Boucher–Neuhäuser syndrome (BNS) is a clinical condition characterized by the triad of hypogonadotropic hypogonadism, cerebellar ataxia, and retinochoroidal degeneration.1,2 BNS is difficult to diagnose in its early stages because the triad components occur at different times in the lives of patients with a wide variability in the few subjects reported to date.3
We report the case of a 26-year-old male patient from the state of Merida (Venezuela) with no family history of consanguinity or significant diseases in his parents or siblings. The patient was referred to the endocrinology unit due to an absence of secondary sexual characteristics, in addition to bilateral visual acuity decrease from 16 years of age and unstable gait from 20 years of age.
A physical examination revealed a body weight of 57kg, a height of 160.0cm, an arm span of 165.7cm, a body mass index of 22.2kg/cm2, eunuchoid habitus, acute voice, bilateral gynecomastia, bilateral horizontal nystagmus with rapid phase to the right, corrected visual acuities in counting fingers at 50cm in the right eye and 20/80 in the left eye, intraocular pressure of 12mmHg in both eyes, Tanner II pubic hair, a 4cm-long penis, and right and left testes of 2 and 3mL respectively (Prader orchidometer), both in hypoplastic scrotal sacs. Neurological examination revealed ataxic gait, truncal ataxia mainly on the right, dystonic posture, dysdiadochokinesia, dysmetry, hypotonia, and global hyperreflexia. No changes were found in superficial or deep sensitivity, nor anosmia or cardiovascular changes.
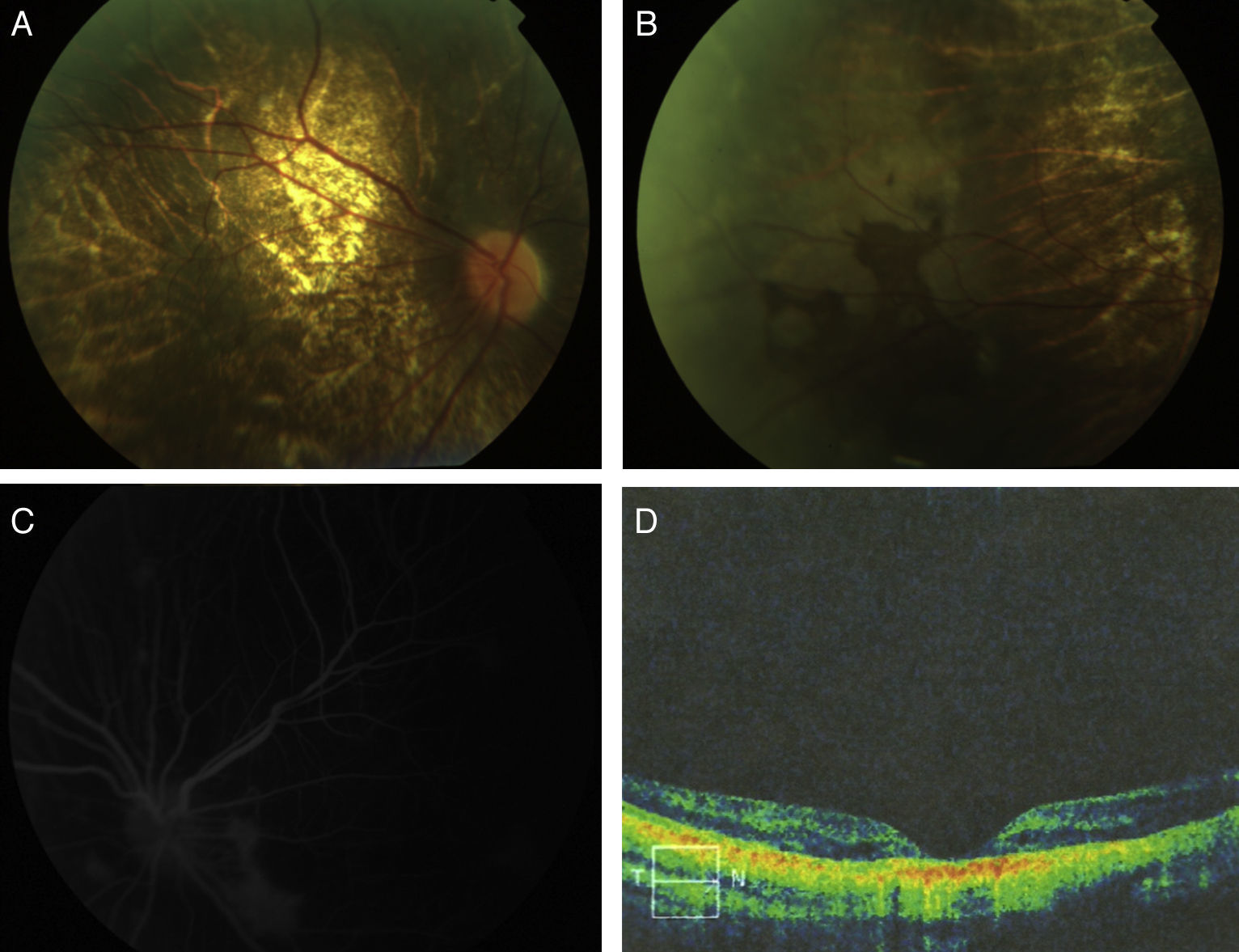
Eye fundus examination showed in both eyes a pale optic disk, retinal vessels with mild tortuosity and retinochoroidal dystrophy characterized by diffuse atrophy of the retinal pigment epithelium, with pigment deposition toward the periphery and visualization of choroidal vessels. Fluorescein angiography showed in both eyes a preserved vascular tree with diffuse atrophy of the retinal pigment epithelium and hyperfluorescent areas with dye accumulation. Optical coherence tomography revealed retinal thinning with changes in the outer layers and thickened and irregular pigmented epithelium, mainly in the fovea, which affected the choriopapillaris (Fig. 1).
(A) Eye fundus photograph showing a pale optic disk, retinal vessels with mild tortuosity, and retinochoroidal dystrophy characterized by diffuse atrophy of the retinal pigment epithelium and visualization of choroidal vessels. (B) Eye fundus photograph showing pigment deposition toward the periphery. (C) Fluorescein angiography showing a preserved vascular tree and hyperfluorescent areas of dye accumulation. (D) Optical coherence tomography showing retinal thinning with changes in the outer retinal layers.
Laboratory tests showed no hematological changes; however, neutrophil hypersegmentation was seen in peripheral blood smears. Blood chemistry tests showed normal values of blood glucose, kidney function, calcium, and phosphorus. Other test results included: follicle-stimulating hormone (FSH), 0.5mIU/mL (normal range [NR], 0.7–11); luteinizing hormone (LH), 0.3mIU/mL (NR, 0.8–7.6); total testosterone, 0.9ng/mL (NR, 2.45–18); prolactin, 8.4ng/mL (NR in males, 0–15); thyrotropin, 1.2mIU/mL (NR, 0.3–4.2); free thyroxine, 0.9ng/dL (NR, 0.8–2). Plasma levels of LH and FSH did not increase 30, 60, and 90min after intravenous administration of 100μg of gonadotropin-releasing hormone (GnRH).
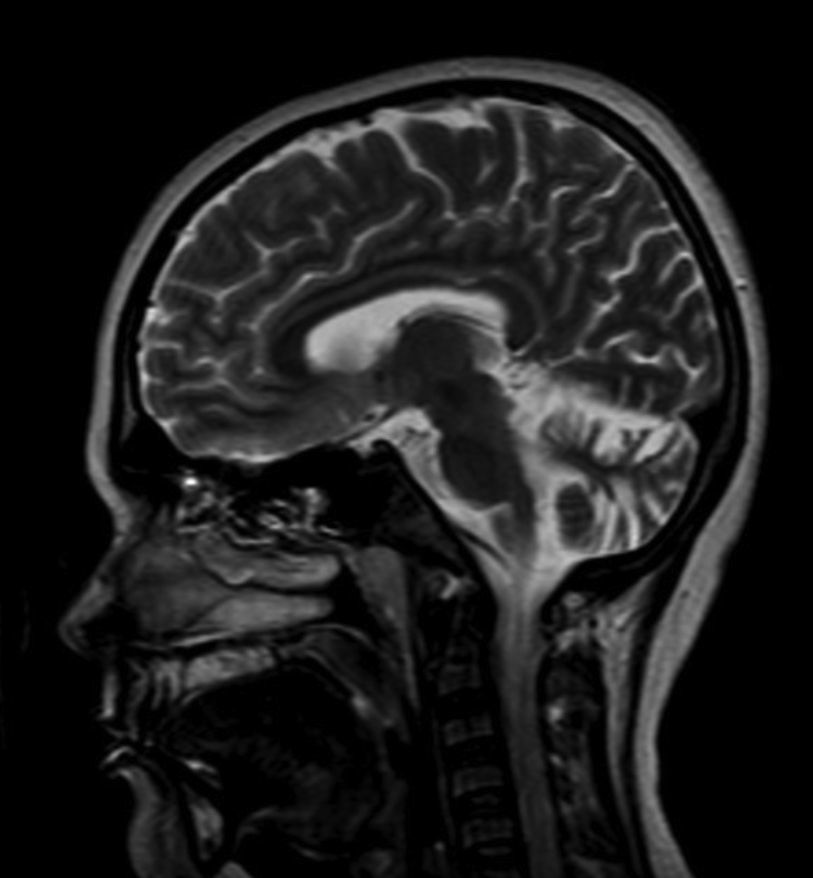
In 50 metaphases analyzed, the karyotype was 46, XY; X-rays of the left hand and wrist revealed a bone age of 16 according to the Greulich and Pyle atlas. Magnetic resonance imaging of the brain showed increased amplitude and depth of both cerebellar hemispheres consistent with cerebellar atrophy (Fig. 2). Bone densitometry showed a bone mineral density of 0.749g/cm2 at L1–L4, corresponding to a Z-score of −3.1 standard deviations (SD) as compared to the population of the same chronological age (26 years) and to a Z-score of −2.5SD as compared to the population of the same bone age (16 years). Total femoral neck bone mass was 0.687g/cm2, corresponding to Z-scores of −2.1 DS and −1.9 DS as compared to the population of 26 and 16 years respectively. Whole body bone mineral density was 1.047g/cm2, corresponding to Z-scores of −1.7 DS (26 years) and −1.2 DS (16 years).
Based on a diagnosis of hypogonadotropic hypogonadism, testosterone enanthate depot was prescribed at an IM dose of 250mg every 21 days. For osteoporosis, teriparatide (human parathyroid hormone [1–34]) 20μg SC daily and calcium and vitamin D supplements were given.
The main characteristics of the reported patient were: retinochoroidal dystrophy, hypogonadotropic hypogonadism, and cerebellar ataxia. This triad has previously been described and is consistent with BNS.1–3 The prevalence of this condition is unknown, but only 17 cases had been reported worldwide up to 1997, with males and females being equally affected.3
Ocular involvement may be the first sign of the syndrome, and occurs between the first and sixth decades of life. The expression and progression of retinochoroidal degeneration also varies widely in the reported cases, but it has been reported that the main ocular sign is diffuse atrophy of the retinal pigment epithelium with pigment deposition in the lower pole and periphery,4 as was found in our patient.
The most common neurological findings in BNS include unstable gait, nystagmus, dysmetry, and dysdiadochokinesia, which form a cerebellar syndrome attributed to significant cerebellar atrophy.3 As in our patient, global hyperreflexia and a variable extent of muscle hypotony with no impairment in sensitivity have been reported in some cases.3 Neurological signs and symptoms are commonly not progressive and occur late, after 15 years of age.1–3
As regards the endocrine characteristics, the absence of secondary sexual characteristics is the main reason for consultation in these patients, suggesting a hypogonadal state, which in BNS corresponds to hypogonadotropic hypogonadism. This results in some cases from GnRH deficiency,5 while in other cases (such as the one reported) a lack of response to GnRH stimulus has suggested gonadotroph cell dysfunction.3 In any case, androgen deficiency, in addition to compromising fertility in those affected, also alters bone metabolism, decreasing bone mass, which causes a significant risk of vertebral and nonvertebral fractures. The Work Group on Mineral Metabolism of the Spanish Society of Endocrinology and Nutrition (SEEN) recently proposed a number of practical recommendations for the evaluation and treatment of osteoporosis associated with different endocrine diseases. For osteroporosis secondary to male hypogonadism, the group recommended the restoration of testosterone levels, the maintenance of adequate calcium and vitamin D levels, and regular physical activity.6 In addition, the use of teriparatide as a therapeutic option is recommended for patients with very low bone mass (<−3SD).6 In this regard, Orwoll et al.7 showed that daily subcutaneous administration of teriparatide 20 or 40μg significantly increased bone mineral density in the spine and femoral neck in both hypogonadal and eugonadal males with osteoporosis. Similarly, in adolescents and young adults with idiopathic hypogonadotropic hypogonadism, testosterone replacement therapy has been associated with increases in both cortical and trabecular bone mass.8
Neutrophil hypersegmentation has been reported in recent years in subjects with BNS. This phenomenon is defined as the presence of at least 5% neutrophils with five or more lobes.9 This hematological finding in our patient supports the hypothesis that neutrophil hypersegmentation is a common characteristic in this syndrome, and that it does not cause apparent immune dysfunction. In addition, the patient had no megaloblastic anemia, uremia, infection, fever, or chronic renal disease, all of which are causes of hypersegmentation.
Although the tissues involved in the triad are of neuroectodermal origin, the exact relation between the three disorders is unknown. In 1989, Limber et al.10 reevaluated one of the patients initially reported by Neuhäuser and Opitz, and suggested that the defect lies in a single gene with pleiotropic effects, but the nature of the gene and its role in the pathophysiology of the disease are unknown. The lack of similar clinical manifestations in siblings of the patient suggests an autosomal recessive hereditary pattern, as previously described.2 No genetic test is currently available to identify subjects with BNS. Diagnosis of BNS is therefore clinical and should be made based on the classical triad.
Authors wish to thank doctors Rafael Muci-Mendoza, Gustavo Paredes, Daniela Hernández, and Luís Betancourt for their advice concerning the integral evaluation of the patient, and Laboratorio Bioclínico Glorias Patrias for its cooperation in the conducting of hormone tests.
Please cite this article as: Lima-Martínez MM, et al. Síndrome de Boucher-Neuhäuser. Endocrinol Nutr. 2013;60:218–20.