Cushing syndrome (CS), due to an ACTH-secreting pituitary adenoma, adrenal tumors, or ectopic ACTH secretion, causes hypercortisolism. CS is associated with major morbidity, especially metabolic and cardiovascular complications, osteoporosis, psychiatric changes, and cognitive impairment. Despite biochemical “cure” of hypercortisolism and clinical improvement after effective treatment, these complications are only partially reversible. Exacerbation of prior autoimmune diseases is also seen. All of these lead to quality of life impairment and increased mortality. This review addresses the main comorbidities and long-term consequences of CS despite clinical and biochemical “cure”.
El síndrome de Cushing (SC), debido a un adenoma hipofisario productor de ACTH, a tumores suprarrenales o a una secreción ectópica de ACTH, determina hipercortisolismo. Se asocia a mayor morbilidad, especialmente complicaciones metabólicas, cardiovasculares, osteoporosis, alteraciones psiquiátricas y deterioro cognitivo. A pesar de la «curación» bioquímica del hipercortisolismo y la mejora clínica tras tratamiento eficaz, estas complicaciones solo son parcialmente reversibles, observándose también una exacerbación de enfermedades autoinmunitarias. Todo ello conlleva un deterioro de la calidad de vida y un aumento de la mortalidad. En esta revisión se repasan las principales comorbilidades y consecuencias del SC a largo plazo, a pesar de su curación clínica y bioquímica.
Endogenous Cushing's syndrome (CS) results from chronic exposure to high glucocorticoid levels. Cortisol is the end product of the stimulation of the hypothalamo–pituitary–adrenal (HPA) axis by the adrenal cortex in response to the action of adrenocorticotropic hormone (ACTH).
The most common cause of CS is excess ACTH release by a pituitary adenoma (Cushing's disease [CD]), although ectopic ACTH secretion by a neoplasm may also occur. On the other hand, the adrenal gland itself may secrete more cortisol due to one or several adenomas, bilateral adrenal hyperplasia, carcinoma and, more rarely, an adrenal micronodular dysplasia (in Carney's syndrome) or the presence of abnormal receptors that determine a bilateral macronodular hyperplasia independent of ACTH.1 In CS, the normal circadian rhythm of cortisol secretion, with maximum concentrations in the morning and minimum levels (virtually undetectable) at midnight, as well as physiological feedback of the HPA axis between cortisol, ACTH, and the hypothalamic peptide CRH, characteristically disappears.
Evidence accumulated in recent decades shows that complete remission of the associated clinical changes does not occur despite the correction of hypercortisolism after effective treatment of CS.2–4 Regardless of the cause, chronic cortisol hypersecretion causes central obesity, muscle atrophy and fatigue, osteopenia, high blood pressure, glucose intolerance, hyperlipidemia, hypercoagulability, mood changes, and depression, among other problems. This results in increased cardiovascular risk during the active phase of the disease, and very likely also in the long term. This has been increasingly apparent in recent years, so that comorbidities and complications of the active phase of CS, although clearly improving after surgery or medical treatment, partly persist after treatment, with negative consequences on the cardiovascular system, bone, brain, health-related quality of life (HRQL), and even cause increased mortality.2–4 Since adequate diagnosis is often delayed 2–5 years, hypercortisolism exerts its harmful effect for a long time before it is diagnosed and treated.5 The long-term follow-up of these patients is therefore mandatory to control complications due to prior chronic exposure to high cortisol levels.
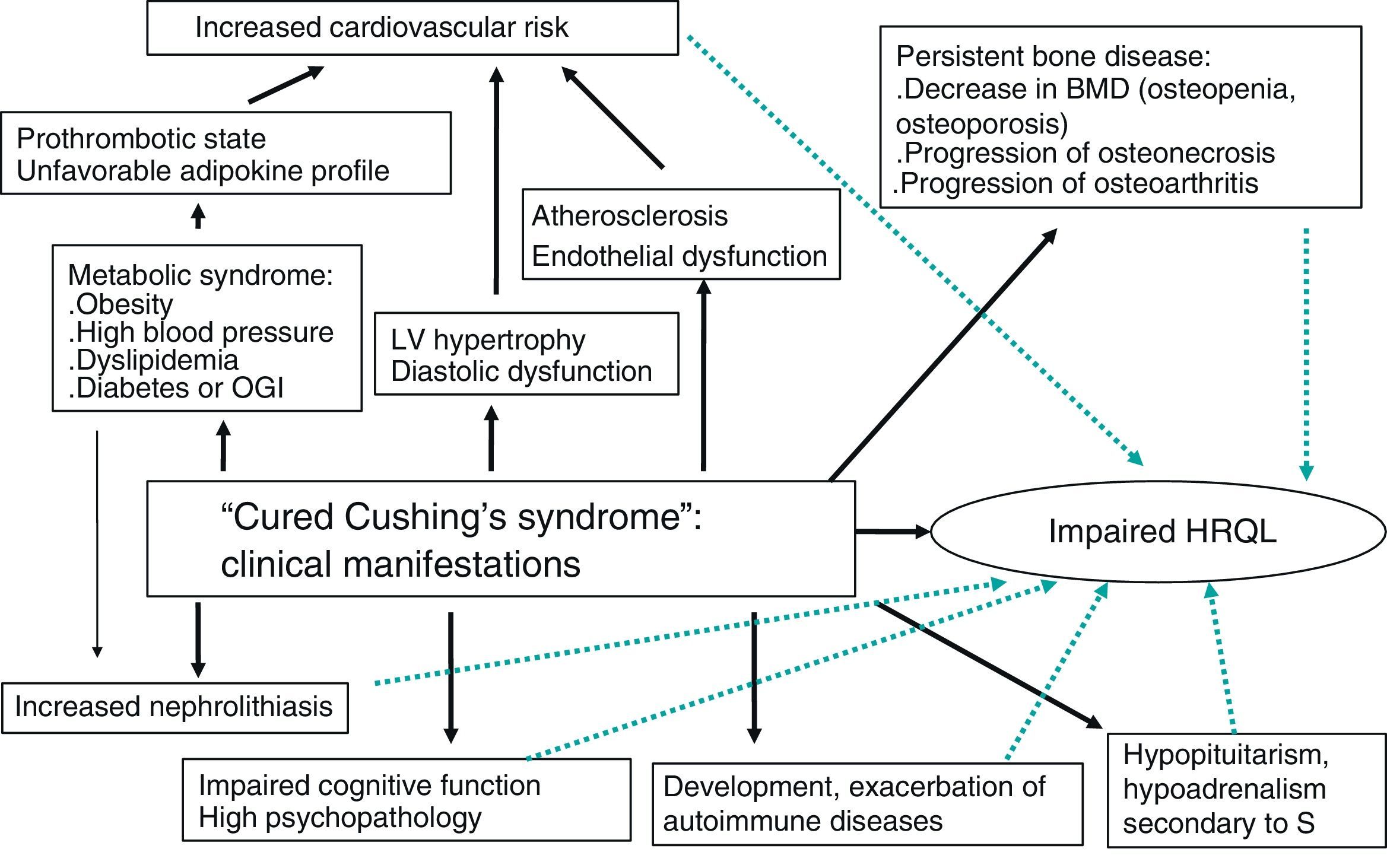
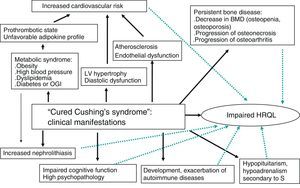
This review is focused on the prognosis and clinical consequences of CS in patients who have been “cured” after adequate treatment and on the impact of these comorbid conditions on HRQL and mortality (Fig. 1).
Cardiovascular systemHypercortisolism enhances cardiovascular risk factors such as central obesity, glucose intolerance, hypertension, dyslipidemia, and hypercoagulability (associated with metabolic syndrome), and is linked to an increased incidence of atherosclerosis. This has an impact on morbidity and mortality and causes heart disease to be the leading cause of death in patients with CS.
Such cardiovascular risk continues to be elevated even five years after the biochemical cure of hypercortisolim.6 A higher body mass index (BMI), waist/hip ratio, blood pressure, fasting blood glucose and insulin, and fibrinogen values were seen in these patients as compared to an age- and sex-matched control group, as well as an unfavorable lipid profile. An increased intima-media thickness and lower distensibility coefficient were also seen as compared to the control group even when matched by BMI. This confirmed the high prevalence of atherosclerosis and cardiovascular risk factors, similar to that seen in active disease, associated with residual abdominal obesity and/or insulin resistance. A study published earlier this year reported that despite the remission of hypercortisolism 11 years before on average, cardiovascular disease was more prevalent, particularly in women (42% CS vs 18% controls; p<0.05) and patients under 45 years of age. Even after excluding patients with hypopituitarism or dyslipidemia, patients under 45 years had a greater prevalence of coronary calcifications and/or non-calcified atheroma plaques as compared to matched healthy controls (30% CS vs 0% controls; p<0.01).7
In another study, a persistence of increased abdominal circumference was seen in patients with CS (irrespective of the cause) one year after hormonal remission.8 Body composition before surgery and on remission six months after glucocorticoid therapy was no longer required for treating inhibition of the HPA axis has recently been compared using whole body magnetic resonance imaging. Although a considerable part of the fat deposits had decreased, most patients continued to have overweight and obesity despite CS remission. Insulin and leptin levels had also decreased, while no improvement was seen in adiponectin or lipid parameters (HDL-C, LDL-C, triglycerides).9
A case-control study also showed that, after a mean of 11 years in remission, patients with endogenous CS continued to have greater total fat and central obesity as compared to age- and sex-matched controls.10
Increased central obesity and visceral fat is a phenotypic characteristic of CS and induces impaired adipokine production. These adipokines may contribute to the pathogenesis of vascular, metabolic, and inflammatory complications such as endothelial damage, high blood pressure, impaired bone remodeling, atherosclerosis, and low grade inflammation.11 Increased levels of leptin, resistin, and proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are associated with greater cardiovascular risk. In vitro studies have shown that leptin increases the activity of the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which converts inactive cortisone into cortisol.12 This and other adipokines and humoral factors may therefore further stimulate circulating cortisol levels, contributing to the typical characteristics of metabolic syndrome, visceral obesity, and hyperleptinemia.13 However, the finding of leptin elevation 10 days after surgery for CD despite a drop in cortisol suggests that factors other than cortisol, such as the persistence of abnormal fat distribution, play a role in leptin hypersecretion.14 In the long term, with remission of hypercortisolism, leptin gradually decreases in parallel to decreases in BMI, fat mass, and insulin.
Adiponectin has anti-atherogenic and anti-inflammatory activity, and is decreased in obesity and in the presence of insulin resistance. Lower adiponectin levels have been noted in patients with cured CS and in those cured 11 years before on average as compared to controls.10 TNF-α is a proinflammatory cytokine with regulatory effects on lipid metabolism, adipocyte function, and insulin signaling. TNF-α elevation has been associated with an increased risk of recurrent coronary acute ischemia. Increases in TNF-α and IL-6 have been seen in patients with active CS, but also years after cure of the condition.10 This unfavorable adipokine profile may contribute to this state of “low grade” inflammation found in patients cured of their CS, with the resultant persistent increase in cardiovascular risk.10,15 If concomitant growth hormone (GH) deficiency exists after pituitary surgery, cardiovascular risk and metabolic changes worsen even more.10 These metabolic parameters, adipokine levels, and abnormal body composition may improve after GH replacement therapy.16,17
Impaired glucose metabolism (insulin resistance, glucose intolerance, and diabetes mellitus) is another significant cardiovascular risk factor, and glucocorticoids promote its occurrence. The prevalence of these changes varies depending on the series. Thus, diabetes mellitus is found in 20–47% of patients, and glucose intolerance in 21–64%.6,18 Although their prevalence decreases with biochemical cure, they often persist, and higher insulin levels are found as compared to the control population.10
Moderate high blood pressure that persists despite the effective treatment of CS appears to be associated with a longer duration of hypertension in the active phase of hypercortisolism. Its pathogenesis appears to be multifactorial: inhibition of the vasodilating system, activation of the renin–angiotensin–aldosterone system, and inhibition of peripheral catecholamine catabolism.19 Moreover, increased cortisol levels may override the capacity of the enzyme 11β-HSD2 (which inactivates cortisol), facilitating cortisol binding to mineralocorticoid receptors, which results in an increased effect of aldosterone and myocardial fibrosis. Patients in remission of hypercortisolism with persistent high blood pressure have been found to have more structural and functional cardiac changes as compared to control hypertensive subjects. This suggests that prior exposure to hypercortisolism worsens the negative effects of high blood pressure.19
Several groups have similarly reported functional and structural cardiac lesions such as left ventricular hypertrophy, diastolic dysfunction, and decreased systolic performance in patients with active CS. Recently, a significant increase in myocardial fibrosis has been seen in patients with active CS as compared to healthy controls and controls with high blood pressure, which suggests that hypercortisolism may have a direct effect on myocardial fibrosis independent of left ventricular (LV) hypertrophy and high blood pressure. This fibrosis appears to be one of the most important factors for the development of cardiac dysfunction, and also one of the greatest determinants of the degree of regression of cardiomyopathy seen in CS.20 Eighteen months after successful treatment of CS, improvements were found in LV systolic and diastolic function in parallel to a reduction in myocardial fibrosis.
A study comparing 15 patients with CS and subclinical LV dysfunction to 30 controls matched by sex, ejection fraction, and hypertension showed that these abnormalities in LV structure and function were reversible at 18 months after the normalization of hyperthyroidism.21 A more recent study found that abnormalities in left ventricular mass parameters seen in 70% of patients with active CS substantially improved during a mean follow-up of four years after the remission of hypercortisolism, although they continued to be more marked as compared to controls.22 In 25 patients with CD, persistent metabolic syndrome, greater vascular damage, and the presence of a greater number of atherosclerotic plaques in the common carotid artery (31.2% vs 6.2%, respectively) were found one year after the remission of hypercortisolism.23
Thus, both excess cortisol and high blood pressure contribute to alter cardiac mass and increase the prevalence of damage in target organs. The importance of controlling high blood pressure and other cardiovascular risk factors before surgery to improve long-term prognosis should be emphasized.
Patients with CS have a greater risk of venous thrombosis (thromboembolism), especially in the postoperative period. Patients who undergo transsphenoidal surgery for CD have been found to have a greater risk of thromboembolism than those undergoing surgery for a non-functional pituitary adenoma, which suggests the role of cortisol (or ACTH) in changes in hemostatic factors.24 Patients with CS have been found to have higher levels of factor VIII, factor IV, and von Willebrand factor, as well as increased synthesis of the plasminogen activator inhibitor type 1 (PAI-1), the main inhibitor of the fibrinolytic system.25 This increased risk of thrombosis is probably enhanced not only by this hypercoagulability induced by hypercortisolism, but also by surgery itself and by the obesity of most of these patients. An improvement in hemostatic system parameters has been seen one year after successful surgery, but complete normalization of hemostasis does not occur.26 Some studies recommend the administration of thromboprophylaxis in the first month after surgery, but further studies are needed to assess the time required to reverse this hypercoagulable state after curative surgery.
It should be noted that both associated hormone deficiencies and replacement therapy, and in some cases incomplete CS cure, may be implicated in these cardiovascular complications. Specifically, patients with no hypocortisolism after transsphenoidal adenoma resection may have subclinical hypercortisolism similar to that seen in some adrenal adenomas (mainly incidentalomas) where this subclinical hypercortisolism is correlated to metabolic syndrome and increased cardiovascular risk.27–29
In conclusion, cardiovascular risk may continue to be high despite the biochemical control of hypercortisolism. The available evidence provides a rationale for the investigation and adequate treatment of cardiovascular disease. Although more extensive studies are needed to devise specific management strategies to decrease the negative cardiovascular impact of prior exposure to high cortisol levels, we think it is justified to treat these patients similarly to other patients with high cardiovascular risk, as is done in diabetes.
BoneThe prevalence of bone disease, mainly osteoporosis, is high and often underestimated in patients with CS.30 Approximately 30–50% of patients with CS experience fractures, particularly vertebral fractures.5 In addition to osteoporosis, osteoarthritis and osteonecrosis have also been reported in patients with iatrogenic CS, but rarely in patients with endogenous hypercorsitolism.31–33
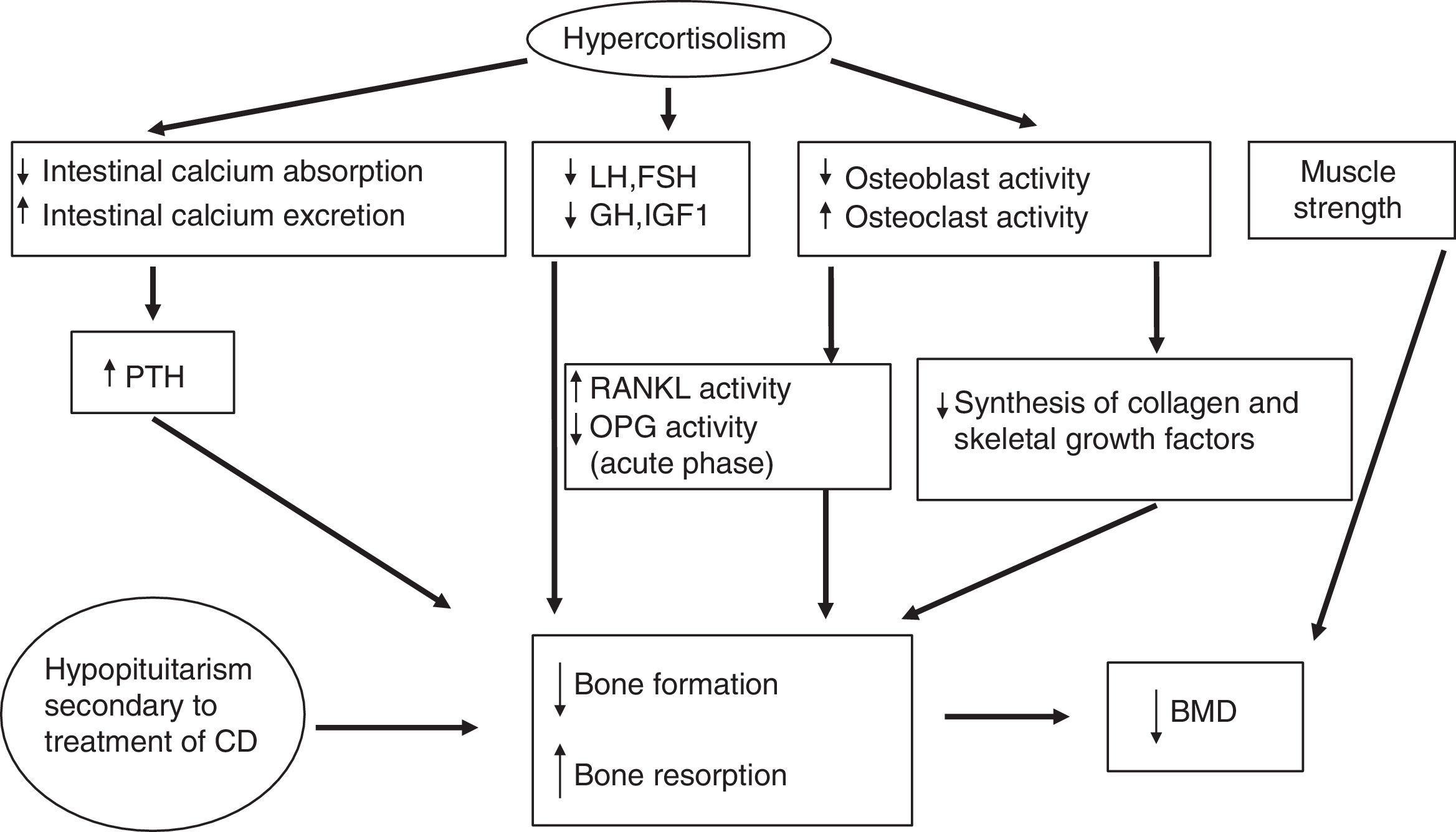
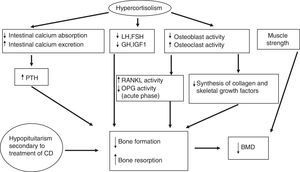
Glucocorticoids significantly affect bone metabolism, decreasing bone mineral density (BMD). They directly decrease collagen synthesis and increase collagen degradation because they inhibit osteoblast action, replication, and differentiation. Glucocorticoids also increase the action and survival of the osteoclasts responsible for the degradation.
Moreover, osteoblasts express 11β-HSD1 in vivo, which locally amplifies the effects of glucocorticoids. 11β-HSD1 therefore has significant implications for glucocorticoid action on bone. This loss of BMD may also partly be due to secondary hypogonadism and/or decreases in GH or insulin-like growth factor type I (IGF-I) levels induced by excess cortisol.
Glucocorticoids indirectly affect the metabolism of calcium, phosphate, vitamin D, and parathormone (PTH) (they decrease their metabolism by a mechanism independent of vitamin D and renal calcium reabsorption, which induces in both cases a modest increase in PTH levels). They also induce a loss of muscle strength and mass. The early effects of glucocorticoids at cell level include an increased production of RANKL (receptor activator of nuclear factor-kappa B-ligand, promoting osteoclastogenesis) and a decreased production of osteoprotegerin (OPG) (a natural antagonist of RANKL),34 although higher OPG levels have been found in patients with chronic endogenous hypercortisolism as compared to controls. OPG levels remain high after the normalization of hypercortisolism, especially in patients with a high coronary risk.35,36 The RANKL/OPG system acts as a paracrine regulator of vascular calcification, because it is also produced in endothelial cells, and may be a marker of subclinical atherosclerosis.37 These elevated OPG levels in CS could therefore probably be more related to cardiovascular risk than to bone status. The pathophysiology of bone disease in CS is detailed in Fig. 2.
Pathogenesis of bone disease in CD. BMD: bone mineral density; CD: Cushing's disease; FSH: follicle-stimulating hormone; GH: growth hormone; IGF-1: insulin-like growth factor type 1; LH: luteinizing hormone; OPG: osteoprotegerin; PTH: parathyroid hormone; RANKL: receptor activator of nuclear factor-kappa B-ligand.
This bone mass loss induced by excess cortisol has been seen to be more prominent in trabecular bone, present in the lumbar spine or the femoral neck.38 In fact, patients with CS have a special predisposition to experience vertebral fractures together with abdominal pain or height decrease due to vertebral compression. Not uncommonly, inappropriately high BMD values are found in the spine.
Most studies report a partial recovery of BMD after treatment for CS, although the series are small and median follow-up is relatively short (no longer than two years). In the series with the longest follow-up after remission of hypercortisolism (mean follow-up of 11 years), decreased BMD values were seen in estrogen-sufficient women as compared to age-, sex- and BMI-matched controls, but not in women with estrogen deficiency (due to menopause or hypogonadism). This suggests that the protective effect of estrogens on bone mass is lost with hypercortisolism. Prior exposure time to excess endogenous cortisol and the duration of postoperative glucocorticoid replacement therapy were predictors of low BMD.39 Persistently decreased osteocalcin levels after the remission of hypercortisolism also suggest decreased osteoblastic activity, which promotes the non-complete recovery of BMD.
Hypercortisolism has also been seen to be able to slow the normal bone mass peak in growing patients, contributing to an increase in osteoporotic fractures, even in the long term, after remission in young people “cured” of CS.40 Additional treatments are probably needed to try and maximize bone mass peak in patients with CS in childhood or adolescence in order to minimize the long-term side effects.
There is other evidence which suggests that bone mass changes may be reversible after the remission of hypercortisolism. This was probably partly due to the fact that prior exposure time to endogenous hypercortisolism was shorter than in other studies.38,41 A long-term prospective study reported that BMD was even more increased after the remission of hypercortisolism as compared to the baseline state of the active disease, and that this improvement was maintained over the years of remission of hypercortisolism (mean follow-up of 71 months).38 BMD recovery positively correlated to increases in levels of osteocalcin and C-terminal telopeptide of collagen type 1 (CTX-1), bone turnover markers (r=0.92; p<0.001). The mechanisms causing BMD recovery are speculative. Some authors attribute it to an increase in osteocalcin levels when glucocorticoids are normalized and to the preservation of trabecular architecture, despite the thinning induced by corticoids, so that osteoblasts may continue synthesizing new bone. This does not occur in the loss of trabecular bone due to other causes of osteoporosis.42
To sum up, BMD recovery appears to be only partial in most patients with “cured” CS. Particularly in young patients who have not yet completed their growth, high cortisol levels have a very negative impact on bone. Large scale studies assessing the long-term occurrence of fractures in patients diagnosed with CS are lacking.
KidneyNephrolithiasis has been reported in half the patients with active CS and in almost 30% of cured patients, a much higher prevalence as compared to the general population.43 The pathogenesis of nephrolithiasis in CS has not been fully elucidated. It may partly result from hypercalciuria. There is probably a synergistic effect of different metabolic and hemodynamic changes caused by hypercortisolism. In fact, higher rates of obesity, high blood pressure, and diabetes mellitus, all very common conditions in CS, have been seen in patients with kidney stones. Increased urinary excretion of uric acid and cystine is an additional factor promoting nephrolithiasis, and may be the consequence of excess glucocorticoids.44 In a large series investigating the role of the different known lithogenic factors in patients with hypercortisolism, high blood pressure and excess urinary excretion of uric acid were found to be independent risk factors for the occurrence of nephrolithiasis.43
Cognitive function and behaviorChronic hypercortisolism has been related to changes in memory, behavior, verbal learning and speech, neuronal activity, and other processes of the central nervous system. Moreover, psychopathological states such as anxiety, depression, and mania are very prevalent in patients with active CS. Major depression is the most common disorder, with a prevalence ranging from 54% to 81% depending on the series.45
Although few reports assessing psychopathology after effective surgery are available in the literature, most changes improve one year after the remission of hypercortisolism, although they do not appear to be fully reversible in the long term.46,47
Hippocampus, amygdala, and brain cortex, important structures involved in cognitive and emotional function, are very rich in glucocorticoid receptors. These are therefore regions particularly vulnerable to excess cortisol. The pathogenesis of brain volume loss induced by high glucocorticoid levels is probably due to multiple factors, including cell death induced by glucocorticoids, interference with neuronal transmission and metabolism processes, and decreased brain water contents.2 Glucocorticoids may occupy both mineralocorticoid and glucocorticoid receptors. 11β-HSD2 (which converts cortisol into an inactive cortisone molecule) is not expressed in the hippocampus or other limbic structures, which allows for the sustained activation of mineralocorticoid receptors by glucocorticoids. Under conditions of supraphysiological glucocorticoid levels (in which both receptors are occupied), decreased cell excitability and reversible atrophy of apical dendrites of pyramidal neurons occur. If hypercortisolism persists, however, as occurs in endogenous hypercortisolism, cell death may occur.46
Studies with high-field (3Tesla) cerebral MRI have found greater brain atrophy as compared to normal controls of the same age, which is not fully reversible despite cortisol normalization.48 Decreased hippocampal volume was seen in 27% of patients with active CS as compared to controls, with a negative correlation to plasma cortisol levels. This decreased hippocampal volume was associated with memory dysfunctions. Interestingly, hippocampal volume improved one year after surgery as compared to baseline, and some of these patients showed improvements in cognitive function tests.49 On the other hand, glucocorticoids increase glutamate accumulation into synaptic vesicles, conferring increased susceptibility to aggression and cell death. Since dexamethasone is very potent for the treatment of cerebral edema, this volume loss in CS could partly be secondary to a decrease in brain tissue water contents due to excess glucocorticoids.46
Recently, poorer results were found in terms of memory and executive function in a sample of 74 patients with CD followed up for a mean of 13 years after remission as compared to controls matched by age, sex, and educational level. They also showed poorer performance than patients undergoing surgery for non-functioning pituitary adenoma. Results improved the longer the time since the remission of hypercortisolism. Hypopituitarism and hydrocortisone replacement therapy were independent predictors of poorer results in cognitive tests.50 Another recent study similarly found impairments of visual and verbal memory and brain atrophy (decreased volume of total and cortical grey matter) in patients with CS in remission as compared to controls. The patient subgroup with the poorest results in memory tests also had a significant reduction in hippocampal volume.48
Excess cortisol has been associated with changes in some neurotransmitters, such as decreased cerebral serotonin synthesis, increased noradrenergic activity, and low levels of 5-hydroxyindoleacetic acid in cerebrospinal fluid, all of them related to the pathogenesis of depression.51 Twelve months after the correction of hypercortisolism, persistent psychopathology (mainly atypical depression) was seen in 24% of patients, although psychopathology was found in 66% of cases during the active phase of the disease. Panic and suicidal ideation increased during follow-up. Adaptive personality disorders have also been reported after treatment for CS, but not in all series.52
All the foregoing suggests that prior and chronic exposure to high glucocorticoid levels and a persistence of changes in the HPA axis after the restoration of eucortisolism may increase individual vulnerability to stressors.53 However, further studies with larger patient samples are required to reach definitive conclusions, because most series included a limited number of patients with heterogeneous clinical characteristics.
It may be concluded that CS is associated with a high prevalence of psychopathology, mainly atypical depression. Prior and chronic exposure to excessively high cortisol levels may have irreversible effects on central nervous system structures (mainly in the areas of cognitive function and behavior).
Autoimmune diseasesGlucocorticoids have an inhibitory action on the immune system. In fact, lymphoid tissue involution and lymphopenia with increased susceptibility to infection occurs in the active phase of CS.
The contrary situation has been reported after the remission of hypercortisolism, where the exacerbation of previously existing autoimmune diseases was seen. Celiac disease, rheumatoid arthritis, the development of sarcoidosis, or lupus erythematosus have been reported in different forms of CS after the correction of hypercortisolism.54 However, the most commonly reported autoimmune disease is autoimmune thyroiditis (Graves’ disease or Hashimoto's thyroiditis). Thyroid autoimmunity was found in 35% of the patients “cured” of CD as compared to 10% of controls. Autoimmune disease appears to occur more frequently in patients with multinodular goiter or positive anti-thyroid antibodies during the active phase of the disease, suggesting that pre-existent thyroid abnormalities and genetic predisposition to autoimmunity are factors for the future development of autoimmune thyroid disorders after cortisol normalization.55
The exacerbation of autoimmune diseases appears to be related to an improvement in immune activity, suppressed by endogenous hypercortisolism during the active phase of the disease. It should therefore be borne in mind that an immune disease which is silent during the active phase of CS may “reappear” after CS remission.
Health-related quality of lifeQuality of life of patients with CS is decreased in active disease as compared to healthy controls and to patients with other pituitary adenomas without hypercortisolism. Although quality of life improves after biochemical remission, it does not completely normalize, even in the long term.
A recent review of the epidemiology, treatment, and consequences of CD reported that HRQL is compromised despite disease remission.4 In another study of 58 patients cured of CS with a mean remission time of 13 years, generic questionnaires revealed poorer HRQL as compared to the control group in terms of fatigue, physical aspects, anxiety, depression, and perception of well-being, especially in patients with associated hypopituitarism.56,57 No relationship was found with the initial grade of hypercortisolism.56
An HRQL questionnaire specific for CS (CushingQoL) including the aspects causing most concern to patients with CS has recently been developed. The questionnaire refers to the four previous months. In 125 patients with CS, some of them with active disease and others in remission or with adrenal insufficiency secondary to treatment, active hypercortisolism (with elevated urinary cortisol) and female sex were seen to be the greatest predictors of low HRQL. No relationship was seen between HRQL and the time since curative surgery or the presence of hypopituitarism, which suggests that the dimensions assessed with CushingQoL are probably more related to hypercortisolism than to the other hormone changes. These changes were independent of CS origin/adrenal or pituitary).58 The CushingQoL questionnaire has recently been confirmed to have good psychometric properties and sensitivity to change for detecting changes in daily clinical practice.59
In patients with GH deficiency on replacement therapy with recombinant human GH and CD in remission, improvement has been seen not only in metabolic parameters, but also in HRQL, three years after starting treatment with GH.17 HRQL impairment is greater in patients with CD and ÇGH deficiency than in those with GH deficiency for other reasons. This suggests that prior exposure to hypercortisolism has a greater impact on HRQL than other hormonal dysfunctions.60
HRQL impairment, even years after treatment, has social and economic consequences. Interviews with 74 patients with treated CS revealed that only 46% felt fully recovered; 81% could return to work, but 11% were granted permanent disability.61
In conclusion, despite the reversibility of hypercortisolism, the quality of life remains impaired in patients treated for CS, with persistent physical, psychological, metabolic, and cognitive changes.
MortalityCS is a life-threatening disease. Untreated CD is associated with an estimated five-year survival of 50%.62 Several studies have reported a two- to five-fold higher mortality as compared to the reference population, mainly due to cardiovascular causes.16,63,64 A recently published meta-analysis of mortality in CS found a mean mortality rate from CD (standard mortality rate [SMR]) of 1.84 (95% CI: 1.28–2.65). In patients with persistent CD after surgery, SMR is 3.73 (95% CI: 2.31–6.01), while the mortality rate of patients with complete remission after surgery does not differ from that of the general population (SMR: 1.23; 95% CI: 0.51–2.97).64 Mortality especially increases in the first postoperative year in patients with no initial cure after surgery, and in general the longer the exposure time to hypercortisolism.65 These results have been confirmed by the largest surgical series published to date, reporting 31 deaths in 285 patients with CD during a mean follow-up of 11.1 years. As compared to the normal population, patients with persistent disease despite treatment die more frequently. In addition, survival decreases over the years in patients with active disease. This suggests that an attempt should be made to correct hypercortisolism as soon as possible to prevent comorbidities and decrease mortality.66
Another recent meta-analysis assessing only patients with CD over 50 years of age found a two-fold greater overall mortality as compared to the general population (SMR: 2.2; 95% CI: 1.45–3.41) in both patients with active disease and in remission. No differences in mortality were found in patients in remission (SMR: 1.2; 95% CI: 0.45–3.2) compared to the general population, but differences were seen in patients with active disease (SMR: 5.5; 95% CI: 2.7–11.3). Thirteen deaths, nine of them for cardiovascular causes, were seen in a cohort of 60 patients followed up for a mean of 15 years. This represents a higher mortality rate than expected in the general population (SMR 13.8; 95% CI: 7.2–36.5). Active disease, older age at diagnosis, high blood pressure, and the coexistence of diabetes were the greatest determinants of mortality in this series.67
These mortality rates have markedly decreased since the introduction of transsphenoidal surgery. However, cortisol normalization may not be sufficient to normalize mortality, as there may be other coexistent risk factors such as hypoparathyroidism, surgery itself, or the persistence of cardiovascular risk factors.
Age at diagnosis and during follow-up of most patients recruited into the studies ranged from 40 to 60 years. In addition, older age at diagnosis was related to a poorer prognosis.67 It would therefore appear advisable to prolong the follow-up of such patients beyond 30 years to confirm whether the remission of hypercortisolism is consistent with the longevity of the general population. Most of these studies were conducted on only a few patients (due to the rarity of CS) with a low number of deaths and variable follow-up time, and the results should therefore be interpreted with caution, but should lead to reflection on the apparently poorer prognosis of patients with cured CS.
To sum up, the mortality rate in patients with “cured” CD may be similar to that of the general population, as least after 10–20 years of follow-up. There is however recent evidence showing persistently higher cardiovascular risk in patients apparently “cured” of CS. It appears therefore advisable to treat them as high-risk patients, implementing prophylactic measures, as is done in diabetes mellitus.
ConclusionsPersistent hypercortisolism in CS is associated with a high number of metabolic, cardiovascular, and cognitive complications, only partially reversible after the remission of excess cortisol. This results in quality of life impairment despite years of remission.
Chronic long-term monitoring is necessary to control comorbidities and to clarify whether the persistence of high metabolic and cardiovascular risk has an impact on the survival of these patients diagnosed with CS.
Because of the rarity of CS, it would be worthwhile to conduct comprehensive, multicenter epidemiological studies that allow us to ascertain the causes of death and morbidity in these patients, or prospective studies such as the one conducted by the European Register on Cushing's Syndrome (ERCUSYN), which currently includes more than 800 patients. This will be the only way to achieve a complete understanding of the long-term prognosis and the final outcome of these patients.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Aulinas A, Valassi E, Webb SM. Pronóstico del paciente tratado de síndrome de Cushing. Endocrinol Nutr. 2014;61:52–61.