Results of studies on the prevalence of distal diabetic polyneuropathy (DPN) are contradictory. Conventional methods used for the diagnosis of DPN in clinical practice have limited effectiveness. The present study aimed to assess the prevalence of DPN in a population with long-standing diabetes (more than 10 years disease duration) by measuring vibratory, thermal and tactile sensitivities with quantitative sensory devices, as well as their relationship with associated clinical risk factors.
Patients and methodsA total of 1011 diabetic patients were evaluated in a multicenter, cross-sectional, observational study. The three sensitivities were assessed by ultrabiothesiometer, aesthesiometer and thermoskin devices, respectively. The prevalence of neuropathic pain was validated by the DN4 questionnaire.
ResultsOf the 1011 cases included, 400 (39.6%) met the diagnostic criteria of DPN, while no DPN was found in the remaining 611 (60.4%). Of the 400 patients with DPN, 253 (63.2%) showed clinical manifestations, while 147 (36.8%) were diagnosed as subclinical DPN. The prevalence of DPN increased with disease duration. There was a progressive loss of the three sensitivities with increased disease duration, particularly thermal and vibratory sensitivities. This loss was statistically significant for the latter two sensitivities. Among patients with clinical DPN, 84.2% had painful neuropathic symptoms. The prevalence of DPN was positively related to micro- and macroangiopathic complications and with dyslipidemia.
ConclusionThis study reveals a high degree of underdiagnosis of DPN, most likely due to the asymptomatic nature of the disease in a considerable proportion of patients. Our observations provide evidence of the usefulness of specific equipment for quantitative and objective assessment of polyneuropathy.
Los resultados de los estudios sobre la prevalencia de la polineuropatía distal diabética (DPN) son discrepantes. Los métodos convencionales para su diagnóstico tienen una eficacia limitada. Por ello, el presente trabajo pretende estudiar su prevalencia en una población diabética con más de 10 años de evolución de la enfermedad, valorando las sensibilidades vibratoria, térmica y táctil con dispositivos que cuantifican el grado de sensibilidad, a la vez que su relación con los factores de riesgo asociados.
Pacientes y métodosSe evaluaron 1.011 diabéticos en un estudio multicéntrico, transversal y observacional. Se valoraron las tres sensibilidades con un ultrabiotesiómetro, un estesiómetro y un termoskin. Se validó la prevalencia de dolor neuropático con el cuestionario DN4.
ResultadosDel total de 1011 casos, 400 (39,6%) cumplían criterios de DPN, mientras que los 611 restantes (60,4%) no los cumplían. De los 400 enfermos con DPN, 253 (63,2%) presentaban manifestaciones clínicas, mientras que los 147 restantes (36,8%) fueron diagnosticados de DPN subclínica. La prevalencia de DPN aumentaba al avanzar la enfermedad. Había una pérdida progresiva de las tres sensibilidades con el tiempo, sobre todo de la térmica y táctil, cuya pérdida era estadísticamente significativa. Un 84,2% de los casos con DPN clínica aquejaban dolor neuropático. La prevalencia de DPN guardaba una relación positiva con las complicaciones micro y macroangiopáticas, y con la dislipidemia.
ConclusionesEl presente estudio revela que hay un alto porcentaje de DPN sin diagnosticar, lo más probable por la ausencia de síntomas en buena parte de los pacientes. Los resultados muestran la utilidad de dispositivos específicos que valoren de manera objetiva y cuantitativa la presencia de polineuropatía.
Distal symmetric diabetic polyneuropathy (DPN) is one of the complications of diabetes. It is the most common presentation of diabetic neuropathy and it presents an insidious and progressive course, resulting in high morbimortality with a negative impact on the patient's quality of life and high social and health care costs.1,2 Epidemiological studies of its prevalence have yielded very different results: from 22.7% to 54%.3–7 This discrepancy may be due to various causes, such as heterogeneity of studied diabetic populations and differences in the diagnostic criteria and in methodologies used in the assessment. While neuropathic clinical symptoms have only a limited value for DPN screening, due to their own intrinsically subjective component, the diagnostic criteria recommended by the San Antonio Conference and other authors8,9 are not always taken in routine practice. On the other hand, the nerve conduction velocity study, despite being the most determinant and reliable test for detecting DPN,10 is not a widely available technique and requires specialized personnel and too much time to perform. Therefore, it is not practical for screening DPN in the clinical routine.
Quantitative sensitive methods allow for the precise determination of the perception thresholds of various sensitivities. They have also proved useful in detecting subclinical DPN and in assessing its severity and progression.11,12 Some studies have used the vibration perception threshold, measured by biothesiometer, as the reference parameter for the assessment of DPN presence.13,14 Other sensitivities such as thermal and tactile sensitivities have scarcely been studied with these quantitative procedures,12 thus limiting our knowledge on the actual prevalence of DPN.
Faced with the above mentioned diagnostic difficulties, as well as the limited epidemiological data available based on objective and quantitative methods, and the less well known associated risk factors for DPN, the present work aims to study this prevalence in a diabetic population with more than 10 years of evolution since the diagnosis of diabetes, using a standardized and homogeneous quantitative methodology, objective and measurable, regarding vibratory, thermal and tactile sensitivities, as well as their relationship with other micro- and macroangiopathic complications and with other associated clinical risk factors . As a result we will be better able to determine the actual prevalence of DPN, which is probably higher than is commonly thought, due to the presence of underdiagnosed subclinical neuropathies.
Subjects and methodsSubjectsOne thousand one hundred and fifty-nine ambulatory patients suffering from diabetes mellitus were studied. They were recruited from hospital outpatient clinics in 20 endocrinology units in Spain, during a 6-month period in 2007. These patients met the following inclusion criteria: age ranging from 16 to 70, diagnosis of type 1 or type 2 diabetes mellitus according to the American Diabetes Association (ADA) recommendations, more than 10 years of disease duration, absence of any known non-diabetic cause of neuropathy and, lastly, the ability to access their clinical history and fill in the questionnaires with no cognitive damage or psychiatric pathology. Clinical data from each subject were obtained from the clinical records by local investigators at each center.
This was a multicentric, cross-sectional and observational study requiring a detailed clinical history from each patient containing anthropometrical data, physical examination, routine habits, pharmacological treatments, appropriate vascular examinations, presence of neuropathic clinical symptoms and data on blood tests. All patients also filled out a DN4 (Douleur Neuropatique) questionnaire, assessing the presence of neuropathic pain.
Sensitivities evaluated- 1.
Vibratory sensitivity using an ultrabiothesiometer (Meteda, Italy), assessed with the voltage necessary to make the patient perceive the vibration. To accomplish this, increasing voltages were applied at three sites of both feet: the head of the first toe metatarsal bone, and the external and the internal malleolus. Threshold for normality was established at 25V ranging from 5 to 35V and having a 100Hz frequency calibration.
- 2.
Tactile sensitivity through the application of a series of Von Frey Aesthesiometer monofilaments (Somedic, Switzerland), which produce increasing nominal pressure and force. Tactile sensitivity threshold was obtained by means of the force, in grams, needed to be applied on the back of the first toes of both feet for the patient to perceive the pressure. Normal threshold was established at a nominal force of 1.1g and a pressure of 14.1g/mm2.
- 3.
Thermal sensitivity, using thermoskin equipment (Meteda, Italy), which distinguishes between two types of thermal discrimination when applied on the skin of the back of the first toe of both feet:
- •
Qualitative, which allows for differentiation between heat (40°C) and cold (25°C).
- •
Quantitative, which allows for the differentiation between progressive increases and decreases of ±1°C based on the previously measured skin surface temperature. Normal cut-off point was established as the ability to discriminate ±4°C. This test was not performed if the patient had not previously been able to differentiate between heat and cold through qualitative discrimination.
- •
In order to validate the objectivity and homogenization of these sensitive examinations, all the investigators participating in the study were previously trained on how to manage the equipment by the appropriate technical staff of the company providing such equipment (Novalab).
Of the initial 1159 subjects in the study, 148 were excluded due to the lack of compliance with any one of the conditions required in this study, leaving a final total of 1011 subjects: 52% with type 1 and 48% with type 2 diabetes mellitus.
Diagnostic criteria of DPNIn order to meet the diagnosis of DPN, the patient had to fulfill at least two of the following five criteria:
1. presence of DPN clinical symptoms in his/her clinical history and in the DN4 questionnaire,
2–4. alteration of the established normal threshold for vibratory or tactile or thermal sensitivity,
5. decrease in peripheral nerve conduction velocity by means of an electrophysiological study.
DPN was considered to be subclinical when no other neuropathic clinical symptoms were present in the clinical history and in the DN4 questionnaire.
Statistical analysisDescriptive statistics were used for all the parameters obtained with respect to statistical analysis. These calculations included the measurement of the central tendency and dispersion for the quantitative variables, and relative and absolute frequencies for the qualitative variables. In both cases, 95% confidence intervals were used. In order to compare independent data, Student's t test was used for the quantitative variables, while quantitative variables that followed a non-Gaussian distribution were assessed through the Mann–Whitney U test. Chi-square test or Fisher's exact test were used for qualitative variables. Statistical tests were performed with a 5% significance level and were bilateral. All the studies were performed using the SAS statistical pack, version 8.2.
This study was governed by the basic ethical principles contained in the Declaration of Helsinki and approved by the Ethical Committee of the Hospital. All the patients participating in the study had previously and voluntarily signed an informed consent.
ResultsPrevalence of clinical and subclinical DPNThe average age of patients was 49.7±14.8 (SD) years and the average duration of diabetes was 19.6±8.2 (SD) years. From the total of 1011 cases included, 400 met the diagnostic criteria of DPN, while the other 611 had no DPN, which represents 39.6% and 60.4%, respectively. Of the 400 patients with DPN, 253 (63.2%) presented clinical manifestations, while 147 (36.8%) were diagnosed with subclinical DPN as they presented no symptoms.
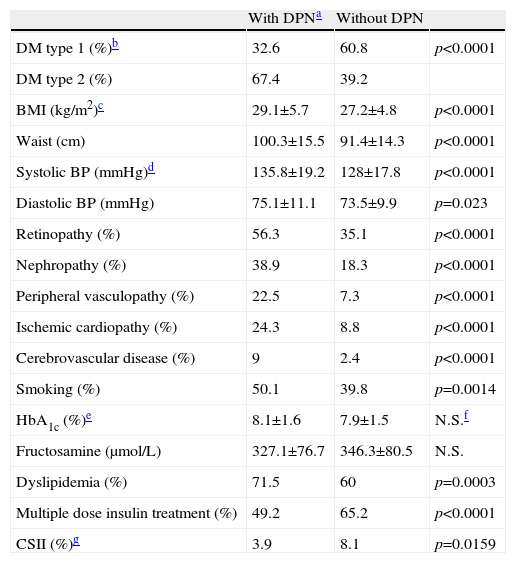
Variables associated with DPNWhen comparing the group of DPN patients (clinical+subclinical) with the group of patients with no DPN, our results showed the following statistically significant differences, displayed in Table 1:
- •
DPN was more common in type 2 than in type 1 diabetic patients. It was also more common in obese patients, as suggested by the results of the body mass index and waist diameter.
- •
DPN prevalence had a positive relationship with the presence of diabetic nephropathy and retinopathy, peripheral vasculopathy, ischemic cardiopathy, cerebrovascular disease, arterial hypertension and dyslipidemia.
- •
The levels of glycated hemoglobin (HbA1c) in blood and fructosamine in plasma showed no association with the existence of DPN, when the observational study was performed. However, its prevalence was lower in patients treated with intensive insulin therapy, both with multiple doses or with a subcutaneous continuous infusion pump.
- •
DPN prevalence was also higher in patients who smoked than in non-smokers.
Results in prevalence percentages (%) or in average ∀SD with their statistical significance.
| With DPNa | Without DPN | ||
| DM type 1 (%)b | 32.6 | 60.8 | p<0.0001 |
| DM type 2 (%) | 67.4 | 39.2 | |
| BMI (kg/m2)c | 29.1±5.7 | 27.2±4.8 | p<0.0001 |
| Waist (cm) | 100.3±15.5 | 91.4±14.3 | p<0.0001 |
| Systolic BP (mmHg)d | 135.8±19.2 | 128±17.8 | p<0.0001 |
| Diastolic BP (mmHg) | 75.1±11.1 | 73.5±9.9 | p=0.023 |
| Retinopathy (%) | 56.3 | 35.1 | p<0.0001 |
| Nephropathy (%) | 38.9 | 18.3 | p<0.0001 |
| Peripheral vasculopathy (%) | 22.5 | 7.3 | p<0.0001 |
| Ischemic cardiopathy (%) | 24.3 | 8.8 | p<0.0001 |
| Cerebrovascular disease (%) | 9 | 2.4 | p<0.0001 |
| Smoking (%) | 50.1 | 39.8 | p=0.0014 |
| HbA1c (%)e | 8.1±1.6 | 7.9±1.5 | N.S.f |
| Fructosamine (μmol/L) | 327.1±76.7 | 346.3±80.5 | N.S. |
| Dyslipidemia (%) | 71.5 | 60 | p=0.0003 |
| Multiple dose insulin treatment (%) | 49.2 | 65.2 | p<0.0001 |
| CSII (%)g | 3.9 | 8.1 | p=0.0159 |
Other results not included in Table 1 are as follows:
- •
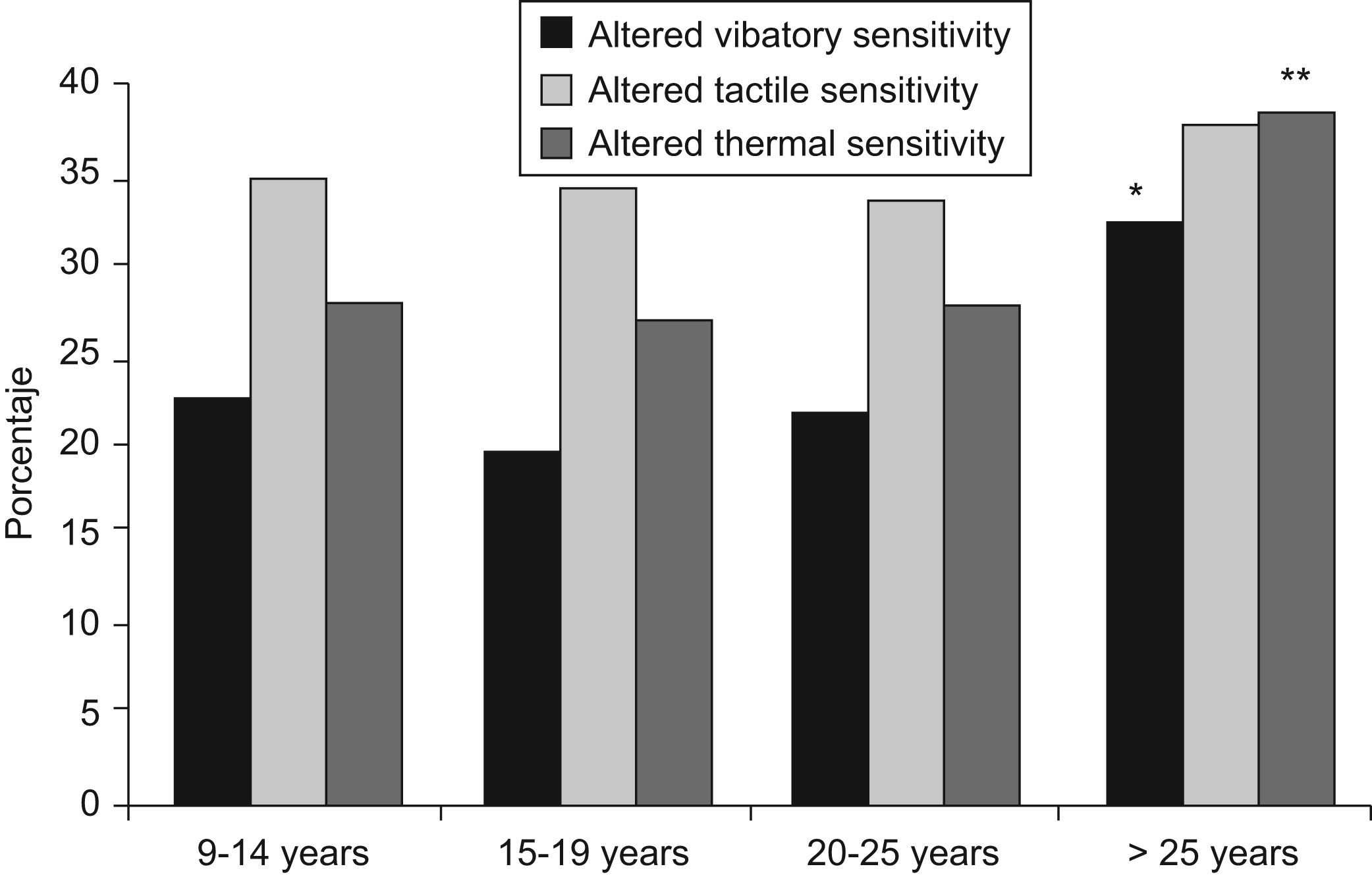
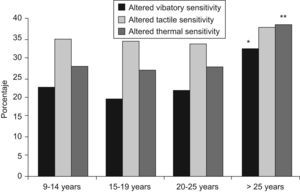
DPN prevalence increased with disease duration, both in type 1 and type 2 diabetic patients, especially when the known duration was longer than 25 years, with 44.1% of the patients with type 1 diabetes presenting DPN and 68.6% of those with type 2 diabetes (p<0.03). In general, the prevalence of DPN increased from 37.4% after 10–14 years of evolution to 52.1% after more than 25 years (p=0.0003). Figure 1 shows the progressive loss of the three sensitivities as the duration of the disease increased, particularly the thermal and vibratory ones. This reached statistical significance for a duration of diabetes longer than 25 years (p=0.0169 and 0.0046, respectively). Although tactile sensitivity loss also increased after this period, it was not statistically significant (p=0.8054).
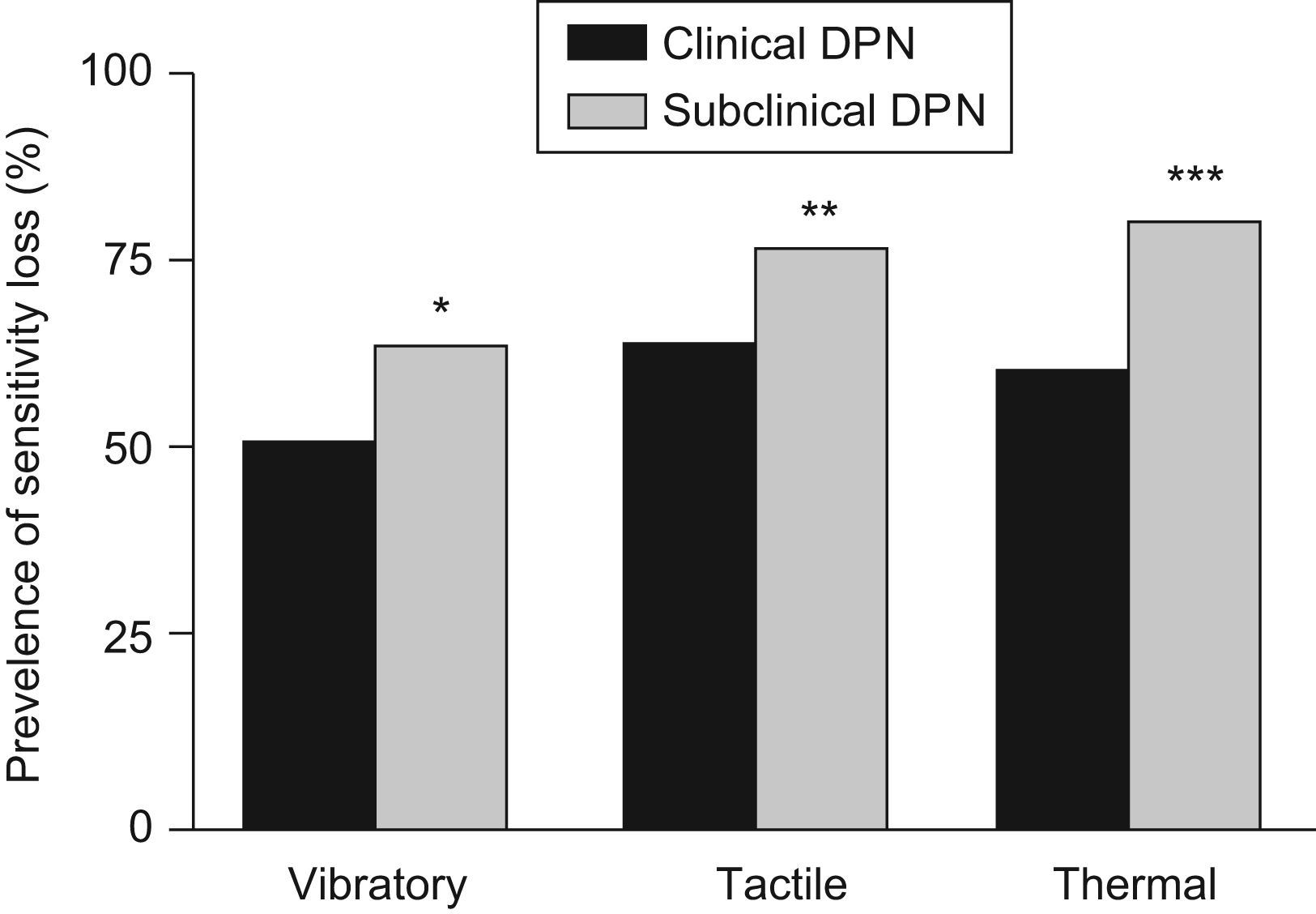
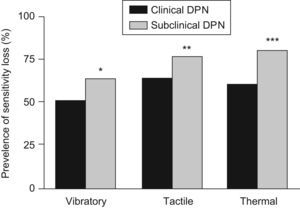
Subclinical DPN was more common in men than in women (73.2% vs. 26.8%; p<0.0001), although no gender differences were shown for clinical DPN. The anthropometrical, clinical and analytical data of both groups presented no differences, with the exception of plasma triglycerides levels, which were higher in the clinical DPN patient group (148.5±116.1 vs. 125.2±82.6(SD)mg/dL; p=0.0224). Regarding the different sensitivities, the subclinical DPN patient group presented a higher prevalence of vibratory (64.1% vs. 50.6%; p=0.0092), tactile (76.9% vs. 63.6%; p=0.0059) and thermal (79.6% vs. 60.1%; p<0.0001) sensitivity losses (Figure 2).
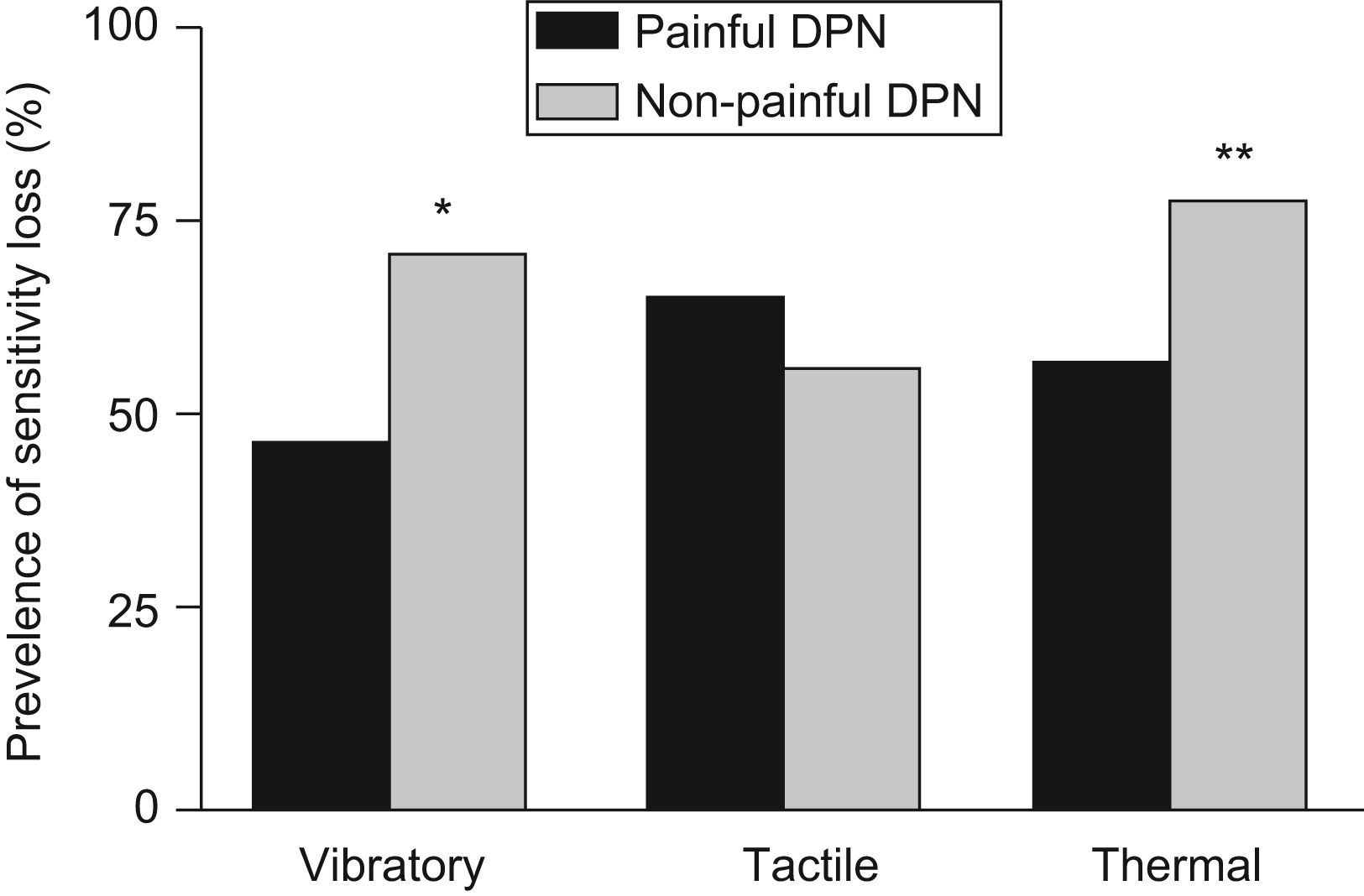
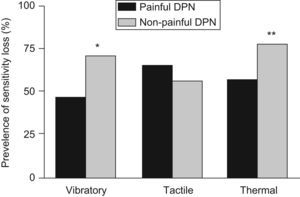
Of the 253 clinical DPN patients, 213 (84.2%) presented with painful neuropathic symptoms in the DN4 questionnaire. The other 40 cases (13.8%) did not complain of pain, independently of the presence of other neuropathic symptoms. Vibratory sensitivity loss was more prevalent (71.1% vs. 46.9%; p=0.0061) in the patient group with non-painful DPN than in those who suffered pain. This was also applicable to the thermal sensitivity loss (77.5% vs. 56.8%; p=0.0142). No statistically significant differences were found with respect to tactile sensitivity assessment (56.4% vs. 64.9%), duration of diabetes, or the other anthropometrical, clinical and analytical parameters. Figure 3 shows the prevalence of loss for each of the three sensitivities in both groups.
DiscussionAlthough the ADA recommendations15 support the use of simple clinical methods for DPN screening—preferably Semmes–Weinstein monofilaments 10g (1.0g monofilament provides superior diagnostic sensitivity) and 128Hz tuning fork—these methods have certain limitations: considerable inter- and intraanalysis variability, subjective interference, lack of universal agreement in the assessment of outcomes and sensitivity far inferior to the diagnostic standard, which is nerve conduction velocity. Therefore, these simple tests are not sufficient to establish a diagnosis of DPN as an isolated criterion alone and patients with polyneuropathy might go unnoticed. At least another diagnostic information is thus needed apart from nerve conduction velocity or from a quantitative sensitive procedure or from histological studies performed through biopsies in order to confirm the diagnosis according to current recommendations.12,16,17
By using a quantitative sensory testing, the overall prevalence of DPN in our study was 39.6%, a value greater than that published by most of the aforementioned series. The greater prevalence in our study is mainly due to two factors: the studied diabetic population had a longer duration of diabetes, with an average of 19.6 years since they were diagnosed (it is widely known that prevalence of metadiabetic complications increases with disease duration); and our methodology, more sensitive, objective, homogeneous and specific than those normally used in usual care, which allows for the detection of more DPN patients. The same argument can account for the fact that DPN prevalence in our series is higher than that previously shown by Cabezas-Cerrato et al.3 in our country. They used only the ankle reflex and cold, pinprick and vibration perceptions with tuning fork, a limited procedure, which understimates the actual prevalence of polyneuropathy.
In this regard, it is worth pointing out that 58% of our DPN patients had not previously been diagnosed, and more than half of them (33.5%) were exclusively diagnosed with the quantitative sensory testing. This reflects the high rate of DPN underdiagnosis, particularly when it is subclinical and goes unnoticed, as highlighted in other studies.18 The knowledge of vibration perception threshold by means of the biothesiometer is a recognized method for early DPN detection.7,14,16 It provides a positive predictive value for the risk of developing neuropathic ulcers and demonstrates good concordance with nerve conduction studies (77–100% sensitivity and 73–81% specificity).19 Diagnostic accuracy increases with thermal perception threshold, which seems to be affected before vibratory sensitivity or electrophysiological studies are also performed.20,21 The combined assessment of thermal, tactile and vibration perception thresholds, as was determined in our study, is thus more sensitive in the early identification of DPN than the isolated assessment of only one of these three sensitivities.
Another noteworthy outcome in our results is the prevalence of subclinical asymptomatic DPN, which accounted for 36.8% of the DPN diabetic patients. In view of this, DPN screenings should be performed carefully and with the use of some of the quantitative sensory devices. The finding that subclinical DPN is more common among males lacks a convincing explanation and requires more study. However, we can assume that this anomaly is apparent and merely reflects a greater degree of concealment of neuropathic symptoms among males.
Our study confirmed that DPN prevalence increases with disease duration, which coincides with other statistics.6,7,22 For example, Young et al.7 have estimated an average prevalence of 36.8% after 10 years of evolution in their diabetic population. The study by Partanen et al.22 estimated a prevalence of 42% after the same period of time in type 2 diabetic patients. The higher apparent prevalence in type 2 diabetes is probably due to the fact that their disease has a longer actual duration, since it is known that many of these patients have been suffering the disease long before they were diagnosed. The results are similar if the prevalence of the alterations in the three sensitivities is analyzed separately (Figure 1), with a significant loss of thermal and vibratory sensitivities in cases of long-standing diabetes. This loss over time is, however, less outstanding with respect to tactile sensitivity, since, despite the tendency towards greater loss after this period of time, this loss shows no significant differences with shorter evolution times. All this likely reflects a greater fragility of the nerve fibers transporting thermal and vibratory sensitivities as a result of the adverse metabolic effects of diabetes over time.
DPN prevalence also demonstrates a positive relationship with the presence of other macro- and microagiopathic complications, which is not surprising since DPN remains a part of metadiabetic complications. In addition, our study demonstrated a higher prevalence in patients who smoked. In this sense, tobacco could be considered an additional vascular risk factor, since its adverse events on the cardiovascular system are well known. It should not be surprising that in our study DPN prevalence bears no relation to HbA1c blood levels and fructosamine in serum since these analytical parameters express the glycemic control level only at the time the observational study took place, regardless of the degree of glycemic control patients experienced during the previous months and years. However, a diminished DPN prevalence is yielded when a good metabolic control is sustained, which is supported by the finding that DPN is decreased when the patient is intensively treated with insulin. Similarly, dyslipidemia is more common in DPN patients than in those patients with no DPN.
Finally, analysis of the vibratory, thermal and tactile sensitivities reveals interesting results. Losses in the three sensitivities are higher in subclinical DPN, as well as in patients with non-painful clinical DPN (except for tactile sensitivity in the latter group; Figures 2 and 3). Even though these results might seem contradictory, they are not. In fact, it is well known that as DPN worsens its subjective symptoms also decrease. The disappearance of these symptoms and the neuropathic pain in patients who had previously suffered them expresses, frequently, progressive axonal damage of the peripheral nerves and a greater loss of sensitivities,23 regardless of diabetes duration. This would account for the greater prevalence of sensitivity loss in patients with no subjective clinical signs and with no neuropathic pain.
Conflict of interestJosé M. Miralles-García, Pedro de Pablos-Velasco and Lucio Cabrerizo have no conflict-of-interest to disclose. María Pérez and Vanessa López-Gómez are employed by Pfizer Spain, the company funding this study.
This study has been funded by an unrestricted grant from Pfizer Spain that covered the costs of the devices used in the study. The authors want to acknowledge Inmaculada Vilardaga (employed by European Biometric Institute, an independent company contracted by Pfizer to collaborate in data analysis and logistic of the study) for her role in study analysis and statistics interpretation, and to all participating investigators (see Appendices 1), whose collaboration was essential for the conduct of the study.
Complejo Hospitalario (León): R. Aguado. Complejo Hospitalario (Vigo): R. Luna, L. Fajar. Complejo Hospitalario de Jaén (Jaén): C. Sánchez, A. Moreno. Hospital Clínico San Carlos (Madrid): A. Calle. Hospital Clínico Universitario (Salamanca): J. Palacio, F. Gómez, V. Villabona. Hospital de Mérida (Mérida): J. Parra, A. Sillero. Hospital de Navarra (Pamplona): L. Forga, J. Martínez, M. Goñi. Hospital Donostia (San Sebastián): T. Abellán. Hospital Dr. Negrín (Las Palmas): P. Soriano. Hospital Doctor Peset (Valencia): A. Hernández, J. Yanini, C. García. Hospital General (Castellón): C. González, P. Cubells, S. Garzón. Hospital Miguel Servet (Zaragoza): R. Albero, P. Diego, C. Zapata. Hospital Son Dureta (Palma de Mallorca): V. Pereg, H. García, M. Codina, S, Tofé, C. Sainz, S. Díaz. Hospital Txagorritxu (Vitoria): E. Rodríguez, A. Antón. Hospital Universitario Carlos Haya (Málaga): F. Soriguer, F. Linares. Hospital Universitario (Tenerife): L. Morcillo. Hospital Universitario Doctor Josep Trueta (Gerona): I. López. Hospital Virgen de la Luz (Cuenca): C. Gómez, D. Calderón, J. Aranda. Hospital Virgen de la Salud (Toledo): A. Marco, E. Cruces, J. Sastre, A. Vicente, M. López, E. Castro, J. López. Hospital Virgen Macarena (Sevilla): C. Morales.