X-linked adrenoleukodystrophy (X-ALD) is the most common peroxisomal disease, with an estimated incidence of 1/40,000 births, and occurs almost exclusively in males.1 Due to the lack of a specific effective treatment, Lorenzo's oil has been used for years as a common therapy for all patients with X-ALD.1 Today, it appears that the most promising characteristic of this substance is its potential preventive action, but it is ineffective once the disease has become manifest.1–4
A 18-year-old male patient with type A hemophilia had been diagnosed with X-ALD at two years of age in Portugal. His family history included hemophilia on his maternal side, as well as a maternal cousin who had died from X-ALD. Despite recommendations, his mother had refused prenatal genetic counseling. At four years of age he started to receive treatment with Lorenzo's oil (35mL/day), which was given for one month only. Treatment was subsequently discontinued when he moved home. From four to seven years of age he underwent regular brain magnetic resonance imaging (MRI) studies and adrenal function tests at the pediatric department, always with normal results. At seven years, the patient discontinued monitoring. The patient remained asymptomatic and with good physical, psychological, and neurological development until 20 years of age. At that age, he attended the emergency room for a syncopal episode and a partial seizure. The imaging tests and electroencephalogram (EEG) performed gave normal results. Two years later, he experienced several generalized tonic–clonic seizures that required admission. On symptom-oriented questioning, the patient reported difficulties in reading and understanding over the previous few months and impaired balance. An EEG confirmed the existence of left temporal focal neurological signs, and MRI revealed bilateral subcortical white matter disease with impaired myelination, secondary to X-ALD. Laboratory tests confirmed lipid profile changes with accumulation of very long chain saturated fatty acids (VLCFAs): C22:0, 2.15 (normal: 0.18–0.48); C24:0/C22:0 ratio, 1.45 (normal <1); C26:0/C22:0 ratio, 0.1 (normal <0.02). Treatment was started with valproic acid (500mg/24h), and the patient was referred to the department of endocrinology and nutrition for hormone testing and assessment of treatment with Lorenzo's oil. Physical examination findings included: weight 68kg, height 172cm, BMI 23.3kg/m2, blood pressure 120/75mmHg. The examination was otherwise unremarkable, with no findings suggesting adrenal insufficiency or hypogonadism. Hormone test results were normal. A dietary survey showed a varied intake with no food restriction. Treatment with Lorenzo's oil was not considered to be indicated because of the neurological signs found, its proven lack of efficacy in these situations, and its potential adverse effects (thrombocytopenia) in a patient with his underlying characteristics (hemophilia). The potential benefit/risk of hematopoietic stem cell transplantation (HSCT) is currently being assessed.
The main metabolic defect in X-ALD is the accumulation of VLCFAs in tissue, mainly in cerebral white matter, adrenal cortex, and testes. This is due to different mutations in the ABCD1 gene, which encodes for a protein in the peroxisome membrane (ALDP) which is involved in the degradation of these fatty acids by beta oxidation.1 A final diagnosis of X-ALD is made by confirming increased levels of VLCFAs (specifically C26:0, C24:0, and C22:0) in plasma and epithelial fibroblast cultures.1 Of these, C26:0 is the most consistently elevated, and is therefore considered to be diagnostic of the disease. Although patients with ketogenic diets also have elevated VLCFA levels, these are only found in plasma, but not in fibroblasts.1 Moreover, measurement of these VLCFAs and polyunsaturated acids allows for the diagnosis of dietary deficiencies during follow-up. Biochemical testing of relatives is crucial, because it allows for the diagnosis of presymptomatic hemizygous males and for providing genetic counseling to heterozygous females.
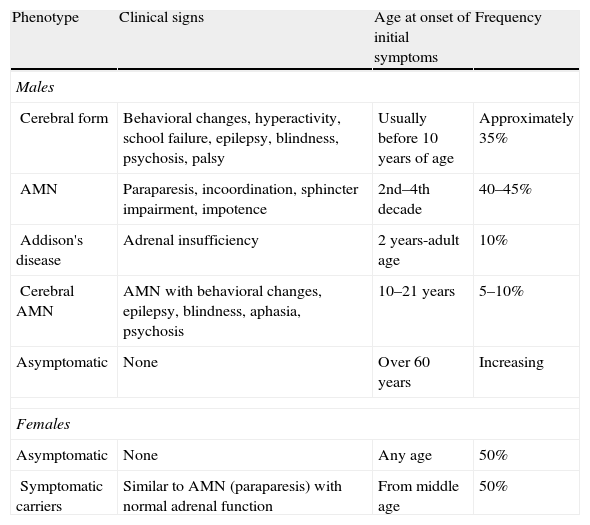
There are several phenotypes (Table 1),5 including the cerebral form (in childhood, adolescence, or adulthood), a form of slowly progressive paraparesis (AMN) in adults, and even asymptomatic cases. Adrenal involvement may occur at any age regardless of the type of neurological involvement. In fact, 10% of patients only have Addison's syndrome.1 Adults may also experience testicular dysfunction with hypogonadism and impotence.
Phenotypes of X-ALD.
| Phenotype | Clinical signs | Age at onset of initial symptoms | Frequency |
| Males | |||
| Cerebral form | Behavioral changes, hyperactivity, school failure, epilepsy, blindness, psychosis, palsy | Usually before 10 years of age | Approximately 35% |
| AMN | Paraparesis, incoordination, sphincter impairment, impotence | 2nd–4th decade | 40–45% |
| Addison's disease | Adrenal insufficiency | 2 years-adult age | 10% |
| Cerebral AMN | AMN with behavioral changes, epilepsy, blindness, aphasia, psychosis | 10–21 years | 5–10% |
| Asymptomatic | None | Over 60 years | Increasing |
| Females | |||
| Asymptomatic | None | Any age | 50% |
| Symptomatic carriers | Similar to AMN (paraparesis) with normal adrenal function | From middle age | 50% |
AMN: slowly progressive paraparesis in adults.
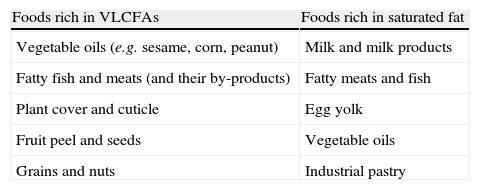
Disease management consists of 1) preventive treatment (diet plus Lorenzo's oil) or 2) symptomatic treatment. The goal of diet is the restriction of VLCFAs and saturated fats to prevent their accumulation (Table 2). For this, 5–10mg/day of C26:0 are given, and fat provision is restricted to 15% of the total calorie supply. A multidisciplinary team, including a dietician who can provide comprehensive dietary education to patients and their relatives and can supervise and give guidance on the preparation of adequate and more palatable menus, is essential to ensure compliance with these recommendations. Since decreased intake is not sufficient to decrease VLCFA levels, so-called Lorenzo's oil (GTO/GTE), a mixture of glyceryl trioleate [GTO] and glyceryl trierucate [GTE] in a 4/1 ratio, is also administered. This decreases or normalizes VLCFA levels. However, we know now that VLCFA levels are not correlated to the degree of neurological involvement, and the normalization of such levels cannot therefore be used as a marker of treatment success. Various reports (using controversial design and results) suggest that the administration of GTO/GTE to asymptomatic patients may prevent the occurrence of neurological symptoms,2,4–8 although further studies are needed. Thus, it appears to be indicated for patients with no neurological symptoms, preferably under 8–10 years of age with normal MRI. In older patients, GTO/GTE is seldom recommended because the risk of developing the cerebral form is low. It is not recommended either in children under one year of age, because it may decrease the levels of essential fatty acids, particularly of docosahexaenoic acid. No benefit has been demonstrated in AMN, although if treatment has been started and is well tolerated, it may be continued. Controversy exists not only on whether Lorenzo's oil is indicated or not, but also on the duration of its administration. Because of the lack of long-term studies (with more than 10 years of follow-up) assessing this aspect, treatment duration should be individualized. Treatment may generally be continued for an indefinite time, since its benefits disappear upon discontinuation, and may be stopped if disease progression and severe side effects occur.
Main foods to be avoided in X-ALD.
| Foods rich in VLCFAs | Foods rich in saturated fat |
| Vegetable oils (e.g. sesame, corn, peanut) | Milk and milk products |
| Fatty fish and meats (and their by-products) | Fatty meats and fish |
| Plant cover and cuticle | Egg yolk |
| Fruit peel and seeds | Vegetable oils |
| Grains and nuts | Industrial pastry |
GTO/GTE is administered at doses of 2–3mL/kg/day to cover 20% of daily calorie requirements. It is usually well tolerated, and its most severe side effects are thrombocytopenia9 (which resolves when treatment is discontinued) and lymphopenia.10 For this reason, a complete blood count is recommended every three months. Every six months, liver enzyme, VLCFA, and monounsaturated and polyunsaturated fatty acid levels should be measured; detailed neurological, neuropsychological/intelligence, and endocrinological (monitoring adrenal function) examinations should be made; and evoked potentials should be recorded.
Once neurological involvement has occurred, GTO/GET is ineffective in preventing disease progression or achieving symptom remission.1–5 It has been speculated that this may occur because, as in phenylketonuria, there is no response to the correction of the biochemical changes once neurological damage has occurred, or because GTO/GTE decreases VLCFA levels in plasma, but not in the brain.11 After the publication of the most recent controlled, randomized studies, the potential clinical benefits of lovastatin12 and immunosuppressant and immunomodulatory treatments such as cyclophosphamide, interferon beta, or intravenous immunoglobulins also appear to be ruled out.13 In this situation, the only treatment that could currently be effective (provided a compatible donor exists) is HSCT in the phase of early cerebral disease, although this does not correct an existing adrenal insufficiency.14 In addition, it has recently15 been found that the combination of Lorenzo's oil with docosahexaenoic acid supplementation appears to increase levels of this acid in plasma and red blood cells and may have a neuroprotective anti-inflammatory mechanism. Novel therapies to manage this disease are expected to be available in the coming years, based on the existence of promising treatments currently being assessed,13 including: a) autologous bone marrow transplantation (useful in the absence of a compatible donor) combined with gene therapy (in this case, bone marrow cells would be genetically modified ex vivo by transferring the ABCD1 gene with the help of a lentiviral vector derived from the modified and inactivated acquired immunodeficiency virus); b) pharmacological induction of ABCD2 (the peroxisomal transporter most homologous with ABCD1) using histone deacetylase inhibitors such as valproic acid (with added antioxidant effects) or 4-phenylbutyrate (in these patients, induction of ABCD2 could compensate for the functional deficiency of ABCD1 and normalize beta oxidation, avoiding the accumulation of VLCFAs); c) combinations of antioxidants such as N-acetylcysteine with lipoic acid and vitamin E (mainly in AMN); or d) the administration of neurotrophic factors such as insulin growth factor-1 or neurotrophin-3.
In conclusion, although additional controlled, large, and long-lasting studies are needed to confirm it, Lorenzo's oil could be effective in preventing neurological manifestations in asymptomatic patients with X-ALD. In patients with neurological involvement, the use of Lorenzo's oil does not change the course of the disease, and early HSCT is currently the treatment of choice. The development of new therapies is expected in the near future.
Please, cite this article as: Prieto Tenreiro A, et al. Tratamiento dietético de la adrenoleucodistrofia ligada a X: ¿es útil el aceite de Lorenzo? Endocrinol Nutr. 2013;60:37–47.