatment.
This knowledge of the reference values for our population and laboratory procedure should help us to both implement and improve the effectiveness of already existing programs of universal screening for thyroid dysfunction associated with pregnancy. It should be taken into account that, in women for whom testing was not requested in the first part of the first trimester, the sample intended for prenatal screening of chromosome diseases may be used to collect thyroid function data and to interpret them based on the specific reference values for weeks 11 to 13. In a geographical area with such significant rates of thyroid autoimmunity and postpartum thyroiditis, a future strategy could be the inclusion of thyroid antibody titers, in addition to TSH levels in first trimester screening tests.
Conflict of interestThe authors state that they have no conflicts of interest in relation to data reported.
This study could not have been possible without the collaboration of Abbott-Diagnostics, which provided all the necessary materials for measurements of TSH, FT4, FT3, and thyroid antibodies; our special thanks to Elena Romero (specialist in immunochemistry from Abbott Científica), whose contribution was indispensable in conducting this study.
Please cite this article as: Sastre-Marcos J, Val-Zaballos F, Ruiz-Ginés MÁ, Saura-Montalbán J, Veganzones-Pérez M. Valores de referencia y cribado universal de la función tiroidea en el primer trimestre de la población de mujeres gestantes del área de Toledo. Endocrinol Nutr. 2015;62:358–360.