Hyperchloremic metabolic acidosis is a complication of urinary diversion using ileum or colon. Its prevalence ranges from 25% to 46% depending on the procedure used and renal function of the patient. It is a consequence of intestinal fluid and electrolyte exchange between intestinal mucosa and urine. The main mechanism is absorption of ammonium and chloride from urine. Long-term chronic metabolic acidosis in these patients may lead to impaired bone metabolism and osteomalacia. Regular monitoring of pH, chlorine, bicarbonate, and calcium–phosphorus metabolism is therefore essential for early diagnosis and treatment.
La acidosis metabólica hiperclorémica es una complicación de las derivaciones urinarias que utilizan ileon o colon, y su prevalencia oscila entre el 25% y 46% de los casos, dependiendo de la técnica utilizada y de la función renal del paciente. Es una consecuencia del intercambio hidroelectrolítico entre la mucosa intestinal y la orina, siendo su mecanismo principal la absorción del amonio y cloruro de la orina. A largo plazo la acidosis metabólica crónica puede conllevar una alteración del metabolismo óseo y producir osteomalacia, por lo que la monitorización del pH, cloro, bicarbonato y metabolismo fosfocálcico es fundamental para un diagnóstico y tratamiento precoz.
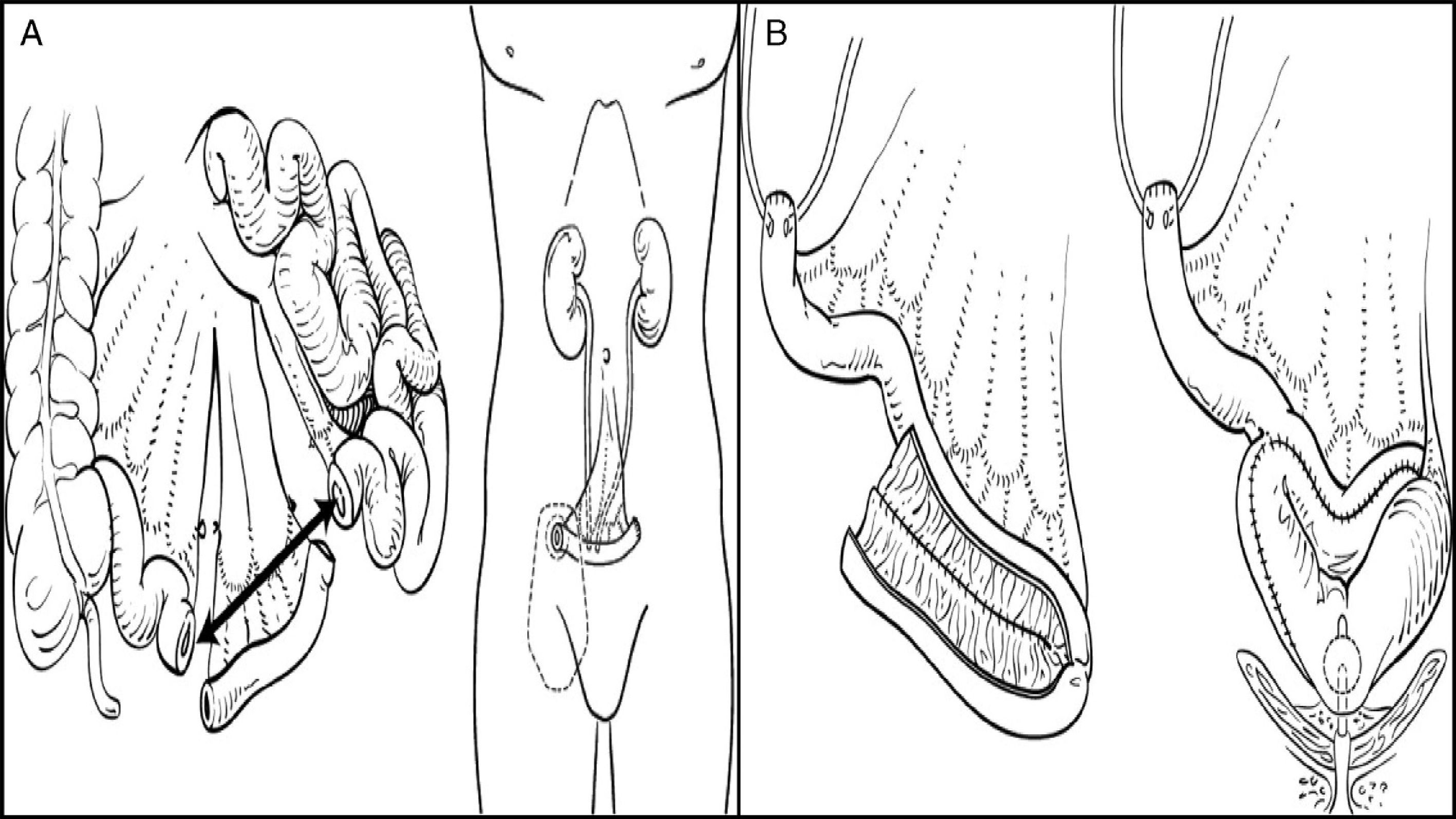
The main indication for urinary diversion is radical cystectomy for bladder cancer. Different segments of the gastrointestinal tract may be used for urinary reconstruction. Ileal and colonic segments are the most commonly used. Stomach and proximal bowel are only used when the rest of the intestinal mucosa is not available, as occurs in some patients with inflammatory bowel disease, short bowel, or a history of pelvic radiation. These segments are used to replace the bladder, either as a conduit to drain urine to the abdominal wall, a urinary stoma, or a reconstruction to form a neobladder, i.e. with diversion to the native urethra.
Urinary diversions may be divided into heterotopic and orthotopic depending on the urine outflow tract. In heterotopic diversions, urine will flow out through a duct different from the urethra (skin, rectum, etc.). Heterotopic diversions may in turn be continent or incontinent. The most commonly used diversion is ureteroileostomy, where an ileal conduit is created: ureters will be attached to one end, while the other end will be taken to the skin to construct a stoma. In orthotopic diversions, the bladder is replaced respecting the natural excretory route, i.e. the urethra. A neobladder is created to store urine, which is passed in a voluntary, controlled way (Fig. 1).
The bowel mucosa differs from the urinary mucosa in its semipermeable and metabolically active nature, so that when it comes into contact with urine, metabolic changes may occur through different electrolyte transport and diffusion mechanisms.
The incidence of water and electrolyte disorders has been estimated to range from 21% to 48% in the different series reported.2–5
Complications derived from bowel resection itself are very few, due to the use of short bowel segments. Malabsorption is very rare with ileal resections shorter than 60cm,6 but vitamin B deficiency12 may occur in the long term in up to 21% of patients.7 That is to say, the risk of the occurrence of these water and electrolyte disorders depends to a large extent on the procedure used, which determines the surface size and contact time of urine with the bowel mucosa.8
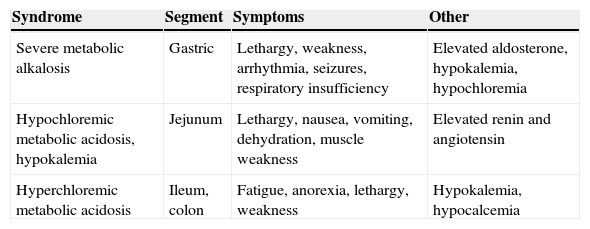
General physiopathogenic conceptsEach gastrointestinal tract segment (stomach, jejunum, ileum, and colon) is physiologically different in type of solute transport, and different changes occur depending on the segment and length used (Table 1). It should also be noted that the absorption capacity of the bowel segment decreases with time, and atrophic areas occur in the mucosa as a defense mechanism against the different osmolarity, pH, and solute concentration of urine.6
Water and electrolyte changes in patients with urinary diversions depending on the bowel segment used.
| Syndrome | Segment | Symptoms | Other |
|---|---|---|---|
| Severe metabolic alkalosis | Gastric | Lethargy, weakness, arrhythmia, seizures, respiratory insufficiency | Elevated aldosterone, hypokalemia, hypochloremia |
| Hypochloremic metabolic acidosis, hypokalemia | Jejunum | Lethargy, nausea, vomiting, dehydration, muscle weakness | Elevated renin and angiotensin |
| Hyperchloremic metabolic acidosis | Ileum, colon | Fatigue, anorexia, lethargy, weakness | Hypokalemia, hypocalcemia |
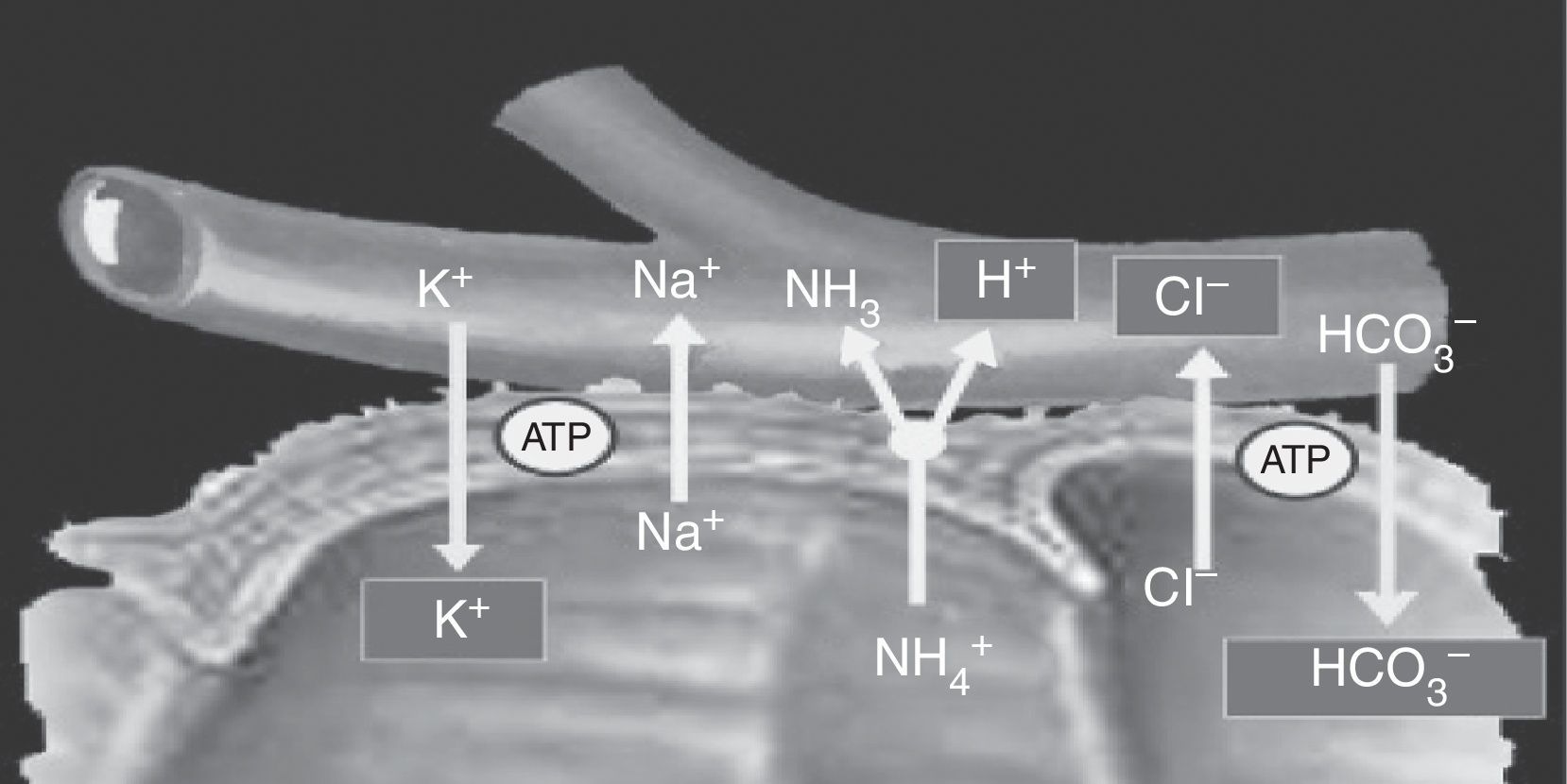
On the one hand, transcellular electrolyte and solute transport occurs in the bowel through transmembrane proteins and electrochemical gradients, co-transporters, and exchange channels. Transcellular sodium (Na+) and hydrogenion (H+) transport occurs through the Na+/K+/ATPase pump and the Na+/H+ exchanger in the luminal membrane of the small and large bowel. This process makes it possible for low intracellular sodium levels to be maintained, establishing the electrochemical gradient required for solute transport. This mechanism promotes Na+ absorption from the intestinal lumen against the concentration gradient existing between the lumen and plasma, with subsequent H+ secretion into the lumen. The efficiency of Na+ absorption increases distally.10 However, potassium transport in the bowel is passive, along the concentration gradient, and potassium absorption therefore depends on luminal levels.
On the other hand, paracellular transport of water and electrolytes occurs along an electrochemical and concentration gradient, which is more important at the proximal level. That is to say, the bowel mucosa is more permeable at the proximal level (stomach, duodenum, and jejunum) than at the distal level (ileum and colon). If proximal segments are used, and depending on urine osmolarity, a rapid movement of water and solutes may occur through the mucosa due to the osmotic gradient, with resultant Na+ and chlorine loss and hypovolemia.10
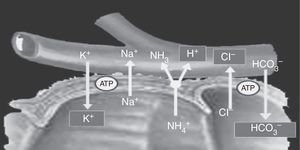
Cells located in the colon and distal bowel have an anion exchange channel that reabsorbs chloride (Cl−) and secretes bicarbonate (HCO3−), and is able to absorb ammonium (NH4+) from urine; the jejunum lacks this capacity. Because of these differences in transport capacity, the metabolic changes that may occur in these patients depend on the bowel segment used, and also on the chemical characteristics of urine (osmolarity, pH, and solute concentration), the contact time of the mucosa with urine, and the presence of renal failure.6 Absorption of NH4+ by the bowel mucosa is the main mechanism of the metabolic acidosis that occurs in patients with urinary diversions. This absorption process of NH4+ is coupled to HCO3− and Cl− exchange. Absorption of NH4+ may also occur through potassium channels (K+), causing the loss of HCO3− and K+8,11 (Fig. 2).
Ammonium and chloride are reabsorbed. Ammonium exchange by a proton is coupled to bicarbonate exchange by chloride; thus, bicarbonate and some potassium are lost.
When the gastric mucosa is used, continuous H+ loss from the gastric mucosa occurs, leading to hypokalemic metabolic alkalosis and hypochloremia. Since urine normally has low Na+, Cl−, and HCO3− levels, high K+ contents and high osmolarity as compared to blood plasma, the use of the jejunum in these diversions causes a depletion of effective circulating volume due to salt and water loss through the bowel mucosa. As a consequence, hyponatremia, hypochloremia, hyperkalemia, and metabolic acidosis occur due to dehydration, followed by metabolic acidosis secondary to hypoperfusion and hypovolemia.6,10,12 Moreover, the more proximal the jejunal segment used, the more severe the metabolic complication.12
The main complication occurring when the ileum and colon are used is hyperchloremic metabolic acidosis due to NH4+ absorption and HCO3− secretion. In some cases, only hyperchloremia with no significant acidosis occurs.13 Other changes reported include hypokalemia, hypomagnesemia, hypocalcemia, hyperammonemia, and increased blood creatinine and urea levels. Isolated cases of muscle palsy secondary to hypokalemic acidosis related to ureterosigmoidostomy have been reported in the literature.14–16
Metabolic acidosis occurs after 26% and 45% of continent diversions and neobladders respectively, and requires hospital admission in up to 4% of cases.4 This proportion decreases to 10–15% with ileal conduits.17,18 Moreover, the incidence of postoperative metabolic acidosis also depends on the length of ileum segment used, as stated above, being higher with segments longer than 40cm.19 In ureterosigmoidostomies, metabolic changes may occur in up to 100% of cases.5
Osteomalacia and bone disease associated with water and electrolyte disorders from urinary diversionsTreatment of metabolic acidosis consists of the oral administration of bicarbonate and should be started early to prevent the development of osteomalacia. Chronic acidosis promotes osteoclast activation and bone resorption and alters the hydroxylation of 25-hydroxyvitamin D (25[OH]D) or calcifediol in the kidney, which favors hypercalciuria and hypermagnesuria, promoting a negative calcium and phosphorus balance, and therefore preventing adequate bone mineralization.20 In addition, metabolic acidosis induces increased protein catabolism, which promotes a negative nitrogen balance and decreased albumin synthesis, so possibly contributing to bone disease development.21 Moreover, patients with pre-existent renal disease have an increased risk of metabolic acidosis and altered vitamin D hydroxylation, and are therefore a group predisposed to develop bone disease. Assessment of this aspect is important because up to 72% of patients undergoing radical cystectomy may experience kidney function impairment over time. Advanced age, high blood pressure, postoperative hydronephrosis, and stenosis of the anastomosis at the ureteroenteric junction are risk factors for such impairment.22
Osteomalacia may occur two to four years after surgery, although it has been reported up to 35 years later.20,23 Patients with osteomalacia after urinary diversion using the ileum or colon usually have elevated serum levels of Cl− and HCO3− deficiency. Alkaline phosphatase (AP) may be increased in plasma due to increased bone turnover, with usually normal (or in the lower limit of normal) calcium and phosphorus levels. Parathyroid hormone (PTH) and 1,25(OH)-vitamin D levels remain normal.20,23–25 Bone disease may also occur in the absence of severe metabolic acidosis due to changes in phosphate, sulphate, and HCO3− levels that promote hypercalciuria and impair bone mineralization.10,26 In patients with osteomalacia, acid–base imbalances should first be corrected. If no improvement is achieved, treatment should also be given with calcium and vitamin D supplements.27
Long-term follow-up of these patients may be difficult because bone changes may be subclinical or asymptomatic. Bone densitometry (BMD) is not able to detect subtle changes, so that bone histology is sometimes the only way to confirm diagnosis.27
Some authors have studied the value of some bone remodeling markers for diagnosis and follow-up. It should be noted that many of these studies excluded women because of the effect of menopause on bone.20,28–30
Koch et al. compared a series of patients undergoing surgery for myelomeningocele and urinary diversion to patients treated by intermittent catheterization 23 and 17 years after surgery respectively. A decreased BMD was seen in both groups. The urinary diversion group had however a greater incidence of complications from orthopedic procedures (intraoperative fracture and non-union) and an increased need of spinal surgery for the correction of spinal deviations. There was no difference between the groups in fracture incidence. The authors therefore concluded that urinary diversions have harmful effects on bone health.31
Giannini et al. studied a series of 25 males with ileal neobladders followed up for 29–75 months and noted increased AP levels and lower BMD. On the other hand, when metabolic variables were adjusted for time since surgery, subjects operated on more recently had lower serum pH and higher urinary hydroxyproline levels. These results prompted the authors to argue that time since surgery is important because metabolic changes caused by the bowel mucosa gradually decrease due to progressive atrophy of the intestinal mucosa in contact with urine. Moreover, BMD was lower in two of the four anatomical sites analyzed in the study group. Thus, significant BMD decreases were found in the femoral neck and Ward's triangle area, but not in the lumbar spine and femoral intertrochanteric region. The authors did not analyze whether these metabolic and BMD changes were associated with an increased risk of fracture.20
In a series of 33 males with neobladders followed up for 4–114 months after surgery, Fujisawa et al. found a negative correlation of the bone markers C-terminal telopeptide of type I collagen in urine and serum deoxypyridinoline with serum pH. In addition, lumbar Z-score (L2–L4) significantly correlated with N-terminal telopeptide and deoxypyridinoline levels.28 Kawakita et al. studied a series of 46 patients with urinary diversions and found that patients with metabolic acidosis had lower BMD and higher urinary levels of pyridinium crosslinks as compared to patients with a normal acid–base status.30 Sevin et al. found lower BMD values in patients with ileal neobladders, but not in those with colonic pouches.25
Other authors, however, found no significant changes in bone metabolism in these cases.29,32,33 Davidsson et al. analyzed bone histology in 20 patients with urinary diversion using ileal conduits and in 19 patients with a continent urinary reservoir created from a cecal segment. Histological study revealed no bone mineralization defects of increased bone resorption, but found a greater trabecular bone volume in the reservoir group and a decreased mineral apposition rate in both groups. The authors concluded that bone turnover was decreased in these patients.32 In subjects with ileal reservoirs and Bricker conduits assessed 2–17 years after surgery, the Campanello et al. group also found no significant changes in BMD or biochemical parameters including vitamin D, PTH, AP, calcium, and osteocalcin as compared to controls.29
None of the above studies found a greater risk of bone fractures in patients with urinary diversions. Gupta et al. recently analyzed a cohort of 50,520 patients with bladder cancer; of these, 4878 had undergone surgery for radical cystectomy and urinary diversion and had a 21% increase in the risk of fracture, especially patients with a prior history of renal disease and who were of younger age.34
On the other hand, in 1992 Wagstaff and Mundy reported that bone disease related to urinary diversions and resulting from metabolic acidosis could cause delayed growth and development.35,36 It should be noted, however, that this was not a single specific consequence of urinary diversions or enterocystoplasties. Gerharz et al. analyzed a series of 123 children with enterocystoplasty and found that four of them had a clinically relevant growth disorder. After a complete endocrine assessment, enterocystoplasty was not considered to be a causative factor in any of them. These authors suggested that growth disorder is non-specific and multifactorial in origin, rather than a direct consequence of surgery. In any case, growth monitoring is generally recommended in these children, because kidney function impairment may alter the mechanisms to compensate for the metabolic changes resulting from enterocystoplasty and potentially affect growth.37
ConclusionsIn urinary diversions, metabolic (and bone) changes largely depend on the time elapsed since surgery, surgical procedure, the gastrointestinal segment used, and segment length.
The most prevalent metabolic complication in urinary diversions is hyperchloremic metabolic acidosis, which may occur both in the short and long term. These patients therefore require long-term follow-up for the early diagnosis and treatment of these complications, with special attention being paid to patients with chronic renal disease.
Although the studies reported to date are heterogeneous (small samples, different surgical procedures, and variable follow-up periods), most authors postulate that the correction of acidosis may prevent and normalize bone mass changes. For this reason, the monitoring of these patients requires the regular measurement of BMD, venous blood gases, and parameters of phosphorus and calcium metabolism.38 Prospective studies of larger samples are required to determine the long-term effects of urinary diversions in bone metabolism.
Conflicts of interestThe authors state that they have no conflicts of interest that may be perceived as detrimental to the impartiality of the research published.
Please cite this article as: Cano Megías M, Golmayo Muñoz Delgado E. Complicaciones metabólicas y óseas de las derivaciones urinarias. Endocrinol Nutr. 2015;62:100–105.