To ascertain the prevalence of metabolic syndrome (MS) in patients with peripheral artery disease (PAD) at the Martorell primary care (PC) center. To analyze the differences in comorbidities and cardiovascular risk factors between patients with PAD with and without MS.
MethodsA cross-sectional, descriptive study on patients diagnosed with PAD according to computerized clinical records of the Martorell PC center. Variables collected included age, sex, high blood pressure (HBP), dyslipidemia (DLP), diabetes (DM), smoking, obesity, cardiovascular disease (CVD), erectile dysfunction (ED), renal failure (RF), and oligoalbuminuria. An analysis comparing patients with and without MS was performed.
ResultsThere were 131 patients diagnosed with PAD, 104 (79%) of whom were male. Sixty-three (48.1%) also had MS. Patients with both PAD and MS had, as compared to those with PAD only, a higher prevalence of HBP (87.3 vs. 60.3%, p: 0.001), DLP (77.8 vs. 60.3%, p: 0.03), DM (69.8 vs. 30.9%, p: <0.001), obesity (25.4 vs. 10.3%, p: 0.03), CVD (42.9 vs. 19.1%, p: 0.004), ED (81.3 vs. 54.3%, p: 0.02), and RF (40.3 vs. 17.9%, p: 0.006).
ConclusionPatients with both PAD and MS had a higher prevalence of HBP, DLP, DM, and obesity. They also had more cardiovascular events and were significantly associated with pathological conditions highly relevant for cardiovascular prognosis such as erectile dysfunction and chronic kidney disease.
Conocer la prevalencia de síndrome metabólico (SM) en pacientes afectos de enfermedad arterial periférica (EAP) en el centro de Atención Primaria (AP) de Martorell. Analizar las diferencias de comorbilidad y factores de riesgo cardiovascular asociados (FRCV) en pacientes con EAP según presenten o no SM.
MetodologíaEstudio descriptivo transversal. Sujetos: pacientes diagnosticados de EAP según historia clínica informatizada de AP de Martorell. Mediciones: edad, sexo, hipertensión arterial (HTA), dislipidemia (DLP), diabetes (DM), tabaquismo, obesidad, enfermedad cardiovascular (ECV), disfunción eréctil (DE), insuficiencia renal (IR) y oligoalbuminuria. Análisis: frecuencias para variables discretas, medias y desviación típica (DS) para las variables continuas. Se realizó análisis bivariante que comparaba pacientes que presentaban EAP y SM con los que solo presentaban EAP.
ResultadosUn total de 131 pacientes diagnosticados de EAP, 104 (79%) varones. De ellos, 63 (48,1%) presentaron SM, de los que 46 (73%) eran varones. Los pacientes que combinaban EAP y SM, en comparación con los que solo tenían EAP, presentaban mayor prevalencia de HTA (87,3 vs. 60,3%; p: 0,001), DLP (77,8 vs. 60,3%; p: 0,03), DM (69,8 vs. 30,9%; p<0,001), obesidad (25,4 vs. 10,3%; p: 0,03), ECV (42,9 vs. 19,1%; p: 0,004), DE (81,3 vs. 54,3%; p: 0,02) e IR (40,3 vs. 17,9%; p: 0,006).
ConclusionesLos pacientes que combinan EAP y SM presentan una mayor prevalencia de HTA, DLP, DM y obesidad; padecen más eventos cardiovasculares a nivel cardiaco o cerebral, y se asocian, también de manera significativa a entidades patológicas tan relevantes desde el punto de vista del pronóstico cardiovascular como la DE y la enfermedad renal crónica.
Peripheral artery disease (PAD) is defined as the manifestation of atherosclerotic disease in peripheral vascular branches, and preferentially affects arteries in the lower limbs.
PAD is currently regarded as a silent epidemic. It is an underdiagnosed, common condition.1 A population study conducted in an area very similar to ours found a 7.6% prevalence of PAD.2 Prevalence exponentially increases from 26% to 54% when patient populations with moderate, high, or very high cardiovascular risk are studied.3,4
The potential of PAD as a predictor of cardiovascular disease (CVD) and vascular death is well-known, and there are authors who consider PAD to be the atherosclerotic complication with the worst vital prognosis.5
The detection of this condition at an early stage, before it becomes clinically evident, and the adequate management of the cardiovascular risk factors (CVRFs) involved in the genesis of atherosclerosis may help to prevent the occurrence of cardiac or cerebral events, which are today the leading cause of death and disability.6
There is ample evidence of the enhancing role of overall cardiovascular risk played by other conditions such as metabolic syndrome (MS), erectile dysfunction (ED), or chronic kidney disease.7–10 MS is defined as the joint occurrence of factors frequently seen in clinical practice: abdominal obesity, dyslipidemia (DLP), and increased blood glucose and blood pressure (BP) levels. Despite the controversy usually associated with this diagnosis because it is far from clear that it has a greater predictive value of cardiovascular morbidity and mortality than some of its individual components, many authors have advocated its value as a prognostic marker, regarding it as even greater than that of the established scales for the stratification of cardiovascular risk.11–13 PAD and MS often occur concurrently,14–17 and quite a few studies recommend more intensive use of the ankle-brachial index (ABI) in these patients in order to detect undiagnosed cases of vascular disease in the lower limbs and to take appropriate action.14
The primary objective of this study was to estimate the prevalence of MS in patients with PAD seen at the Martorell primary care center. The secondary objectives were to analyze any potential differences in behavior of the main CVRFs and other conditions that determine the final risk, such as ED, renal failure (RF), and CVD (ischemic heart disease and cerebrovascular disease) in patients with PAD with or without MS, and to ascertain the degree of control of CVRFs achieved.
MethodsStudy design and populationThis was a descriptive cross-sectional study conducted in 2014 on patients diagnosed with PAD seen at the Martorell (Barcelona) primary care center, covering a population of 30,197, of whom 12.8% were over 64 years of age according to data taken from the Central Registry of the Primary Care Computer System (SIAP).
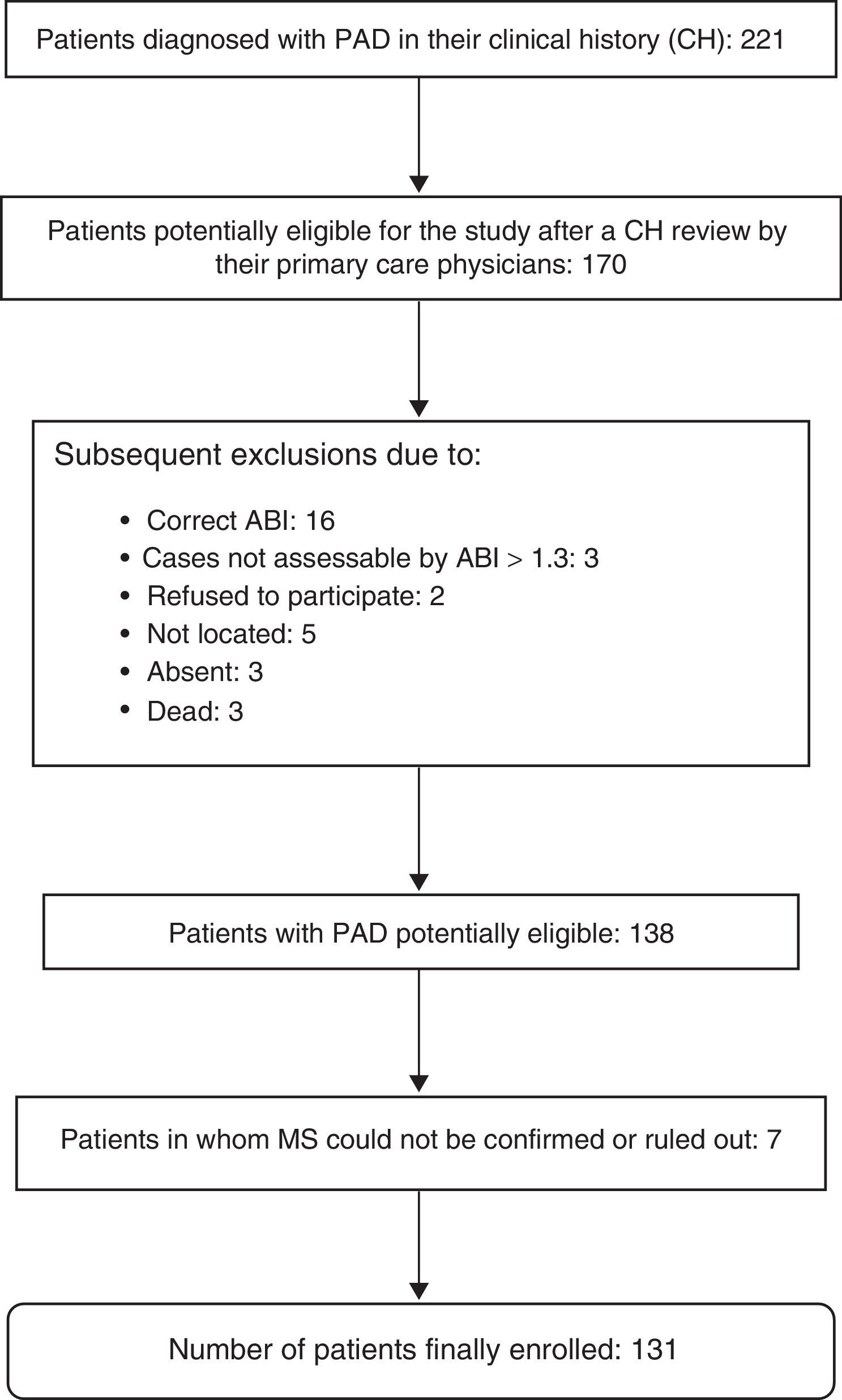
The study population consisted of all patients diagnosed with PAD according to data collected from the e-cap (code 173.9) computerized clinical history, including patients who had undergone revascularization surgery and amputation of one lower limb as a consequence of PAD. Subjects with end-stage disease, severe psychiatric disorders, who were institutionalized, and those subjects for whom adequate monitoring at primary care clinics could not be guaranteed, because, for example, they had no permanent residence in the town of Martorell, were excluded from the study. Patients with clinical histories not including the minimal data required to confirm or rule out the existence of associated MS were also excluded.
A two-stage authentication protocol was applied to verify the accuracy of the PAD diagnosis. First of all, the collaboration of the general practitioners of the patients was requested to review their histories. As a definitive diagnostic measure, the ABI was then measured in all patients with a diagnosis of PAD validated by their physicians, including those who had required revascularization surgery. In the event of amputation affecting one of the limbs, the ABI was measured in the non-amputated limb.
Data recording and diagnostic criteriaData were taken from the patients’ computerized medical histories. The demographic data collected included age and sex. Cardiovascular risk factors: HBP, defined as systolic blood pressure (SBP) ≥140mmHg or diastolic blood pressure (DBP) ≥90mmHg; diabetes mellitus (DM) if one of the two following criteria were met: basal fasting blood glucose ≥126mg/dL (7mmol/L) on two occasions, blood glucose 2h after an oral glucose tolerance test ≥200mg/dL (11.1mmol/L) on two occasions, random blood glucose ≥200mg/dL (11.1mmol/L) together with typical symptoms, or glycosylated hemoglobin (HbA1c) ≥6.5% twice, or once if associated with any of the above criteria; DLP, defined as levels of total cholesterol (total C) ≥200mg/dL (5.8mmol/L) or triglycerides (TG) ≥200mg/dL; obesity, defined as a body mass index (BMI) ≥30kg/m2, and smoking, defined as regular cigarette smoking in the previous month.18 Oligoalbuminuria, diagnosed by estimating the urinary albumin/creatinine ratio in the first morning urine, was also recorded, as was any associated morbidity, including CVD (ischemic heart disease: angina pectoris and myocardial infarction) and cerebrovascular disease (transient ischemic attack and stroke); RF, defined as a glomerular filtration rate (GFR) <60mL/min/1.73m2 estimated by the Modification of Diet in Renal Disease formula19; and ED, which was diagnosed based on a single, direct question to males enrolled into the study about the presence or absence of this health problem.
The most recent values recorded in the clinical history for SBP and DBP, pulse pressure (PP), total C, high and low density lipoprotein cholesterol (HDL-C and LDL-C respectively), TG, GFR, BMI, and glycosylated hemoglobin (HbA1c) in diabetic patients were collected, provided they had been recorded in the two years prior to the start of the study.
A diagnosis of PAD was confirmed based on the ABI. The tests were performed by 10 nurses trained to apply the technique according to the relevant recommendations.20 Portable unidirectional UltraTec PD1 Doppler equipment was used to obtain the ABI, which is the ratio between the highest SBP recorded in each lower limb (posterior tibial or dorsalis pedis) and the highest SBP in any of the upper limbs. A diagnosis of PAD required ABI values of ≤0.9. The condition was categorized as mild, moderate or severe if ABI values were 0.71–0.90, 0.41–0.70, and ≤0.40 respectively. The possibility of arterial calcification was considered if ABI values were higher than 1.3.
A diagnosis of MS was made based on the criteria of the Adult Treatment Panel III (ATP III) modified21 when three of the five following diagnostic criteria were found: abdominal obesity, defined as a waist circumference greater than 102cm in males and 88cm in females or a BMI > 28.8kg/m2, BP ≥130/85mmHg or treatment with antihypertensive drugs, HDL-C <40mg/dL in males and <50mg/dL in females, TG ≥150mg/dL, basal fasting blood glucose ≥110mg/dL, current hypoglycemic treatment or previously diagnosed diabetes.
In accordance with the recommendations of the clinical practice guidelines of the European Society of Cardiology on the diagnosis and treatment of PAD,20 control was considered optimum if the values given for the following CVRFs were reached: SBP/DBP less than 140/90, LDL-C less than 100mg/dL (or 70mg/dL if possible) and HbA1c less than 7%.
Statistical analysisFrequencies were used for qualitative variables, and means and SD for quantitative variables. A bivariate analysis was done to ascertain differences between subjects with and without MS. A Chi-square test was used for qualitative variables, and a Student's t test for quantitative variables. A value of p<0.05 was considered significant. SPSS version 15.1 software was used for the statistical analysis.
Ethical issuesAll the patients enrolled gave their verbal consent for the use of data from their clinical histories in this study.
ResultsOf the 138 subjects diagnosed with PAD, 107 (77.5%) were males, and the mean patient age was 71.56 (1.69) years. Sixteen patients (11.6%) had a history of revascularization, and five (3.6%) had undergone surgical amputation of one or both lower limbs. HBP, DLP, and DM were the most prevalent CVRFs, found in 71.7%, 66.7%, and 48.6% of the patients respectively. Forty-one (29.7%) patients had experienced at least one prior cardiovascular event, either cardiac or cerebral, 69 (75.8%) had oligoalbuminuria, 39 (29.3%) RF, and 46 ED (67.6%).
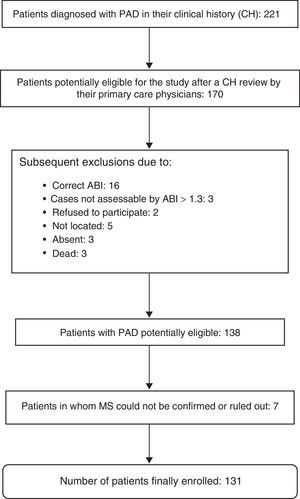
The presence or absence of MS could not be evaluated in seven patients, so that 131 patients were finally enrolled into the study. A diagnosis of MS was confirmed in 63 patients (48.1%). Fig. 1 shows the exclusion process followed until the final study population was obtained.
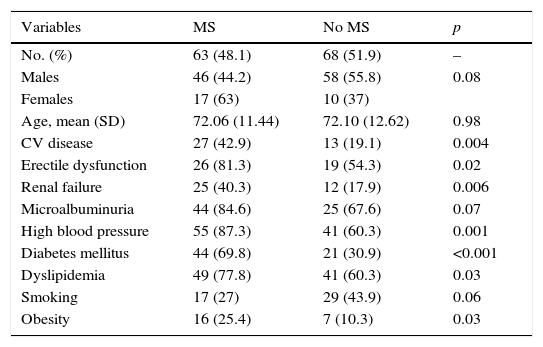
Table 1 shows the differences in distribution of CVRFs and comorbidities between patients with PAD with or without associated MS. Patients with MS had, as compared to those with no MS, a greater prevalence of HBP (87.3% vs. 60.3%; p: 0.001), DM (69.8% vs. 30.9%; p: <0.001), DLP (77.8% vs. 60.3%; p: 0.03), and obesity (25.4% vs. 10.3%; p: 0.03). Patients with MS had had more cardiac or cerebral ischemic events (42.9% vs. 19.1%; p: 0.004) and a greater prevalence of RF (40.3% vs. 17.9%; p: 0.006) and ED (81.3% vs. 54.3%; p: 0.02).
Risk factors, major cardiovascular disease, kidney disease, and erectile dysfunction in patients with peripheral artery disease with or without metabolic syndrome.
| Variables | MS | No MS | p |
|---|---|---|---|
| No. (%) | 63 (48.1) | 68 (51.9) | – |
| Males | 46 (44.2) | 58 (55.8) | 0.08 |
| Females | 17 (63) | 10 (37) | |
| Age, mean (SD) | 72.06 (11.44) | 72.10 (12.62) | 0.98 |
| CV disease | 27 (42.9) | 13 (19.1) | 0.004 |
| Erectile dysfunction | 26 (81.3) | 19 (54.3) | 0.02 |
| Renal failure | 25 (40.3) | 12 (17.9) | 0.006 |
| Microalbuminuria | 44 (84.6) | 25 (67.6) | 0.07 |
| High blood pressure | 55 (87.3) | 41 (60.3) | 0.001 |
| Diabetes mellitus | 44 (69.8) | 21 (30.9) | <0.001 |
| Dyslipidemia | 49 (77.8) | 41 (60.3) | 0.03 |
| Smoking | 17 (27) | 29 (43.9) | 0.06 |
| Obesity | 16 (25.4) | 7 (10.3) | 0.03 |
SD, standard deviation; CV disease, cardiovascular disease (ischemic heart disease or cerebrovascular disease); MS, metabolic syndrome.
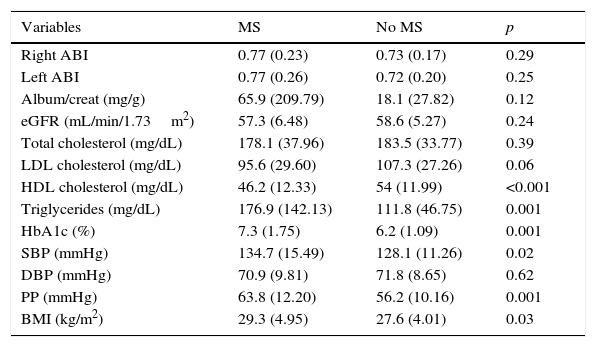
Table 2 shows the mean control values of the different CVRFs, the ABI, and kidney function parameters in patients with PAD with or without coexisting MS. Patients with concurrent PAD and MS had lower levels of HDL-C (46.18 vs. 54.03mg/dL; p: <0.001) and higher levels of TG (176.9 vs. 111.8mg/dL; p: 0.001), HbA1c (7.3% vs. 6.2%; p: 0.001), SBP (134.7 vs. 128.1mmHg; p: 0.02), PP (63.8 vs. 56.2mmHg; p: 0.001), and the BMI (29.3 vs. 27.6kg/m2; p: 0.03) than patients with no MS.
Mean control values of the different cardiovascular risk factors in patients with PAD with or without coexisting MS.
| Variables | MS | No MS | p |
|---|---|---|---|
| Right ABI | 0.77 (0.23) | 0.73 (0.17) | 0.29 |
| Left ABI | 0.77 (0.26) | 0.72 (0.20) | 0.25 |
| Album/creat (mg/g) | 65.9 (209.79) | 18.1 (27.82) | 0.12 |
| eGFR (mL/min/1.73m2) | 57.3 (6.48) | 58.6 (5.27) | 0.24 |
| Total cholesterol (mg/dL) | 178.1 (37.96) | 183.5 (33.77) | 0.39 |
| LDL cholesterol (mg/dL) | 95.6 (29.60) | 107.3 (27.26) | 0.06 |
| HDL cholesterol (mg/dL) | 46.2 (12.33) | 54 (11.99) | <0.001 |
| Triglycerides (mg/dL) | 176.9 (142.13) | 111.8 (46.75) | 0.001 |
| HbA1c (%) | 7.3 (1.75) | 6.2 (1.09) | 0.001 |
| SBP (mmHg) | 134.7 (15.49) | 128.1 (11.26) | 0.02 |
| DBP (mmHg) | 70.9 (9.81) | 71.8 (8.65) | 0.62 |
| PP (mmHg) | 63.8 (12.20) | 56.2 (10.16) | 0.001 |
| BMI (kg/m2) | 29.3 (4.95) | 27.6 (4.01) | 0.03 |
Data are given as mean (SD). Album/creat, the albumin/creatinine ratio in the first morning urine; SD, standard deviation; eGRF, the estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL, high density lipoprotein; BMI, the body mass index; ABI, the ankle-brachial index; LDL, low density lipoprotein; DBP, diastolic blood pressure; SBP, systolic blood pressure; PP, pulse pressure; MS, metabolic syndrome.
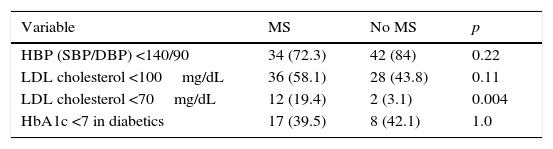
Table 3 provides the percentages of optimum BP, LDL-C, and HbA1c control achieved in both groups; only for LDL-C <70mg/dL did we find statistically significant differences favoring patients with PAD and MS (19.4% vs. 3.1%; p: 0.004).
Percentages of optimum blood pressure, LDL cholesterol, and HbA1c control depending on the presence or absence of coexisting MS.
| Variable | MS | No MS | p |
|---|---|---|---|
| HBP (SBP/DBP) <140/90 | 34 (72.3) | 42 (84) | 0.22 |
| LDL cholesterol <100mg/dL | 36 (58.1) | 28 (43.8) | 0.11 |
| LDL cholesterol <70mg/dL | 12 (19.4) | 2 (3.1) | 0.004 |
| HbA1c <7 in diabetics | 17 (39.5) | 8 (42.1) | 1.0 |
Data are given as n(%). HbA1c, glycosylated hemoglobin; HBP, high blood pressure; LDL, low density lipoprotein; DBP, diastolic blood pressure; SBP, systolic blood pressure; MS, metabolic syndrome.
Patients with PAD have a high prevalence of MS. Patients with both diseases have a greater prevalence of the main classical CVRFs, and also of RF, ED and established CVD as compared to those with PAD alone. The prevalence of MS is 48%, lower than the 63% recently reported by Estirado et al. in a multicenter study conducted on almost 4000 patients with PAD but with no associated ischemic cardiac or cerebral disease.22 These data contrast with those reported in the CLYDIA study conducted in Spain on patients diagnosed with any type of CVD (ischemic heart disease, cerebrovascular disease, or lower limb ischemia), in which 17.4% of patients with PAD also had MSM.23 Outside of Spain, the subject addressed here has similarly attracted the interest of many authors, who have also reported high prevalence rates, ranging from 50% to 60%.14,24,25
In our study, the fact that PAD coexists or not with MS did not determine statistically significant differences as regards the distribution of this disease by sex and age: the mean age was 72 years in both groups, while females were more likely to have MS (17 [63%] vs. 10 [37%]) with a trend to statistical significance, which was not reached.
All CVRFs, except smoking, for which a reverse relationship was shown, were significantly more prevalent in the group of patients with MS, which was as expected because most CVRFs are part of the diagnostic criteria of the condition. Similarly, HDL-C levels were significantly lower in patients with MS.
Both oligoalbuminuria and GFR values less than 60 were more prevalent in the group with concurrent PAD and MS; statistical significance was reached for RF.
ED is more prevalent in patients with MS. This association is documented and presumable, as clear relations have been established between this disease and the most relevant CVRFs, which are constituents of MS itself.8,26 A history of cardiac or cerebral ischemic CVD is more commonly seen in the group of patients with combined MS and PAD, a finding which is consistent with those reported in the studies reviewed by us, all of which consider MS and its components to be predictors of ischemic heart disease and cerebrovascular disease.20–26
In both groups, mean ABI severity was mild. Thus, in the reported case MS did not appear to be associated with more severe vascular involvement, unlike in other studies14 where patients with MS had more severe vascular disease.
As regards control of the different CVRFs, the high rates of optimum BP control achieved in both groups should be stressed. PP values were significantly higher in patients with MS, which agrees with the reports in other studies27–29 and is not surprising considering that a greater difference between systolic and diastolic pressure results in a greater predisposition to the occurrence of target organ lesion and CVD. No statistically significant differences were found in optimum control rates of HbA1c <7% and LDL-C <100mg/dL between the two groups, but a significant difference favoring the group of patients with MS was seen for LDL-C levels <70mg/dL. The results of the degree of control seen in our patients did not generally agree with the data reported in the literature reviewed, where patients with MS showed significantly worse control than patients with no MS, despite their greater use of drugs.22,28 The explanation may be that the healthcare professionals at our center are usually highly focused on cardiovascular prevention. Patients with MS were significantly more obese than those without MS, which was also found in the studies previously reviewed and discussed. This was to be expected because, like elevated BP and blood glucose, overweight and abdominal obesity are part of the diagnostic criteria of MS.
This study has several limitations. One of the most important limitations was the small patient sample: the 138 patients diagnosed with PAD at our center at the start of the study illustrate the high degree of underdiagnosis of the condition. This are various possible reasons for this, such as PAD being erroneously considered to be merely a “second category disease” when compared to the immediate risks associated with ischemic heart disease and CVD, or to the capacity of CVD to induce severe sequelae. The reason may lie in an inadequate understanding of the disease and of its role as a predictor of cardiac or cerebral events. Another limitation was the uncommon use of the ABI at our center before this study was conducted. When done manually, as occurred when data collection was performed, the ABI is a procedure that requires skills that were not well-developed at the time, as well as an examination time of approximately 20min, which is too long for the current organizational and care structure of primary care clinics. Therefore, the results should be taken with a certain caution, and in any case are only representative of the patients seen at our center. This, as well as the fact that this was a cross-sectional study, only allows us to venture associations between MS and PAD, although it is true that the results found differ little, in general, from those reported in a good number of the studies listed in the references.
On the other hand, the underreporting of waist circumference in the clinical histories forced us to use the BMI > 28.8kg/mm2 as a diagnostic criterion of MS, in the context of the modified definition proposed by the National Cholesterol Education Program,21 and supported by studies such as the one by Sattar et al.,30 amongst others. We are, however, aware of the fact that the BMI does not estimate abdominal obesity and, thus, insulin resistance as unequivocally as the measurement of waist circumference. This limitation may partly be compensated for by the fact that the study population consisted of patients monitored at clinics and consulting rooms for CVRFs other than obesity, so that the diagnosis of MS was made in most cases without the need for the BMI criterion.
Many patients are seen daily at primary care clinics, and a high proportion of them have other concurrent risk factors such as obesity, DM, HBP, and DLP. These patients often meet the diagnostic criteria for MS, and as this is undoubtedly associated with PAD, an estimation of the ABI should be considered to be a priority in these cases.17–26 The ABI is a simple, noninvasive technique well accepted by its users and rapidly performed with the new electronic Doppler models. We therefore think that the routine use of the ABI in patients with MS is indicated for the detection of patients with occult vascular disease.
Family physicians should be aware of the importance of making an early diagnosis of PAD both because of its local impact and for its predictive power regarding cardiac and cerebral ischemic events. We are prepared to provide integral care to patients, and occupy a strategic position within the healthcare system. Just because of that position and training, we should play a role in promoting joint strategies with the specialties concerned; specifically, with departments such as endocrinology and vascular surgery.
Conflicts of interestThe authors state that they have no financial relation or any other type of personal conflict as regards this study.
We thank the PC physicians who collaborated in the review of the clinical histories of their patients (Sonia Granado, María Isabel Fuentes, Belén Henares, Francisco Javier Molinero, Cristina Paíno, Hypatia Mejía, Maite Juan, José Luis Tandaipán, Sahara Benere, María del Claustre Roselló, Elizabeth Moreira, Flora López, and Sonia Miravet) and the nurses who confirmed the diagnosis of PAD by performing the ABI (Pere Trigos, Soledad Rosende, Eva Fontiverio, Susana Migueles, Natalia Mingorance, Ángeles López, Gemma Comerma, Ariadna Lapena, and Rubén Casas). We acknowledge the collaboration of all of them.
Please cite this article as: Oriol Torón PÁ, Badía Farré T, Romaguera Lliso A, Roda Diestro J. Síndrome metabólico y enfermedad arterial periférica: 2 enfermedades relacionadas. Endocrinol Nutr. 2016;63:258–264.