To describe baseline characteristics of diabetic patients who were started on insulin pump and real time continuous glucose monitor (CSII-rtCGM) in a specialized center in Medellin, Colombia.
Materials and methodsAll patients with diabetes with complete data who were started on CSII-rtCGM between February 2010 and May 2014 were included. This is a descriptive analysis of the sociodemographic and clinical characteristics.
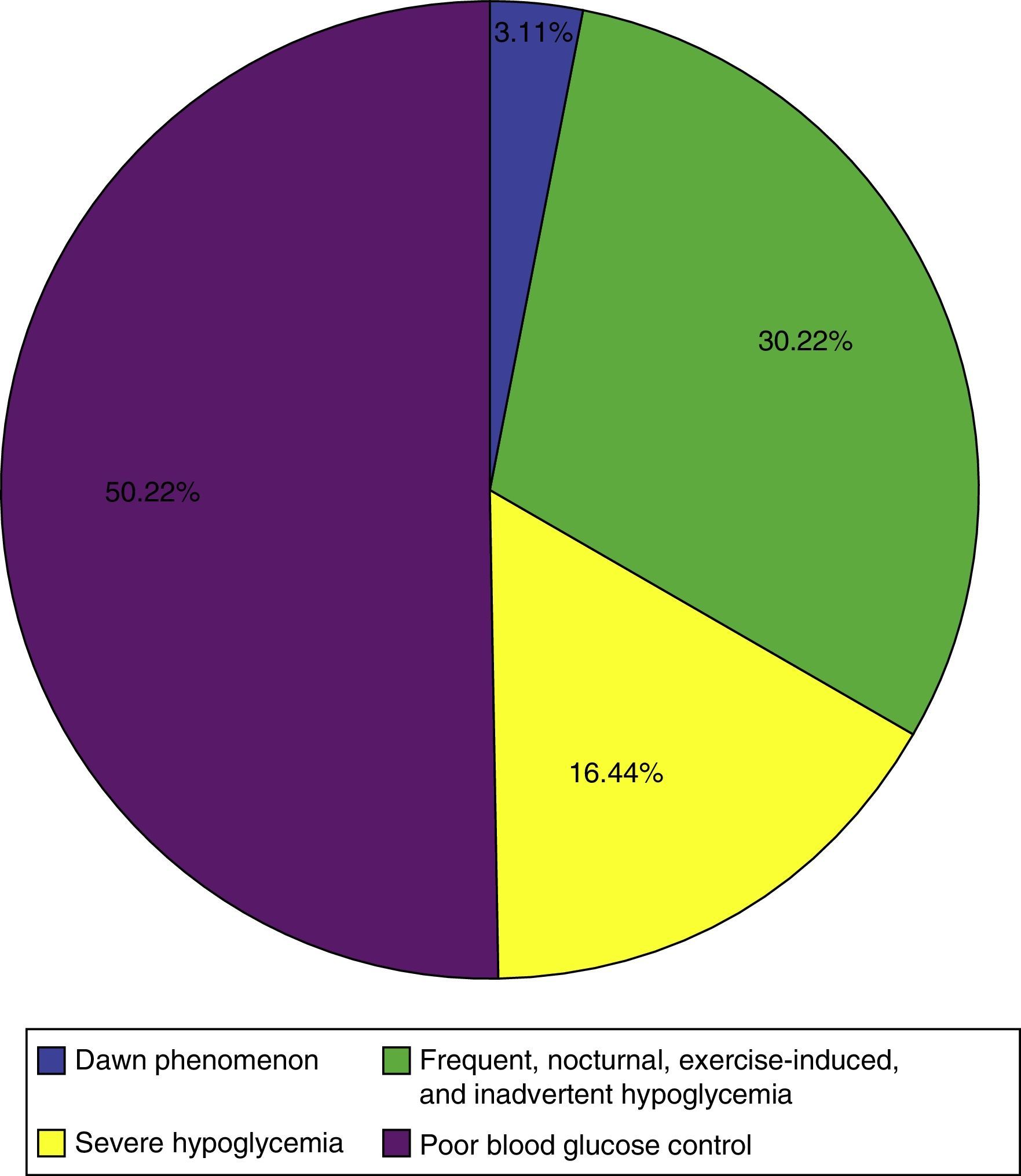
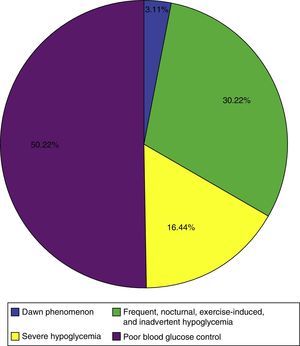
Results141 of 174 patients attending the clinic were included. 90.1% had type 1 diabetes (T1D). The average age of T1D patients at the beginning of therapy was 31.4 years (SD 14.1). 75.8% of patients had normal weight (BMI<25), 21.0% were overweight (BMI 25–30) and 2.3% were obese (BMI>30). The median duration of T1D was 13 years (P25–P75=10.7–22.0). 14.2% of the patients were admitted at least once in the year preceding the start of CSII-rtCGM because of diabetes related complications. Mean A1c was 8.6%±1.46%. The main reasons for starting CSII-rtCGM were: poor glycemic control (50.2%); frequent hypoglycemia, nocturnal hypoglycemia, hypoglycemia related to exercise, asymptomatic hypoglycemia (30.2%); severe hypoglycemia (16.44%) and dawn phenomena (3.1%).
ConclusionBaseline characteristics of patients included in this study who were started on CSII-rtCGM are similar to those reported in the literature. The Clinic starts CSII-rtCGM mainly in T1D patients with poor glycemic control, frequent or severe hypoglycemia despite being on basal/bolus therapy.
Caracterizar los pacientes con diabetes que iniciaron terapia con bomba de insulina y monitorización continua de glucosa en tiempo real (CSII-rtCGM) en un centro especializado de Medellín, Colombia.
Materiales y métodosSe evaluaron los pacientes con diabetes que recibieron entrenamiento e instalación del dispositivo en el centro entre febrero de 2010 y mayo de 2014. Se realizó un análisis descriptivo de las variables sociodemográficas y clínicas.
ResultadosSe incluyeron 141 pacientes de los 174 pertenecientes al programa. El 90,1% tenía diabetes tipo 1 (DT1), siendo la edad promedio al inicio de la terapia de 31,4 años (SD: 14,1). El 75,8% de los pacientes tenía peso normal (IMC<25), el 21,0% sobrepeso (IMC=25–30) y el 2,3% era obeso (IMC≥30). La mediana de duración de la enfermedad fue de 13 años (P25–P75=10,7–22,0). El 14,2% de los pacientes fueron hospitalizados al menos una vez en el año previo al inicio de la terapia con CSII-rtCGM por causas relacionadas con la diabetes. El promedio de hemoglobina glucosilada era de 8,6%±1,46%. Las principales indicaciones para la formulación de CSII-rtCGM fueron: mal control glucémico (50,2%); hipoglucemias frecuentes, nocturnas o asociadas al ejercicio y sin síntomas de alarma (30,2%); hipoglucemias severas (16,44%) y fenómeno del alba (3,1%).
ConclusiónLas características de los pacientes que inician terapia con CSII-rtCGM son similares a las reportadas en la literatura. Los resultados muestran que el centro especializado inicia esta terapia principalmente en pacientes con DT1 con mal control glucémico a pesar de la terapia intensiva, hipoglucemias leves persistentes o hipoglucemias severas.
Diabetes is a disease where the pancreas does not adequately satisfy insulin demand. This causes increased blood glucose levels which lead to the occurrence of acute consequences (hyperglycemic crises) and chronic complications.1 The current worldwide prevalence of diabetes is 6.4%,1 and its predominant form is type 2 diabetes mellitus (T2DM).2,3 A considerable increase in diabetes incidence and prevalence is predicted in developing countries as the result of sedentary lifestyles and obesity.
Although no curative therapy is available for the disease, it is well known that strict blood glucose control decreases the associated complications.4–6 Intensive insulin schemes based on blood glucose levels and on the amount of carbohydrates to be ingested, implying multiple daily injections, have therefore been used.4,7,8 Continuous subcutaneous insulin infusion with real time continuous glucose monitoring (CSII-rtCGM), as a new approach that mimics pancreatic physiology, provides rapid-acting insulin analogs as basal supplies 24h a day, with the administration of higher doses before meals, making it possible to know glucose values in interstitial tissue as a reflection of real time blood glucose, and indicating when the patient needs to take action.4,9 The use of CSII-rtCGM has allowed for improved blood glucose control and has provided a tool for continuous insulin provision with continuous glucose monitoring. There is currently no consensus as to which patients benefit most from this therapy, although some medical indications are recognized, including poor blood glucose control, the dawn phenomenon, and severe, unnoticed, nocturnal and exercise-induced hypoglycemia. Patient adherence and empowerment regarding his/her disease are essential.9,10
Approximately 400,000 patients with diabetes in the United States are receiving therapy with continuous subcutaneous insulin infusion,11 including children, adults, elderly, and pregnant women. Consequently, it is common there to see patients with this device in clinical practice.12,13 In Colombia, only a few centers specialize in the training, qualification, installation, and monitoring of patients on therapy with CSII-rtCGM. The purpose of this study is to report the epidemiological characteristics of diabetic patients who were started on this therapy at one of the specialized centers in Medellín, Colombia.
Materials and methodsAn observational, retrospective study was conducted at a center specializing in endocrinology in Medellín, Colombia. The study sample consisted of all patients, children and adults, with type 1 and 2 diabetes mellitus in the CSII-rtCGM program who received training and had the device implanted at the center between February 1 2010 and May 31 2014. Patients with devices implanted at other centers or with incomplete insulin pump formulation parameters were excluded.
Sociodemographic, anthropometric, clinical, and laboratory variables were collected. The clinical history was considered the primary information source for the study. An investigator reviewed the eligibility criteria of the patients and recorded information on the variables of interest in a recording instrument specifically designed for this purpose; the information was subsequently entered into a Microsoft Access® database. The data were analyzed using IBM SPSS Statistics 22® statistical software.
To minimize potential information bias, an investigator reviewed any inconsistencies between the information in the clinical history and the information included in the recording instrument and databases.
A descriptive analysis was made of the study variables using such measures as the absolute and relative frequencies for qualitative variables (sex, social security affiliation, education level, comorbidities, type of diabetes mellitus, severe and non-severe hypoglycemic episodes, any history of hospitalizations for complications and/or acute decompensation associated with the disease, treatment received before device implantation, microvascular and macrovascular complications from diabetes at the start of treatment with the insulin pump).
For the quantitative variables of age, the body mass index, glycosylated hemoglobin (HbA1C) at device implantation, the annual number of hospitalizations for causes related to diabetes, the time from the diagnosis of diabetes to the start of treatment with CSII-rtCGM, and the time from the start of CSII therapy to the insertion of the real time continuous glucose monitoring (rtCGM) sensor, measures of central tendency (mean or median) and dispersion (standard deviation interquartile range: 25th–75th percentile) were obtained according to the normal distribution of the assessed variable in the Kolmogorov–Smirnov test. No comparisons were made and no measures of association were established in the study.
Severe hypoglycemia was defined as an episode that required the assistance of another person for the active administration of carbohydrates or glucagon or any other corrective action. Although plasma measurements may not have been available during the episode, neurological recovery following the normalization of glucose levels was considered adequate evidence of hypoglycemia. Symptomatic hypoglycemia was defined as any episode of glucose levels less than 70mg/dL in a diabetic patient associated with neuroglycopenic and autonomic symptoms and signs which was resolved with carbohydrate intake (Whipple's triad). Asymptomatic hypoglycemia was defined as any episode of glucose levels less than 70mg/dL in a diabetic patient not associated with the symptoms. Symptomatic and asymptomatic hypoglycemia not meeting severity criteria were considered as non-severe, and could be or not be nocturnal and/or associated with exercise.
All patients were trained by a multidisciplinary group including a diabetes educator, a nutritionist, and an endocrinologist. A Paradigm VEO system from Medtronic was used in all cases.
The research protocol was evaluated and approved by the university ethics committee of the center specializing in endocrinology.
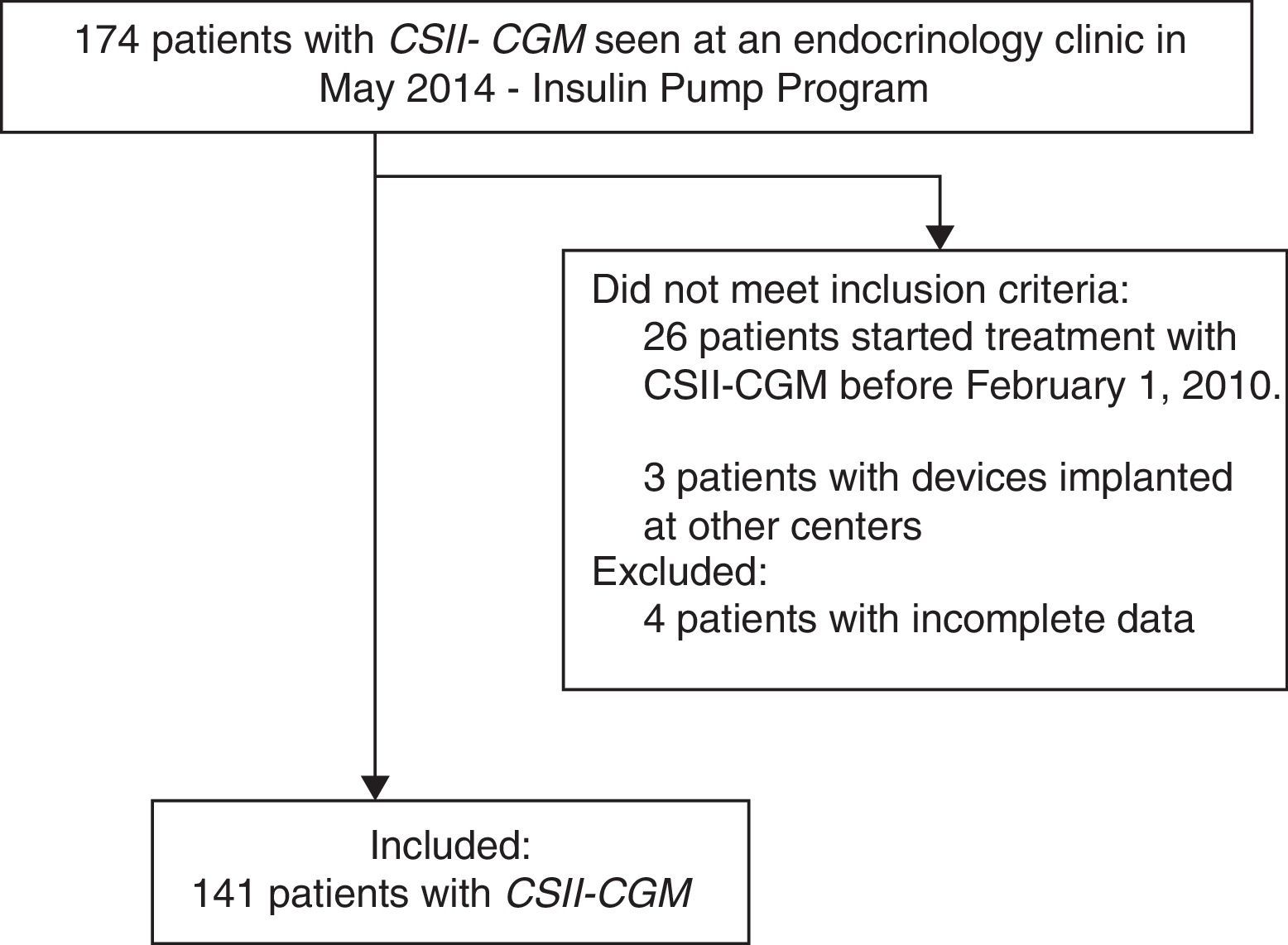
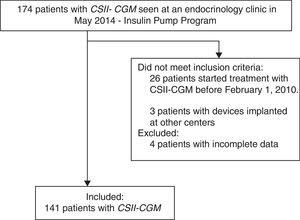
ResultsA total of 174 patients treated with CSII-rtCGM seen at the institution until May 2014 were identified. One hundred and forty-one diabetic patients meeting the eligibility criteria were recruited, and four patients were excluded due to incomplete records (Fig. 1). 90.1% (127/141) of patients had T1DM, three patients were pregnant at the time of device implantation, and 9.9% (14/141) had T2DM.
The mean patient age was 33.6±15 years, 54.0±13.7 years in patients with T2DM and 31.4±14.1 years in those with T1DM; 20.6% of the patients were under 18 years of age. 70.2% of patients were female; 56.0% had received secondary education, 30.5% professional education, 12.1% primary education, and 1.4% higher education. All study patients were affiliated to the Colombian social security system. At device implantation, 4.3% of patients were active smokers and 6.4% former smokers. In their clinical history, 41.1% of the patients reported taking physical exercise.
The median body mass index (BMI) was 23.3 (P25–P75=20.7–24.8); 75.8% of patients had normal weight (BMI<25), 21.0% were overweight (BMI>25 and <30), and 2.3% were obese (BMI≥30). Analyzing patients with T1DM alone, the median BMI was 22.0 (P25–P75=20.0–24.0), while the median BMI in patients with T2DM was 28.0 (P25–P75=23.0–29.0).
The median diabetes duration in the evaluated patients was 13.0 years (P25–P75=10.7–22.0). As regards the microvascular complications associated with diabetes, 9.9% of the patients had diabetic retinopathy, which was proliferative in most cases (12/14). Nephropathy was found in 15.6% of diabetic patients, and one of them had a kidney transplant for this reason. Neuropathy was found in 14.2% of patients. Sensitive neuropathy was most common (17/20), followed by erectile dysfunction (2/20) and gastroparesis (1/20). A single patient had diabetic foot. There were three patients with at least one macrovascular complication such as cerebrovascular disease, heart failure secondary to coronary disease, occlusive arterial disease, and intermittent claudication.
In addition to diabetes, 31.2% of the patients had thyroid disease (autoimmune thyroid dysfunction requiring treatment), 27.0% had dyslipidemia, 20.6% high blood pressure, and 6.4% an associated autoimmune disease other than thyroid disease.14.2% of the patients were admitted to hospital at least once in the year before the start of therapy with CSII-rtCGM for causes related to diabetes. The observed hospitalization rate was 0.3 hospitalizations/patient/year (range=0–13).
Median daily blood glucose measurements were 6.0 (P25–P75=4.0–6.0). Before CSII-rtCGM therapy was implemented, 95.0% of the patients reported at least one hypoglycemic episode in the previous year, and 39.0% of the patients reported at least one episode of severe hypoglycemia. The severe hypoglycemia rate was 14.7 episodes/patient/year. Non-severe hypoglycemia episodes were reported as symptomatic in 49.6% of cases, while 40.6% were not classified, and 9.8% were rated as asymptomatic based on the finding of a self-monitoring value less than 70mg/dL and the absence of neuroglycopenic symptoms reported by the patient.
All patients were receiving insulin analogs in a basal/bolus regimen to treat the disease before the start of therapy with CSII-rtCGM. Insulin combined with oral hypoglycemic drugs, mainly metformin, was received by 7.8% of the patients.
The mean HbA1C level was 8.6%±1.4 at treatment start, 8.5±0.1 in patients with T1DM and 9.1±0.3 in those with T2DM.
The main indications found for continuous insulin infusion with continuous glucose monitoring are shown in Fig. 2. More than one indication for the pump was found in 59.5% of the patients; poor blood glucose control and severe hypoglycemia was the most common combination.
As regards the formulation parameters of the continuous subcutaneous insulin infusion device, in agreement with the general rules for starting therapy with CSII-rtCGM, the median basal rate (the number of basal rates programmed for each patient during the day) at the start was 1.0 (P25–P75=1.0–1.0) and median sensitivity (the insulin sensitivity factor) was 50.0 (P25–P75=50.0–50.0); this was calculated using the formula 1700/total daily insulin dose.
The sensor was inserted some days before the device in four patients, but a majority of patients (61%) received both simultaneously. In 49 patients (34.7%) the sensor was inserted within three months of the device being inserted, and in two patients more than one year later (457 and 744 days). Patient education was the key criterion when choosing the time for sensor insertion.
DiscussionIntensive insulin treatment traditionally involves multiple daily injections,5 and although it improves blood glucose control, it also tends to increase the frequency of hypoglycemic episodes.4 Regimens that allow for adequate insulin provision and minimize the risk of hypoglycemia through frequent glucose monitoring are therefore required. Innovations in insulin administration and glucose monitoring have been designed in an effort to improve blood glucose control and patient quality of life. These advances include CSII-rtCGM therapies, which allow for continuous subcutaneous infusion and real time continuous glucose monitoring.5,7
The mean age of all patients at the start of therapy with CSII-rtCGM in our study was 33.6±15 years (90.1% with T1DM), similar to that previously reported in another study where 91.7% of patients had T1DM (34.1±17.1 years);14 if patients with T1DM are considered alone, the mean age was 31.4±14.0, which agrees with most studies reported in the literature,15–24 while the mean age was higher in patients with T2DM (54.0±13.7), as has also been reported.25–27 The efficacy and safety of therapies with CSII-rtCGM has been shown in elderly patients with T2DM26 and in patients over 50 years of age with T1DM.28
Few data are available on pregnant women using these therapies, but they appear to be promising in this group of patients.12 In our study, three patients with T1DM were pregnant. In the population analyzed, the BMI was similar to that reported in other studies evaluating diabetic patients who started therapy with CSII-rtCGM.14,15,17,18,20–23 Studies on patients with T2DM alone reported higher BMI values.25–27
The proportion of smoking diabetic patients who started therapy with CSII-rtCGM has been reported as ranging from 4.0% to 22.8%.17,21,25,26
Although disease duration or time from the diagnosis of diabetes to device insertion was approximately 13.0 years,14,17–23,25–27 there are studies in patients with T1DM reporting the start of CSII-rtCGM at diagnosis or shortly thereafter (3.3±3.0 years).16
Chronic complications associated with diabetes are common. Thus, retinopathy is reported in 21.0–77.0% of diabetic patients.14,15,20,25–27 The reason for such a variable percentage is that the occurrence of retinopathy depends on the type of diabetes and the time since disease onset. However, a much lower proportion was found in our cohort. The incidence of nephropathy and neuropathy agreed with that reported in other studies.15,20,25–27 Interestingly, retinopathy was less common than nephropathy in our cohort. This may be explained by the fact that ophthalmology reports were not available for all patients, or by the trend to start therapy with CSII-rtCGM in patients with poorly controlled diabetes and evidence of diabetic nephropathy, and could represent a selection bias.
In addition, the proportion of macrovascular complications in our study agreed with that reported in patients with T1DM,14,15 but was lower than that reported in patients with T2DM.25,26
Hospitalization rates of 8.5 days/patient/year before the start of CSII-rtCGM have been reported in the literature.20 Hospitalization days per patients could not be quantified in our study, but it was established that 14.2% of our patients had at least one hospitalization episode for reasons related to diabetes in the year before the new treatment was started. With a hospitalization rate of 0.3 hospitalizations (events)/patient/year, it cannot be ruled out that this variable was underestimated due to reporting problems.
Our study patients had a median of 6.0 glucose measurements daily. This measurement was made after the educational program was started, just before device implantation, and suggested that patients adhered to the treatment and that the education provided by our group probably influenced this result. In our study, 95.0% of patients reported hypoglycemia before therapy, and this was the indication for the use of the device in almost half the cases. New insulin pumps with a continuous glucose monitoring sensor make it possible to set a reference glucose threshold below which insulin infusion is stopped, thus avoiding severe hypoglycemic episodes. Recent studies assessing this strategy have shown that nocturnal hypoglycemia episodes are decreased without altering blood glucose control.18 In one study, up to 10.6% of diabetic patients treated with multiple insulin doses reported at least one episode of severe hypoglycemia in the previous month, and the monthly frequency of hypoglycemia episodes reported (both severe and non-severe) was 3.3±3.5 (mean±SD).17 Thirty-nine percent of patients in the assessed population reported at least one episode of severe hypoglycemia in the previous year, and the severe hypoglycemia rate was 14.7 episodes/patient/year. In another study, 13.6% of patients reported that they had experienced severe hypoglycemia the year before the start of the new treatment with the device.19
Patients with T1DM and inadvertent hypoglycemia and/or a marked increase in glucose levels at dawn despite adjustments in intensive therapy with regimens of multiple daily insulin injections are good candidates for CSII-rtCGM.24 In most studies evaluating the efficacy and safety of CSII-rtCGM, the main indication is poor blood glucose control despite the implementation of conventional intensive therapies.14–17,19–28 Other studies, however, have examined the appropriateness of these devices for patients with severe, inadvertent, or nocturnal hypoglycemia.14,18
Regarding its efficacy and safety compared with that of standard intensive regimens, the mean glycosylated hemoglobin values reported in the literature at the time of the start of treatment with insulin pump in clinical trials in patients with T1DM were 8.3±0.5 and 8.4±0.9 (mean±SD), very close to those found by us.19,21 Retrospective evaluations found mean HbA1C levels of 8.0%±0.9 and 8.9±1.9 at the start of therapy with this device.14 HbA1C levels were much lower (7.2%±0.7) in patients in whom the start of treatment was related to nocturnal hypoglycemia.18
Approximately 30.0% of patients with T2DM have poor blood glucose control despite the implementation of clinical practice guidelines, and they have HbA1C levels higher than 8.0%.25 Treatment with CSII-rtCGM could be an alternative in this setting because patients with T2DM may be trained to use it, and it may be considered when intensive insulin regimens are indicated.26,27 One study reported the use of CSII-rtCGM associated with oral hypoglycemic drugs in a group of patients with T2DM with a mean HbA1C value of 9.4±0.8%.25 Initial HbA1C values of 8.2%±1.3% and 8.4±1.1% (mean±SD) have also been reported in the literature as adequate for starting therapy with CSII-rtCGM in patients with T2DM.26,27
A significant finding was that one third (31.2%) of our patients had autoimmune thyroid dysfunction amenable to treatment in addition to diabetes, and since 90.1% of the patients have T1DM, thyroid function screening tests are warranted in this population according to the guidelines. Other comorbidities such as high blood pressure, which was reported in up to 76.4%25 of patients with T2DM, were less common in our cohort, which is probably explained by the greater number of patients with T1DM, but the proportion of patients with dyslipidemia was similar to that previously reported.25,26 Although 6.4% of the patients in our study reported at least one autoimmune disease other than thyroid disease, no data have been reported in the literature on the frequency of other autoimmune diseases in diabetic patients who are started on treatment with CSII-rtCGM.
At our center, all therapies with CSII-rtCGM were started using insulin analogs. Insulin analogs result in a significant decrease (0.2%) in HbA1C values as compared to crystalline insulin when used in continuous subcutaneous glucose infusion devices, and are preferred by patients.29 Some studies report fewer hypoglycemia episodes with insulin analogs, but this varies depending on the definition used; no differences are found in insulin dose or weight when analogs are compared with crystalline insulin.29
The start of continuous glucose monitoring at the same time or very close in time to the insertion of the system for continuous subcutaneous insulin infusion may promote adherence. As efficacy is directly related to days of use, either both systems are inserted at our center simultaneosly, or the monitoring device is inserted within three months of the infusion device implant, which is authorized by the Colombian health system. A controlled clinical trial is currently ongoing in children and adolescents (aged 5.0–18.0 years) with T1DM diabetes who will start treatment with CSII-CGM. This trial will compare the simultaneous start of both systems to sensor insertion six months after pump infusion is started. The objective is to assess whether this has implications for treatment efficacy and safety in this population groupl,16 which is among the least adherent.30
The limitations of our study include its retrospective nature and potential bias, as patients attended a specialized endocrinology clinic in Medellín (Colombia). Our description of these subjects is warranted by the novelty of the therapy. It should be noted, however, that only four patients who met the study inclusion criteria were excluded for incomplete data, which suggests that the clinical histories were complete.
In our experience, the characteristics of the patients who started treatment with CSII-rtCGM are similar to those reported in the literature. The results show that the specialized center mainly starts this therapy in patients with T1DM with poor blood glucose control despite intensive treatment, mild persistent hypoglycemia, or severe hypoglycemic episodes.
Conflicts of interestWe state that none of the investigators has any conflict of interest.
Please cite this article as: Aristizábal N, Ramírez A, Hincapié-García J, Laiton E, Aristizábal C, Cuesta D, et al. Caracterización epidemiológica de pacientes diabéticos en terapia con infusión subcutánea continua de insulina y monitorización continua de glucosa en tiempo real. Endocrinol Nutr. 2015;62:451–457.