Hypophysitis represents a heterogeneous group of inflammatory lesions affecting the pituitary gland with a complex pathogenesis and is insufficiently characterized. The different types belong to the group of non-hormone secreting sellar masses, with which they share clinical, and sometimes also radiographic, presentation. These similarities make differential diagnosis difficult in the absence of surgery, which is not indicated in hypophysitis but allows for pathological study.1,2 The clinical signs of hypophysitis are similar to those of other sellar masses and include headache, visual disturbances, and variable degrees of hypopituitarism and diabetes insipidus.
Hypophysitis may be classified based on various criteria. Adenohypophysitis, infundibuloneurohypophysitis, and panhypophysitis are distinguished based on the anatomical location of the condition. There may be lymphocytic, granulomatous, xanthomatous, and mixed hypophysitis depending on the histopathological characteristics. Based on etiology, a distinction between primary hypophysitis, either isolated or as part of a systemic disease; hypophysitis secondary to sellar diseases such as germinoma, Rathke cyst, craniopharyngioma, or pituitary adenoma, or occurring in the setting of systemic diseases such as Wegener's granulomatosis, tuberculosis, sarcoidosis, and syphilis; finally, hypophysitis secondary to immunomodulatory drugs such as interferon and antibodies to cytotoxic T-lymphocyte antigen-4 (CTLA-4) has been reported.1
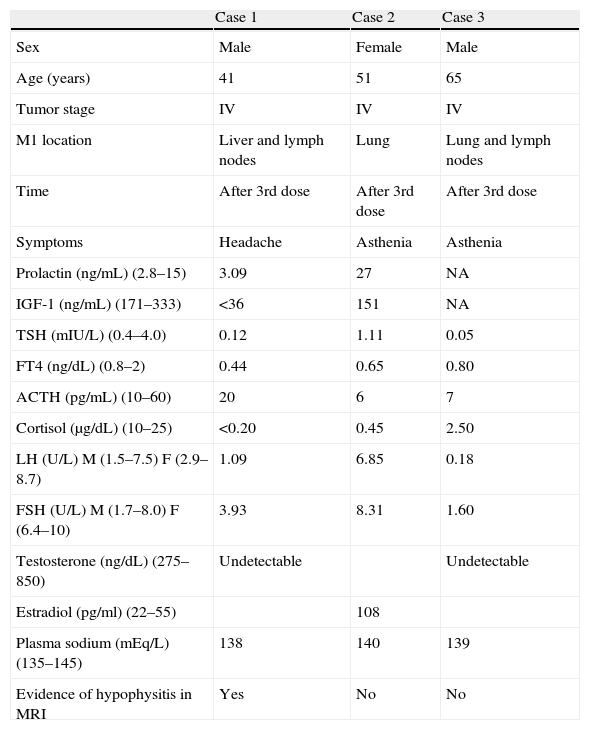
In the context of the use of immunomodulatory therapies, we report three cases of autoimmune hypophysitis secondary to ipilimumab occurring during the treatment of metastatic melanoma. Thirteen patients with metastatic melanoma have been treated to date with ipilimumab at our hospital. Table 1 summarizes the clinical and laboratory data.
Clinical and laboratory characteristics of the case series.
| Case 1 | Case 2 | Case 3 | |
| Sex | Male | Female | Male |
| Age (years) | 41 | 51 | 65 |
| Tumor stage | IV | IV | IV |
| M1 location | Liver and lymph nodes | Lung | Lung and lymph nodes |
| Time | After 3rd dose | After 3rd dose | After 3rd dose |
| Symptoms | Headache | Asthenia | Asthenia |
| Prolactin (ng/mL) (2.8–15) | 3.09 | 27 | NA |
| IGF-1 (ng/mL) (171–333) | <36 | 151 | NA |
| TSH (mIU/L) (0.4–4.0) | 0.12 | 1.11 | 0.05 |
| FT4 (ng/dL) (0.8–2) | 0.44 | 0.65 | 0.80 |
| ACTH (pg/mL) (10–60) | 20 | 6 | 7 |
| Cortisol (μg/dL) (10–25) | <0.20 | 0.45 | 2.50 |
| LH (U/L) M (1.5–7.5) F (2.9–8.7) | 1.09 | 6.85 | 0.18 |
| FSH (U/L) M (1.7–8.0) F (6.4–10) | 3.93 | 8.31 | 1.60 |
| Testosterone (ng/dL) (275–850) | Undetectable | Undetectable | |
| Estradiol (pg/ml) (22–55) | 108 | ||
| Plasma sodium (mEq/L) (135–145) | 138 | 140 | 139 |
| Evidence of hypophysitis in MRI | Yes | No | No |
ACTH, adrenocorticotropic hormone; M, male; IFG-1, insulin-like growth factor type 1; FSH, follicle-stimulating hormone; LH, luteinizing hormone; F, female; M1, primary metastasis; NA, not available; MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone; FT4, free thyroxine.
Case 1: A 41-year-old male with stage IV melanoma and hepatic and nodal metastases. After the third dose of ipilimumab, he experienced holocranial headache and decreased libido. Laboratory tests showed adrenal insufficiency and secondary hypothyroidism and hypogonadism. MRI revealed overall pituitary gland enlargement with heterogeneous uptake of intravenous contrast, with several low uptake foci, consistent with hypophysitis.
Case 2: A 51-year-old female with stage IV melanoma and lung metastases. After the third dose of ipilimumab, marked asthenia occurred. Hormone tests showed ACTH and TSH deficiency, and the pituitary image in MRI was normal.
Case 3: A 65-year-old male with stage IV melanoma and nodal and lung metastases. After the third dose of ipilimumab, he experienced asthenia and temporospatial disorientation. Hormone tests showed secondary corticotropic and gonadotropic deficiency, but FT4 levels were within normal limits. The involvement of all other axes showed the central origin of the thyrotropic deficiency. The pituitary image was normal.
All three patients were treated with high-dose glucocorticoids (dexamethasone 4mg/6h), which were tapered over one month until the replacement dose was reached. They also received hormone replacement therapy with levothyroxine and testosterone depending on the deficiencies found. At the date of submission for publication, these patients showed no recovery of the affected hormonal axes. They all received the fourth dose of ipilimumab as planned in their treatment schedule.
Disruption of the tolerance of the immune system to antigens located on tumor cells is one of the new approaches to cancer treatment.3
In the activation of cell-mediated immune response, T lymphocyte (TL) receptors interact with molecules of the major histocompatibility complex of antigen-presenting cells (APCs). Co-stimulation induced by binding of the B7 ligand of APCs to CD28 of TLs allows for activation of the latter. On the contrary hand, TL activation and proliferation are inhibited if a negative signal mediated by binding of the same B7 ligand of APCs to CTLA-4 occurs,4 with modulation of autoantibody formation.5
Ipilimumab (Yervoy; Bristol-Myers Squibb, Princeton, NJ, USA), a fully human monoclonal immunoglobulin (IgG1) against CTLA-4, prevents binding of the latter to the B7 receptor. This leads to TL activation and proliferation, which result in an antitumor effect and increased autoantibody formation. Ipilimumab was approved in March 2011 by the FDA and in July 2011 by the EMA for the treatment of metastatic melanoma. In addition, 47 clinical trials with this agent in various neoplasms are currently ongoing worldwide.6 Induction treatment consists of a cycle of four intravenous doses of 3mg/kg, repeated every 3 weeks.
Disruption of immune tolerance to neoplastic cell antigens is not free from adverse consequences related to the activation of autoimmune phenomena. Autoimmune hypophysitis secondary to treatment with ipilimumab has been reported in up to 17% of patients with melanoma and renal cell cancer receiving the drug.3 Hypophysitis occurs on average at week 11 of treatment, usually before the fourth dose, suggesting a cumulative effect. The clinical presentation does not differ from hypophysitis from other causes. ACTH and TSH are affected in all reported cases of ipilimumab-induced hypophysitis. Most males have hypogonadotropic hypogonadism, and only one case associated with diabetes insipidus3 and one with inappropriate arginine vasopressin secretion7 have been reported.
Pituitary MRI may show the typical changes found in autoimmune hypophysitis, such as global, uniform, and homogeneous gland enlargement, usually moderate. Decreased hyperintensity of the characteristic signal of neurohypophysis is sometimes seen. Cases of hypophysitis induced by ipilimumab with normal neuroimaging have been reported.
The treatment recommended for immune-mediated adverse effects is based on the use of high-dose glucocorticoids (dexamethasone 4mg/6h or methylprednisolone 1–2mg/kg/day), with the dose tapering over one month until glucocorticoid replacement doses are reached. It remains to be established whether these high glucocorticoid doses are needed, or whether treatment could be started at doses closer to physiological ones. On the contrary, levothyroxine and testosterone should be used at adequate replacement doses depending on the findings in each case.4,8
To date, a recovery of corticotropic function has been reported in only one case.3 Thyroid function is recovered in 37–50% of cases,3,9 and male gonadal function in 57%.10
In conclusion, we report three clinical cases of immune-mediated hypophysitis in the setting of the treatment of metastatic malignant melanoma with ipilimumab.
Because of the advent of new immunomodulatory therapies that may potentially induce adverse endocrine effects, it is important to identify and report this cause of hypophysitis and to remain alert to its potential occurrence. This will make it possible to prevent delays in treatment and to avoid the consequences of hypopituitarism, particularly unreplaced adrenal insufficiency.
Please cite this article as: de Hollanda A, Aranda GB, Mora M, Gaba L, Halperin I. Ipilimumab, una causa de hipofisitis autoinmunitaria. Endocrinol Nutr. 2013;60:604–606.