This study was intended to assess the effectiveness and predictors factors of inpatient blood glucose control in diabetic patients admitted to medical departments.
Material and methodsA retrospective, analytical cohort study was conducted on patients discharged from internal medicine with a diagnosis related to diabetes. Variables collected included demographic characteristics, clinical data and laboratory parameters related to blood glucose control (HbA1c, basal plasma glucose, point-of-care capillary glucose). The cumulative probability of receiving scheduled insulin regimens was evaluated using Kaplan–Meier analysis. Multivariate regression models were used to select predictors of mean inpatient glucose (MHG) and glucose variability (standard deviation [GV]).
ResultsThe study sample consisted of 228 patients (mean age 78.4 (SD 10.1) years, 51% women). Of these, 96 patients (42.1%) were treated with sliding-scale regular insulin only. Median time to start of scheduled insulin therapy was 4 (95% CI, 2–6) days. Blood glucose control measures were: MIG 181.4 (SD 41.7) mg/dL, GV 56.3 (SD 22.6).
The best model to predict MIG (R2: .376; p<.0001) included HbA1c (b=4.96; p=.011), baseline plasma glucose (b=.056; p=.084), mean capillary blood glucose in the first 24h (b=.154; p<.0001), home treatment (versus oral agents) with basal insulin only (b=13.1; p=.016) or more complex (pre-mixed insulin or basal-bolus) regimens (b=19.1; p=.004), corticoid therapy (b=14.9; p=.002), and fasting on admission (b=10.4; p=.098).
ConclusionPredictors of inpatient blood glucose control which should be considered in the design of DM management protocols include home treatment, HbA1c, basal plasma glucose, mean blood glucose in the first 24h, fasting, and corticoid therapy.
Nuestros objetivos fueron evaluar el control glucémico intrahospitalario de pacientes con diabetes mellitus (DM) y determinar sus factores predictores.
Material y métodosEstudio de cohortes retrospectivo analítico con inclusión de pacientes dados de alta de medicina interna con un diagnóstico relacionado con la DM. Se recogieron variables clínicas (demográficas y relacionadas con el manejo intrahospitalario del paciente) y analíticas relacionadas con el control glucémico (HbA1c, glucemia plasmática inicial, glucemias capilares durante el ingreso). Se evaluó la probabilidad de recibir insulina programada mediante curvas de Kaplan Meier y los factores predictores de la glucemia media (GM) y de su variabilidad (desviación estándar [VG]) mediante regresión múltiple.
ResultadosSe incluyeron 228 pacientes (edad media 78,4 [DE 10,1] años, 51% mujeres); 96 (42,1%) recibieron solo pauta correctora. La mediana del tiempo hasta el inicio de insulina programada fue 4 días (IC 95%: 2–6). Las medidas de control fueron: GM 181,4 (DE 41,7) mg/dl, VG 56,3 (DE 22,6) mg/dl.
El mejor modelo predictor de la GM (R2: 0,376; p<0,0001) incluyó HbA1c (b=4,96; p=0,011), glucemia plasmática inicial (b=0,056; p=0,084), glucemia media de las primeras 24h (b=0,154; p<0,0001), tratamiento domiciliario (versus antidiabéticos orales) con insulina basal (b=13,1; p=0,016) o mezclas o basal-bolo (b=19,1; p=0,004), tratamiento con corticoides (b=14,9; p=0,002) y ayuno al ingreso (b=10,4; p=0,098).
ConclusiónLos determinantes del control glucémico intrahospitalario, que deberían considerarse en protocolos de actuación, son el tratamiento previo, la HbA1c, la glucemia inicial y media de las primeras 24h de ingreso, el ayuno y la utilización de corticoides.
Diabetes mellitus (DM) is a chronic disease of carbohydrate metabolism affecting 371 million people worldwide and with an increasing prevalence.1 According to a recent study, DM prevalence in Spain is 13.8%.2 The hospital management of patients with DM is a common problem, as they are estimated to account for 30–40% of patients attending the emergency room and for up 25% of admissions to both medical and surgical hospital wards.3 When these figures are interpreted, however, one should also bear in mind that this is still an underdiagnosed disease, because this diagnosis is not reported at discharge in one third of patients with hyperglycemia upon hospital admission.4
DM is not only a common problem, but also a relevant one, because hyperglycemia upon admission is associated with greater morbidity and mortality5 and resource utilization, with a resultant increase in costs.6 Despite this epidemiological association, few interventional studies intended to show the efficacy of intensive blood glucose control in inpatients not admitted to intensive care units (ICUs) are available; a recent meta-analysis7 showed that intensive hospital treatment of hyperglycemia outside the ICU decreased infections (RR=0.41; 95% CI: 0.21–0.77), but not mortality, with a borderline significant increase in the risk of hypoglycemia (RR=1.58; 95% CI: 0.97–2.57).
Based on the available evidence on the risks and benefits of metabolic control in non-ICU hospitalized patients, medical bodies8 now recommend preprandial and random blood glucose levels of <140mg/dL and <180mg/dL respectively (<200mg/dL in patients with limited life expectation or a high risk of hypoglycemia). These goals may be difficult to achieve9 due to the sometimes unpredictable nutritional intake, the stress from disease, with an increase in counterregulatory hormones, and the use of hyperglycemic drugs, especially corticosteroids.
An additional factor that impairs hyperglycemic control in hospitals is clinical inertia, due to the difficulty in persuading staff responsible for patients to change their beliefs and behavior.3 To overcome this inertia, educational programs have been suggested. These would be reinforced by the distribution of hospital management protocols, followed by regular evaluations of their implementation and efficacy at the hospital.10 The basic rules for preparing a protocol for hyperglycemia include the establishment of glycemic goals, the calculation of total insulin dose (TID) with its basal and prandial components (avoiding the use of corrective rapid insulin regimens [RIRs] only), and guidance on insulin adjustment during hospital stay and when treatment on discharge is being planned.11
This study was undertaken to assess whether the treatment of patients with DM at our hospital complied with the recommendations made by national and international medical associations.3,8 The specific objectives included:
- (1)
To assess the use of scheduled insulin versus RIR alone in patients with DM.
- (2)
To assess glycemic control in patients with DM.
- (3)
To find out the predictors of inpatient glycemic control, which would help us to design specific medical protocols for improving the management of patients with DM.
This was a retrospective descriptive and analytical cohort study.
PatientsAll patients discharged from the internal medicine department of Hospital Clínico Universitario Lozano Blesa in Saragossa for approximately four consecutive months (from January 3 to April 25, 2010) with some diagnosis (primary or secondary) included in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) related to DM (DM, secondary DM, abnormal glucose, and postoperative hypoinsulinemia) were enrolled into the study. Patients in whom management upon admission required treatment at the ICU and patients with a primary diagnosis of diabetic ketoacidosis or hyperosmolar hyperglycemic nonketotic decompensation requiring the use of initial intravenous insulin were excluded.
VariablesThe following variables were collected from each patient.
- (1)
Clinical variables: age at admission, sex, reason for admission, Charlson comorbidity index, type of DM, time since DM onset, type of outpatient treatment for DM, length of hospital stay, intake or fasting state upon admission, use of corticosteroids during admission, and type of treatment at hospital discharge.
- (2)
Variables related to glycemic control: use or otherwise (and day of start) of scheduled insulin, plasma blood glucose at admission, HbA1c measured during hospital stay or in the previous three months, glucose values obtained by capillary glucose monitoring, and frequency and severity of hypoglycemia (hypoglycemia was defined as blood glucose values <70mg/dL, and considered severe if glucose values were <40mg/dL or concomitant loss of consciousness occurred).
- (3)
Generated variables: the Charlson score was codified using three categories <3 (reference category), 3 and >3. Outpatient treatment before admission was recodified using three categories (diet and/or oral antidiabetic drugs as reference category, basal insulin with or without oral antidiabetic drugs and mixtures or basal-bolus) because of the small number of patients on basal-bolus treatment. To assess inpatient glycemic control, the following were estimated from capillary blood glucose values: maximum blood glucose level (the highest capillary blood glucose value during hospital stay), mean blood glucose (MBG) in the first 24h, MBG during hospital stay, defined as the mean of all capillary blood glucose values, corrected MBG (the mean of the daily means of capillary blood glucose values), standard deviation of all capillary blood glucose values of the patient (glucose variability, GV) and coefficient of variation (CV), defined as the ratio between standard deviation and mean blood glucose (SD/MBG).
Plasma blood glucose was measured using an enzymatic method with hexokinase, HbA1c by high-performance chromatography, and capillary blood glucose using an Optium Xceed® meter with a precision of 3–3.6% (CV), an accuracy r=0.98 for plasma blood glucose, and a 99% compliance with the ISO standard.
Statistical methodsQuantitative variables are presented with the mean and standard deviation (SD), while qualitative variables are given as frequency distribution. Quantitative variables were compared using a Student's t test for independent samples, ANOVA, or non-parametric Mann–Whitney or Kruskal–Wallis tests. Qualitative variables were compared using a Chi-square test or Fisher's exact test. The correlation between quantitative variables (mean blood glucose and corrected mean blood glucose) was evaluated using the Pearson correlation coefficient.
The probability of receiving scheduled insulin during the hospital stay was determined using Kaplan–Meier survival curves. A log-rank test was used to compare the curves depending on the outpatient treatment received.
The predictors of inpatient glycemic control were determined using univariate and multivariate linear regression. The dependent variables taken into account included MBG, GV, and CV. To determine the best predicting models, variables were included in multivariate models based on statistical criteria (p<0.1 in univariate analysis) and on clinical meaning. The measure of variability selected was the standard deviation of all capillary blood glucose values of the patient (GV) instead of CV, because the R2 of the predictive model was higher.
An association with a value of p<0.05 was considered significant. SPSS version 17.0 software was used for the statistical analysis.
ResultsA total of 255 patients who met the inclusion criteria were initially selected. However, the clinical histories of 27 patients (10.6%) could not be reviewed, which left 228 valid patients for data analysis. Diagnoses related to DM included type 2 diabetes (n=198), secondary diabetes (n=14), and stress hypoglycemia (n=16).
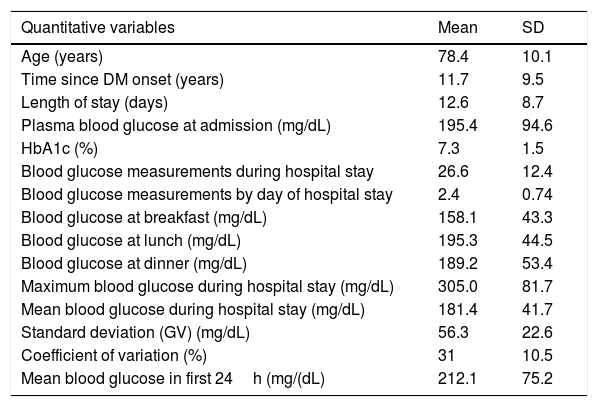
The mean age was 78.4 (SD 10.1) years, with a similar proportion of males (49%) and females (51%). Table 1 shows the clinical characteristics of the patients at admission. The main reasons for admission were dyspnea (42%) and febrile syndrome (12.3%).
Patient characteristics and metabolic control at admission.
| Quantitative variables | Mean | SD |
|---|---|---|
| Age (years) | 78.4 | 10.1 |
| Time since DM onset (years) | 11.7 | 9.5 |
| Length of stay (days) | 12.6 | 8.7 |
| Plasma blood glucose at admission (mg/dL) | 195.4 | 94.6 |
| HbA1c (%) | 7.3 | 1.5 |
| Blood glucose measurements during hospital stay | 26.6 | 12.4 |
| Blood glucose measurements by day of hospital stay | 2.4 | 0.74 |
| Blood glucose at breakfast (mg/dL) | 158.1 | 43.3 |
| Blood glucose at lunch (mg/dL) | 195.3 | 44.5 |
| Blood glucose at dinner (mg/dL) | 189.2 | 53.4 |
| Maximum blood glucose during hospital stay (mg/dL) | 305.0 | 81.7 |
| Mean blood glucose during hospital stay (mg/dL) | 181.4 | 41.7 |
| Standard deviation (GV) (mg/dL) | 56.3 | 22.6 |
| Coefficient of variation (%) | 31 | 10.5 |
| Mean blood glucose in first 24h (mg/(dL) | 212.1 | 75.2 |
| Qualitative variables | No. | % |
|---|---|---|
| Home treatment | ||
| Diet and/or oral antidiabetic drugs | 135 | 59.2 |
| Basal insulin | 57 | 25 |
| Mixtures | 29 | 12.7 |
| Basal-bolus | 3 | 1.3 |
| Charlson index | ||
| <3 | 101 | 44.3 |
| 3 | 62 | 27.2 |
| >3 | 65 | 28.5 |
| Fasting at admission | 39 | 17.1 |
| Hypoglycemia | ||
| None | 161 | 70.6 |
| Mild (40–70mg/dL) | 30 | 13.2 |
| Severe (<40mg/dL or loss of consciousness) | 34 | 14.9 |
| Corticosteroid treatment during hospital stay | 85 | 37.3 |
| Treatment at discharge | ||
| Diet and/or oral antidiabetic drugs | 126 | 55.2 |
| Basal insulin | 72 | 31.6 |
| Mixtures | 26 | 11.4 |
| Basal-bolus | 4 | 1.8 |
| Place of discharge | ||
| Home | 203 | 89 |
| Death | 19 | 8.3 |
| Another hospital | 3 | 1.3 |
| Voluntary discharge | 3 | 1.3 |
Forty-one patients (18%) were treated with oral antidiabetic drugs during their hospital stay. Scheduled insulin regimens were received by 132 patients (57.9%) during their hospital stay, while 96 patients (42.1%) received RIR alone. The median time to the start of scheduled insulin regimens (the day of the hospital stay when ≥50% of patients with DM were receiving such regimens) was four days (95% CI: 2–6).
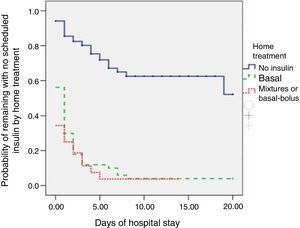
The proportion of patients who received scheduled insulin regimens was statistically higher (p<0.001) in patients on home treatment with insulin (93%) as compared to those treated with diet or oral antidiabetic drugs (35%). The difference in survival curves, which reflected the rate of administration of scheduled insulin which was itself dependent on the initial treatment of patients, was also statistically significant (p<0.0001; Fig. 1). In a follow-up restricted to three weeks of hospitalization, the probability of receiving scheduled insulin was 49% for patients taking oral antidiabetic drugs at home and virtually 100% for patients on insulin therapy before admission.
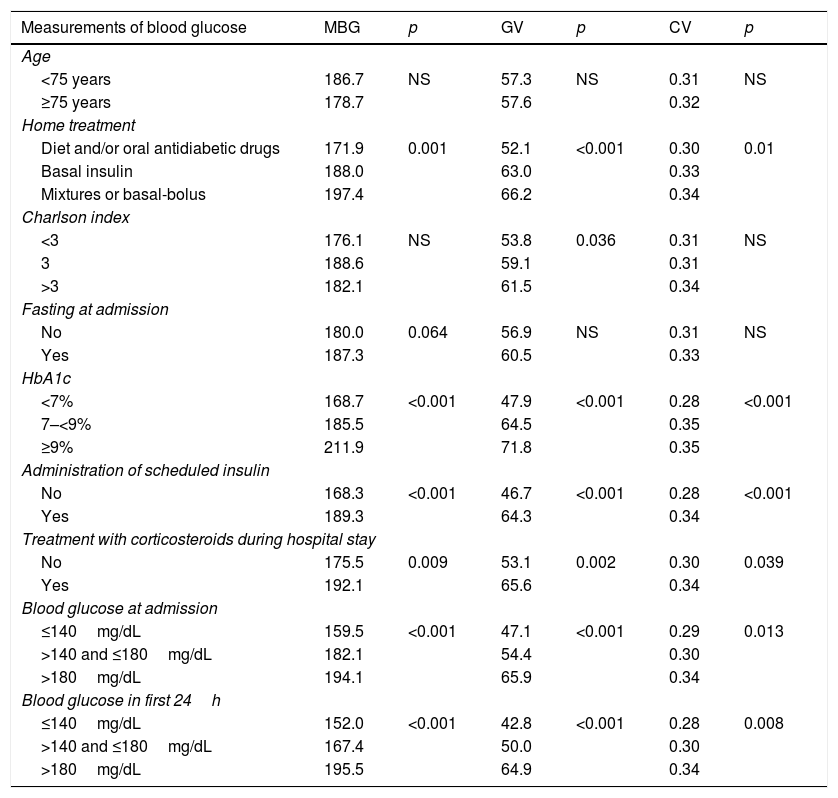
MBG was 181.4mg/dL (SD 41.7), GV 56.3mg/dL (SD 22.6), and CV 31% (SD 10.5). Table 2 shows blood glucose values depending on the baseline characteristics of the patients. Corrected mean blood glucose was 182.4mg/dL (SD 41.6), with a correlation coefficient of 0.992 (p<0.0001) as compared to MBG. MBG was therefore used for all calculations of regression analyses.
Measurements of mean blood glucose (MBG), standard deviation (GV), and coefficient of variation (CV) depending on the baseline characteristics of patients.
| Measurements of blood glucose | MBG | p | GV | p | CV | p |
|---|---|---|---|---|---|---|
| Age | ||||||
| <75 years | 186.7 | NS | 57.3 | NS | 0.31 | NS |
| ≥75 years | 178.7 | 57.6 | 0.32 | |||
| Home treatment | ||||||
| Diet and/or oral antidiabetic drugs | 171.9 | 0.001 | 52.1 | <0.001 | 0.30 | 0.01 |
| Basal insulin | 188.0 | 63.0 | 0.33 | |||
| Mixtures or basal-bolus | 197.4 | 66.2 | 0.34 | |||
| Charlson index | ||||||
| <3 | 176.1 | NS | 53.8 | 0.036 | 0.31 | NS |
| 3 | 188.6 | 59.1 | 0.31 | |||
| >3 | 182.1 | 61.5 | 0.34 | |||
| Fasting at admission | ||||||
| No | 180.0 | 0.064 | 56.9 | NS | 0.31 | NS |
| Yes | 187.3 | 60.5 | 0.33 | |||
| HbA1c | ||||||
| <7% | 168.7 | <0.001 | 47.9 | <0.001 | 0.28 | <0.001 |
| 7–<9% | 185.5 | 64.5 | 0.35 | |||
| ≥9% | 211.9 | 71.8 | 0.35 | |||
| Administration of scheduled insulin | ||||||
| No | 168.3 | <0.001 | 46.7 | <0.001 | 0.28 | <0.001 |
| Yes | 189.3 | 64.3 | 0.34 | |||
| Treatment with corticosteroids during hospital stay | ||||||
| No | 175.5 | 0.009 | 53.1 | 0.002 | 0.30 | 0.039 |
| Yes | 192.1 | 65.6 | 0.34 | |||
| Blood glucose at admission | ||||||
| ≤140mg/dL | 159.5 | <0.001 | 47.1 | <0.001 | 0.29 | 0.013 |
| >140 and ≤180mg/dL | 182.1 | 54.4 | 0.30 | |||
| >180mg/dL | 194.1 | 65.9 | 0.34 | |||
| Blood glucose in first 24h | ||||||
| ≤140mg/dL | 152.0 | <0.001 | 42.8 | <0.001 | 0.28 | 0.008 |
| >140 and ≤180mg/dL | 167.4 | 50.0 | 0.30 | |||
| >180mg/dL | 195.5 | 64.9 | 0.34 | |||
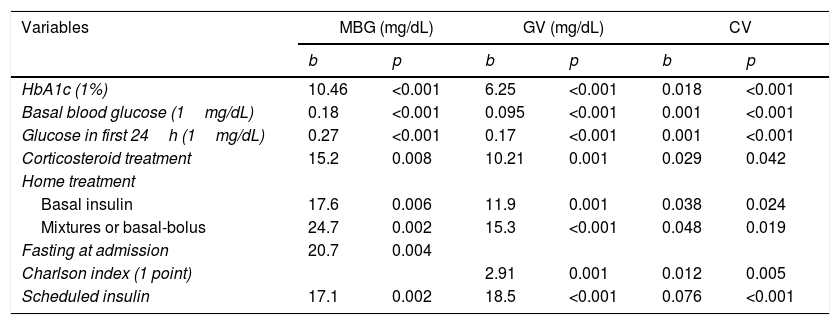
Table 3 shows the univariate predictors of blood glucose measurements. Variables consistently affecting all blood glucose measures during the hospital stay (mean blood glucose and variability) were those related to the metabolic state of the patient before admission (treatment for DM and HbA1c), the initial degree of decompensation (basal plasma blood glucose and capillary blood glucose in the first 24h after admission), and the need for corticosteroid treatment. Patients in whom scheduled insulin regimens were started also had higher mean blood glucose and glucose variability during hospitalization. In this case, the scheduled insulin regimen was probably the consequence of the greater degree of metabolic decompensation, rather than the cause. Therefore, since our primary objective was to identify factors that should be taken into consideration when designing hospital management protocols, this variable was not included in multiple regression models with predictive purposes.
Univariate predictors of mean blood glucose (MBG), standard deviation (GV), and coefficient of variation (CV).
| Variables | MBG (mg/dL) | GV (mg/dL) | CV | |||
|---|---|---|---|---|---|---|
| b | p | b | p | b | p | |
| HbA1c (1%) | 10.46 | <0.001 | 6.25 | <0.001 | 0.018 | <0.001 |
| Basal blood glucose (1mg/dL) | 0.18 | <0.001 | 0.095 | <0.001 | 0.001 | <0.001 |
| Glucose in first 24h (1mg/dL) | 0.27 | <0.001 | 0.17 | <0.001 | 0.001 | <0.001 |
| Corticosteroid treatment | 15.2 | 0.008 | 10.21 | 0.001 | 0.029 | 0.042 |
| Home treatment | ||||||
| Basal insulin | 17.6 | 0.006 | 11.9 | 0.001 | 0.038 | 0.024 |
| Mixtures or basal-bolus | 24.7 | 0.002 | 15.3 | <0.001 | 0.048 | 0.019 |
| Fasting at admission | 20.7 | 0.004 | ||||
| Charlson index (1 point) | 2.91 | 0.001 | 0.012 | 0.005 | ||
| Scheduled insulin | 17.1 | 0.002 | 18.5 | <0.001 | 0.076 | <0.001 |
In the multivariate analysis, the best predictive model of MBG (R2 of the model 0.376; overall p<0.0001) consisted of HbA1c (b=4.96; 95% CI: 1.17–8.76; p=0.011), baseline plasma blood glucose (b=0.056; 95% CI: 0–0.12; p=0.084), mean blood glucose in the first 24h (b=0.154; 95% CI: 0.08–0.23; p<0.0001), home treatment with basal insulin (b=13.1; 95% CI: 2.5–23.8; p=0.016) or basal-bolus mixtures (b=19.1; 95% CI: 6.2–32; p=0.004), the need for corticosteroid treatment (b=14.9; 95% CI: 5.4–24.4; p=0.002), and fasting at admission (b=10.4; 95% CI: 1.9–22.7; p=0.098). The best predictive model for GV (R2 of the model 0.488; overall p<0.0001) consisted of age (b=0.28; 95% CI: 0.04–0.52; p=0.023), HbA1c (b=3.59; 95% CI: 1.78–5.4; p<0.0001), mean blood glucose in the first 24h (b=0.136; 95% CI: 0.1–0.17; p<0.0001), home treatment with basal insulin (b=8.35; 95% CI: 2.8–13.9; p=0.003) or basal-bolus mixtures (b=9.44; 95% CI: 2.75–16.1; p=0.006), and the need for corticosteroid treatment (b=12.2; 95% CI: 7.3–17.2; p<0.0001).
DiscussionA review of the clinical histories of patients discharged from our hospital with a diagnosis related to the presence of DM showed that the management of these patients did not completely follow the recommendations of the medical bodies. Eighteen percent of patients continued to take oral antidiabetic drugs after hospital discharge, and 42.1% received rapid insulin regimens (RIRs) for correction alone, without scheduled insulin. The preprandial MBG of patients was approximately 180mg/dL, above the recommended goal of 140mg/dL. After identifying where the management of these patients could be improved, the main factors predicting for MBG and glucose variability were found to be those related to prior outpatient management (HbA1c and type of treatment) and to the degree of initial metabolic decompensation (plasma blood glucose at baseline and in the first 24h after admission), and the need for corticosteroid treatment.
It is currently agreed that the best treatment for patients with DM is the replacement of oral antidiabetic drugs by insulin. Insulin should ideally be administered as a scheduled regimen of basal insulin (fasting condition) or as basal insulin plus prandial insulin (preserved intake condition). Except in special cases, the use of insulin as RIR only is not recommended.3,8,11 Despite these recommendations, we found at our hospital that more than 40% of patients, especially those treated on an outpatient basis with oral antidiabetic drugs, were not receiving scheduled insulin. A study conducted in 44 US hospitals12 showed that 16% of patients with type 1 DM and 35% of patients with type 2 DM only received insulin as RIR. The lack of use of scheduled insulin has been attributed to unfamiliarity with insulin therapy on the part of the professionals responsible for patient care, the fear of hypoglycemia, clinical inertia, and the belief that intensive treatment is not a priority in the context of the disease that causes admission.11 We therefore think that there is room for improvement in the management of patients with DM admitted to the hospital ward, and agree with Pérez et al. that the clinical inertia that prevents the administration of scheduled insulin regimens should be overcome from the time of patient admission,3 i.e. in the emergency room.
The patients in our hospital showed deficient metabolic control according to the recommended control criteria,8 but this should be put into context, bearing in mind the advanced age and high comorbidity of the patients. In a recent study13 of 620 non-ICU hospitalized patients, glycemic control (MBG: 182mg/dL; SD: 57.9mg/dL; CV: 31.9%) was virtually identical to that seen in our patients, which confirms the difficulties involved in DM management in hospitalized patients.
Although intensive blood glucose treatment has not been shown to decrease mortality in hospitalized patients,14 there are epidemiological studies showing that various glycemic control parameters may impact on patient prognosis. The most consistent of these parameters is MBG: in the Kosiborod et al. study15 patients admitted to hospital with acute myocardial infarction and preexistent DM had an increased inpatient mortality risk (OR=4.1; 95% CI: 1.8–9.3) when MBG was >200mg/dL. Therefore, and because of its simple calculation, MBG is the control parameter that could become the reference for assessing the quality of the hospital care of patients with DM, facilitating best-practices benchmarking16 among hospitals. However, the importance of inpatient glycemic variability both for the mid-term (one year) development of cardiovascular events17 and 90-day mortality has recently been recognized13. We therefore decided to assess the factors associated with measures not only of MBG, but also of variability.
The variables selected in the three models were those related to prior outpatient management (HbA1c as a marker of long-term glycemic control and type of treatment as a marker of time since onset and, probably, of the insulin reserve of patients), to the degree of decompensation shortly after admission (plasma blood glucose at baseline and in the first 24h after admission) and the need for corticosteroid treatment. Such variables should be taken into account in the design of the treatment algorithm included in hospital management protocols. It is generally agreed that baseline blood glucose, diabetes type and/or prior treatment, and the possibility of oral intake condition the initial insulin therapy of patients.3 Special mention should be made of corticosteroid treatment, because its marked impact on glycemic control requires specific protocols adapted to this situation.10,18 The high power of mean blood glucose in the first 24h for predicting MBG throughout the hospital stay emphasizes the importance of an adequate treatment approach from the time of hospital admission.
It should also be borne in mind that the dissemination of any protocol should be combined with an education program. In one survey,19 69% of surgeons admitted the need to improve their training in the management of hyperglycemia. The Institute for Safe Medication Practices20 has recognized that insulin is a drug associated with risks in the hospital, and in order to improve patient safety, specifically recommends the implementation of protocols which lay down the rules for insulin use as well as courses for all hospital professionals using insulin.
Factors supporting the validity of our study include the selection of a large patient sample representative of the patients seen in a medical ward, a careful review of the clinical histories together with the systematic collection of all capillary blood glucose values during the hospital stay, and the evaluation of the factors that determine both average inpatient glycemic control and its variability. Our study also has limitations, such as the impossibility of collecting the clinical history data of 10% of the patients, the non-inclusion of patients with type 1 diabetes (which was probably due to the exclusion of discharges from the endocrinology department, although an error in the codification of the medical histories at hospital discharge cannot be excluded), and the non-inclusion of surgical patients.
In conclusion, we have identified an opportunity for improving the quality of care of patients with DM admitted to the internal medicine departments of our hospital. Therapeutic inertia needs to be overcome by implementing management protocols that increase the use of scheduled insulin to improve metabolic control in patients, starting at the time of admission from the emergency room. Factors that should be included in the protocol for deciding initial treatment and its changes during the hospital stay include prior outpatient treatment, HbA1c, baseline plasma blood glucose, mean blood glucose in the first 24h after admission, whether or not oral intake is possible, and the use of corticosteroids.
Conflict of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Sáenz-Abad D, Gimeno-Orna JA, Sierra-Bergua B, Pérez-Calvo JI. Factores predictores del control glucémico promedio y de su variabilidad en pacientes diabéticos ingresados en el hospital. Endocrinol Nutr. 2015;62:257–263.