Research in animal models has demonstrated the role of osteocalcin, a bone formation marker, in regulation of energy metabolism. Those studies have led to a new concept of the bone acting as an endocrine organ by secreting osteocalcin, which acts by increasing insulin secretion, lowering plasma glucose, and increasing insulin sensitivity and energy expenditure. Results in humans have been conflicting. On the other hand, antiresorptive drugs used against osteoporosis decrease osteocalcin levels, while anabolic drugs increase osteocalcin levels. However, the effects of these therapies on energy metabolism have not been investigated.
La investigación en modelos animales ha demostrado el papel de la osteocalcina, marcador de formación ósea, en la regulación del metabolismo energético. Estos trabajos han dado lugar a un nuevo concepto del hueso como órgano endocrino mediante la secreción de osteocalcina, que actúa incrementando la secreción de insulina, disminuyendo la glucosa plasmática, así como aumentando la sensibilidad a la insulina y el gasto energético. Los resultados en humanos han sido diversos y en ocasiones contradictorios. Por otro lado, los fármacos antirresortivos frente a la osteoporosis disminuyen los niveles de osteocalcina mientras que los osteoanabólicos la incrementan. No obstante, no se han investigado los efectos de estas terapias sobre el metabolismo energético.
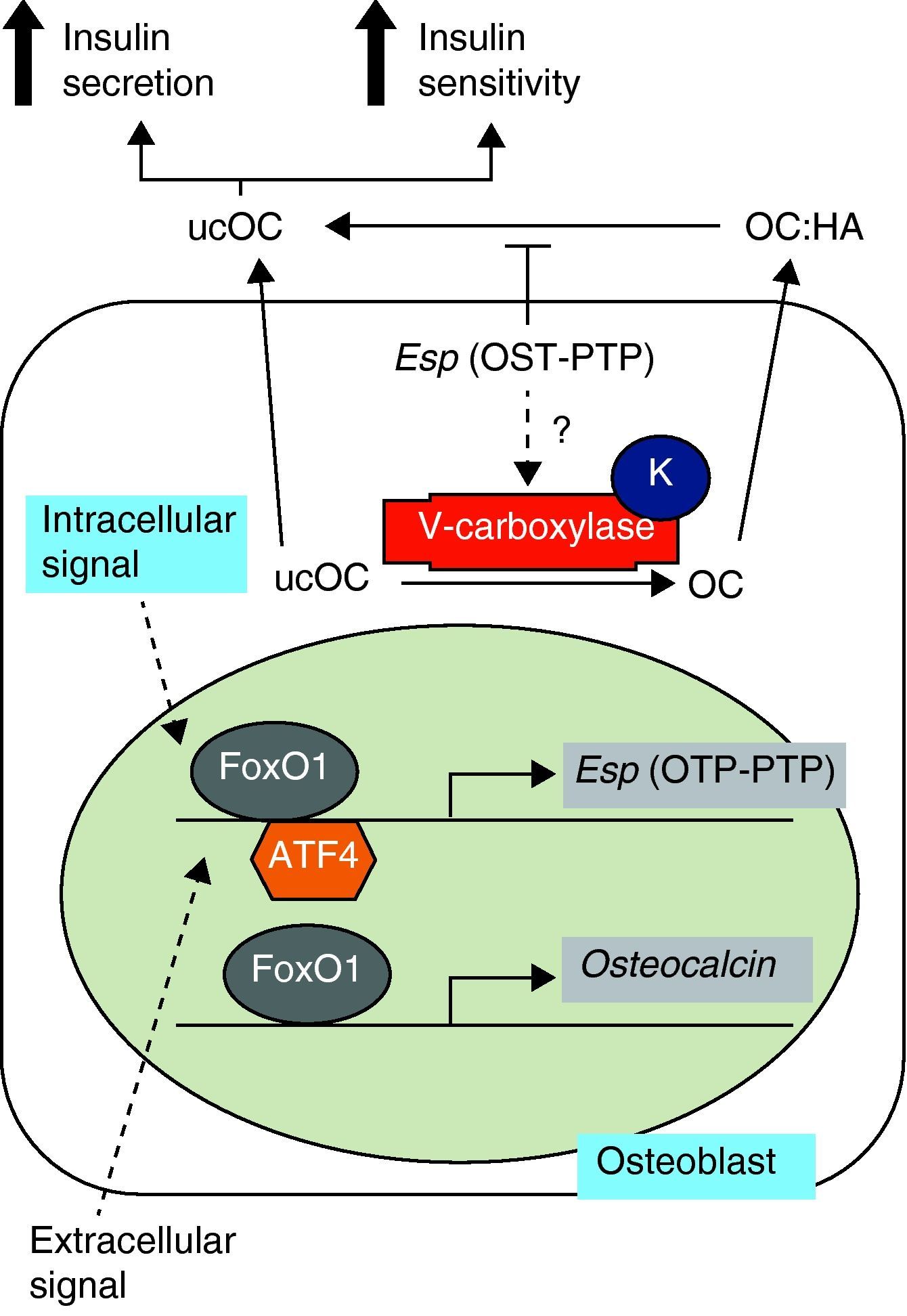
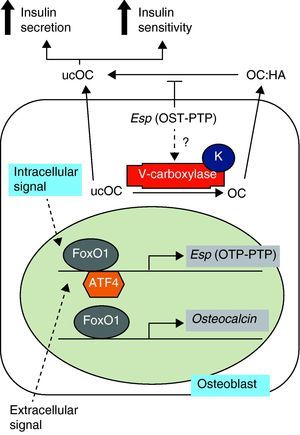
Studies in animal models have shown the role of osteocalcin in the regulation of energy metabolism. This 5-kDa noncollagenous protein, also known as bone GLa protein (BGP), is characteristic of bone, is secreted by cells of the osteoblastic line, and is related to the bone mineralization process.1 Once synthesized, most osteocalcin is incorporated into bone extracellular matrix, but small amounts are released into circulation and are considered a bone formation marker. Its post-translational modification by vitamin K-dependent gamma-carboxylation allows osteocalcin to strongly bind to hydroxyapatite calcium ions. However, the undercarboxylated fraction, with less than three carboxyl residues, has less affinity for bone. Thus, a greater proportion of undercarboxylated osteocalcin is in the circulation and may directly act upon pancreatic beta cells and adipocytes.2 These findings have led to a new concept of bone as an endocrine organ based on the secretion of osteocalcin, which increases insulin secretion, decreases plasma glucose, and increases insulin sensitivity and energy expenditure (Fig. 1).3
Osteocalcin synthesis and carboxylation. Adapted from Motyl et al.4 HA: hydroxyapatite; K: vitamin K; OC: osteocalcin; OTP-PTP: osteotesticular protein tyrosine phosphatase; ucOC: undercarboxylated osteocalcin.
Thus, osteocalcin inactivation in mice results in increased visceral fat with carbohydrate intolerance, low insulin levels, changes in insulin response to glucose, and a decreased mass of pancreatic beta cells.4 These signs are associated with decreased serum levels of adiponectin, an adipokine known to improve insulin sensitivity. The product of the Esp gene is osteotesticular protein tyrosine phosphatase (OST-PTP), which is expressed in osteoblasts and Sertoli cells only. This is important for osteoblast maturation and appears to influence the production of undercarboxylated osteocalcin. Suppression of the Esp gene in mice therefore results in the opposite phenotype to that of mice -/- for osteocalcin with hypoglycemia, insulin increase in response to glucose, a greater mass of pancreatic beta cells, and protection against obesity. Alternatively, the overexpression of OST-PTP in mouse models results in a phenotype identical to that of osteocalcin inactivation.4,5
The clinical implications of these findings for type 2 diabetes mellitus (T2DM) and metabolic syndrome are extremely significant, and many observational studies are therefore available.6,7 This research had already established that diabetic patients have lower osteocalcin levels as compared to nondiabetics, and an inverse relation had been found between osteocalcin and basal glucose, basal insulin, glycosylated hemoglobin (HbA1c), insulin resistance index (HOMA), high sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), body mass index, and body fat percentage, as well as a direct relation to adiponectin. In this same study,6 the inverse relation between osteocalcin and metabolic phenotype and adiposity markers was maintained for three years. It is therefore postulated that this marker may predict for osteocalcin changes. Similar results were seen in a large Chinese cohort with different osteocalcin levels as the result of changes in carbohydrate metabolism8 and in postmenopausal women.9,10 However, other authors did not confirm the association between undercarboxylated osteocalcin and glucose levels or insulin resistance in humans.11 Osteocalcin has also been related to atherosclerosis parameters in T2DM based on the correlation seen between osteocalcin levels and the values of intimal thickness and brachial-ankle pulse wave velocity in males12 and established atherosclerotic disease.13
As regards the relation of osteocalcin to adiposity parameters, its role in adiponectin secretion by adipose tissue, leading to increased insulin sensitivity, has already been mentioned. The positive relationship between both substances is significant in postmenopausal women.10 However, the role of osteocalcin in the regulation of triglyceride and cholesterol levels is not well established. In a study in a Chinese population,14 percent fat and high density lipoprotein cholesterol were independently associated with osteocalcin in males, and triglyceride levels were an independent factor with a positive influence on osteocalcin in premenopausal women. In postmenopausal women, an inverse relationship was found between osteocalcin and abdominal obesity parameters.15
The most novel in vitro and in vivo experimental studies are aimed at determining whether the administration of osteocalcin affects the different aspects of energy metabolism. The Ferron et al. study2 showed that osteocalcin acts directly upon culture cells and that different amounts regulated cell proliferation and insulin secretion on the one hand, and fat mass and insulin sensitivity on the other. In addition, intermittent osteocalcin administration partially restored insulin sensitivity and glucose tolerance and increased pancreatic beta cell mass in mice with a fat-rich diet. It also increased energy expenditure, protected against obesity, and reversed hepatic steatosis.16 Most interestingly, evidence of improved blood glucose management and fat mass reduction with intravenous osteocalcin administration was provided in animal models.
In this regard, an interventional clinical study in nondiabetic subjects17 assessed the effect of a high-calorie diet and regular physical activity on osteocalcin levels and concluded that weight loss through diet and regular physical activity resulted in a significant osteocalcin increase, which was associated with changes in visceral fat mass. The association between changes in circulating levels of undercarboxylated osteocalcin occurring during treatment for osteoporosis (PTH 1–84 vs alendronate) and changes in metabolic parameters has recently been examined with results consistent with those seen in animal models.18 In this study, the group given PTH experienced a small but significant body weight decrease at 12 months of treatment and a decrease in fat mass, while no significant changes occurred in weight or fat mass in the group treated with alendronate. Moreover, in the group treated with PTH, a significant correlation was found between the increase in undercarboxylated osteocalcin levels and decreases in body weight and fat mass. Similar results were seen in the group treated with alendronate, but they were not statistically significant. Moreover, in the overall sample, changes in undercarboxylated osteocalcin positively correlated to adiponectin changes, but no association was shown with leptin, insulin, glucose, or the insulin/glucose ratio.
On the other hand, vitamin K is a co-factor for the enzyme glutamate carboxylase, responsible for the carboxylation of osteocalcin,19 and lower dietary levels of vitamin K are associated with higher levels of undercarboxylated osteocalcin, while vitamin K supplements decrease undercarboxylated osteocalcin levels.20 Warfarin, an anticoagulant drug, inhibits vitamin K-dependent carboxylase, preventing post-translational carboxylation of factors in the coagulation cascade and osteocalcin, therefore increasing the levels of the undercarboxylated portion and decreasing blood glucose in mice. However, warfarin also regulates osteocalcin gene expression, so that treatment with warfarin hinders the interpretation of studies of this protein and its role in carbohydrate metabolism.21
In conclusion, the finding in animal models that osteocalcin produced by osteoblasts influences insulin secretion and sensitivity opens up new perspectives for understanding the biological mechanisms of carbohydrate and energy homeostasis. Studies conducted to date are however inconclusive, and specifically designed research should be performed in humans to confirm the hypothesis relating bone to energy metabolism.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: García-Martín Antonia, et al. Osteocalcina: nexo de unión entre homeostasis ósea y metabolismo energético. Endocrinol Nutr. 2013;60:260–3.