Specific TSH and free thyroxine ranges are needed to adequately assess maternal thyroid function during pregnancy.
AimThe aim of this review is to review studies reporting data on references TSH and free thyroxine levels in Spanish women during the first trimester of pregnancy.
Material and methodsLiterature search and selection of studies providing reference TSH ranges in the first trimester of pregnancy.
ResultsThe TSH cut-off point to define hypothyroidism (P97.5) was different depending on the immunoassay used. Gestational age, thyroid autoimmunity, and maternal iodide nutritional status can determine the variability seen in the results.
ConclusionsDifferences found in the studies do not allow for establishing a unified TSH reference range. However, results suggest that the TSH upper reference limit is close to 4μU/mL. Reference TSH ranges specific for each population and immunoassay during pregnancy should be defined.
Durante la gestación, se requieren rangos de referencia específicos de TSH y de tiroxina libre para la correcta valoración de la función tiroidea materna.
ObjetivoRevisión de estudios sobre valores de referencia para TSH y tiroxina libre durante el primer trimestre de gestación de población española.
Material y métodosBúsqueda bibliográfica y selección de estudios que contengan rangos de referencia de TSH en el primer trimestre de gestación.
ResultadosEl punto de corte de TSH para definir hipotiroidismo (P97,5) varió según el inmunoanálisis utilizado. La edad gestacional, la autoinmunidad tiroidea y el estado nutricional de yodo de las poblaciones utilizadas condicionaron la variación observada en los resultados.
ConclusionesLas diferencias encontradas en los estudios revisados no permiten establecer un rango de referencia unificado para TSH. No obstante, se observa que el límite superior del rango para TSH está próximo a 4μU/mL. Sería conveniente disponer de rangos de referencia propios para cada población y específicos para cada inmunoanálisis.
Pregnancy causes significant physiological changes in the maternal thyroid gland, which must adapt to greater iodine requirements and increased thyroid hormone production.1 The measurement of serum thyroid stimulating hormone (TSH) concentration is a very sensitive test for diagnosing alterations in thyroid function. However, because of the physiological changes that occur during pregnancy, specific reference ranges for TSH and free thyroxine (FT4) are required in order to allow for the adequate assessment of maternal thyroid function.
TSH concentrations decrease in pregnancy, particularly during the first trimester, due to the stimulating effect of the TSH receptor secondary to the action of human chorionic gonadotropin (hCG). The opposite trend is seen in the case of FT4, with levels that rise in the first trimester and fall during the second and third trimesters.
Based on the upper limit of the TSH reference values during the first trimester of pregnancy, established from studies involving large pregnant population samples and the numerous maternal and fetal complications associated with subclinical gestational thyroid hypofunction, the Endocrine Society,2 together with other international bodies, in 2007 stated that in the absence of intrinsic reference values, the upper limit for TSH during the first trimester of pregnancy should be regarded as 2.5μU/ml, versus 3.0μU/ml during the second and third trimesters. As a result of the subsequent confirmation of a higher rate of maternal-fetal complications in pregnant women with TSH>2.5μU/ml in the first trimester,3 these reference values remained unchanged in the different international guides published up until 2012.4–6 In Spain, the Spanish Society of Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición [SEEN]) in 20097 and in a subsequent review published in 2013,3 recommended the monitoring during pregnancy of the TSH levels established to date by the international guides.
Since 2009, several authors in Spain have published reference ranges for TSH and FT4, mainly in the first trimester, obtained in their respective populations of pregnant women. Following the recommendation to universally screen for thyroid dysfunction in the pregnant population, proposed by the SEEN in 2012,8 there has been an even greater increase in the number of publications and reports on thyroid function reference values in pregnant women. However, the plasma TSH concentrations recorded are influenced by many factors (ethnicity, positive thyroid autoimmunity, iodine nutritional status, the body mass index [BMI], smoking, age, parity, etc.),9 as well as by the measurement methods used. Adequate calculation of the TSH and FT4 reference ranges in the pregnant population requires at least the consideration of adequate iodine nutrition and negative thyroid autoimmunity.10
Material and methodsA literature search was made for TSH and FT4 reference values in the pregnant population in Spain covering the period from 1 January 2009 to 31 December 2017. The PubMed database was consulted, the following key words being used in the search: “pregnancy, TSH reference values, Spain”, “pregnancy, TSH, Spain”, “pregnant women thyroid function Spain”. This search yielded 151 studies of which 9 were finally selected.
We also consulted the database of articles published in Endocrinologiá, Diabetres y Nutrición (Elsevier) and in the journal published by the Sociedad Española de Médicos de Atención Primaria (Spanish Society of Primary Care Physicians [SEMERGEN]) during that period, using the keywords “reference values, thyroid hormones, pregnancy” in the search. Two further publications were obtained from this second search. In addition, a consultation of the communications at Spanish congresses of Endocrinology and Nutrition, and Clinical Laboratories, yielded another four articles. We also asked the 20 members of the Gestational Thyroid Dysfunction working group of the Thyroid Knowledge Area of the SEEN to submit all their studies on TSH and FT4 reference values in pregnant women published in journals or reported at international, national or regional congresses during that period of time.
Initially, the minimum requirement of the available studies for calculating TSH and FT4 reference values during pregnancy was that they had been published in journals or reported to adequately referenced congresses. However, bearing in mind that the aim of the study was to collect the largest possible body of data on TSH and FT4 reference values in pregnancy in the Spanish population, we also included four studies that had not been previously published or reported at congresses, contributed by members of the Thyroid Knowledge Area of the SEEN (the hospitals of Málaga, Virgen del Rocío and Valme in Seville, and Puerto Real in Cádiz).
The selection criteria considered essential for the inclusion of studies for review were:
- -
Specification of the analytical method used.
- -
Definition of percentiles P2.5 and P97.5 corresponding to the TSH levels in the first trimester of pregnancy.
- -
Studies conducted in Spain.
In order to perform the analysis, we collected the following data from the selected studies: the author, the year of publication, the city/town, the number of pregnant women included in the study, the week of pregnancy in which blood sampling was made for measuring TSH, the presence or absence of thyroid autoimmunity (anti-thyroid peroxidase [TPO] and anti-thyroglobulin [Tg] antibodies), the immunoassay used, the iodine nutritional status (based on ioduria measurement), and percentiles P2.5 and P97.5 corresponding to TSH and (if available) FT4.
A descriptive analysis was made of TSH P97.5 in the first trimester of pregnancy. Differences were reported by grouping the studies according to the immunoassay used. Likewise, we analyzed the data by selecting studies with n>400 pregnant women, those with negative anti-TPO and anti-Tg antibodies, and studies in pregnant women with ioduria >150μg/l to check for possible differences with respect to the global data.
It was not possible to obtain the individual data of each study or the TSH P97.5 confidence intervals for most of them. This fact, and the heterogeneity of the populations of the different studies, did not allow us to conduct a meta-analysis with the data obtained.
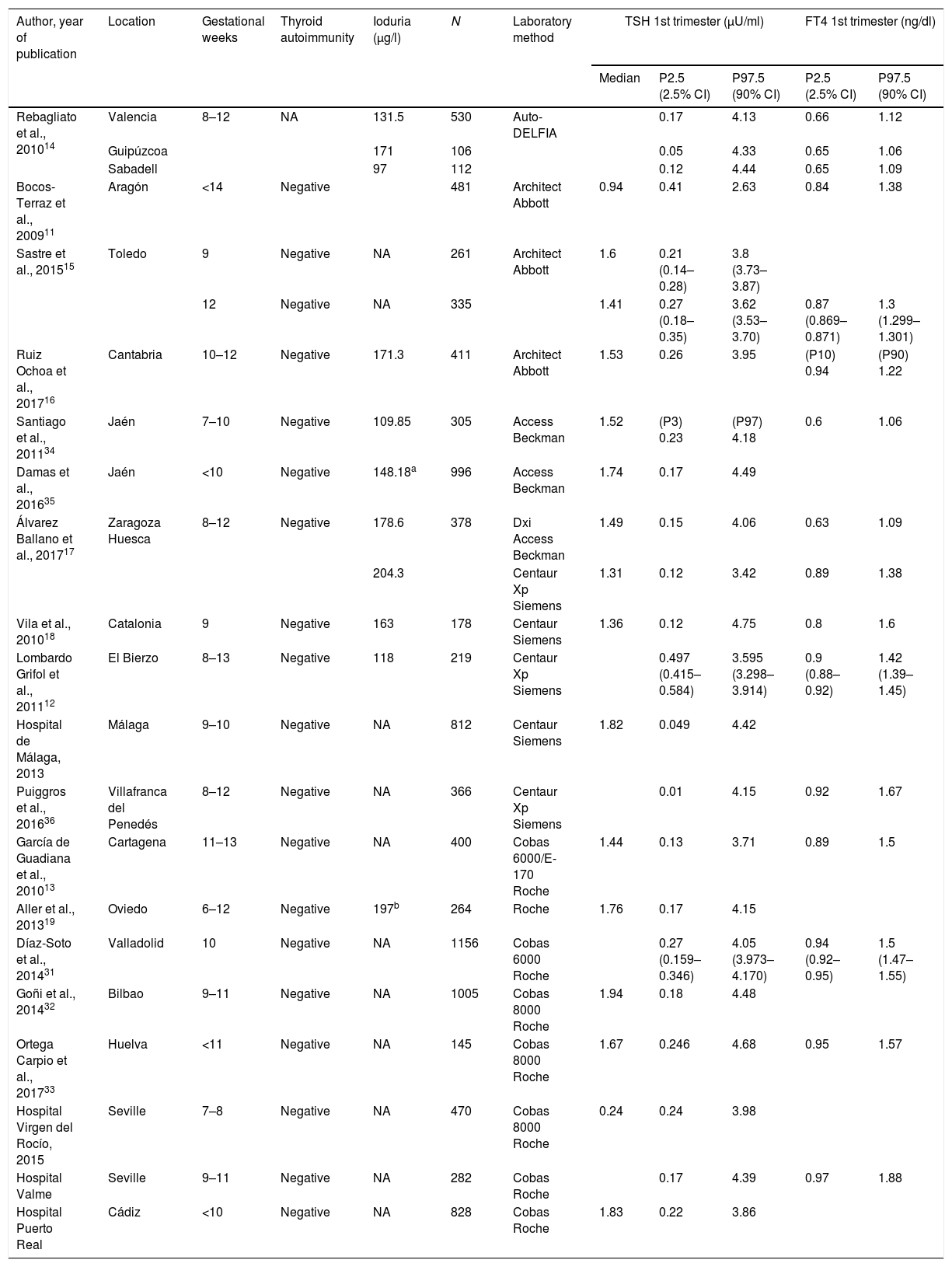
ResultsA total of 19 studies were included. Table 1 shows the TSH and FT4 reference values of the different studies according to the immunoassay used.
Reference TSH and FT4 concentrations in pregnant Spanish women.
| Author, year of publication | Location | Gestational weeks | Thyroid autoimmunity | Ioduria (μg/l) | N | Laboratory method | TSH 1st trimester (μU/ml) | FT4 1st trimester (ng/dl) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | P2.5 (2.5% CI) | P97.5 (90% CI) | P2.5 (2.5% CI) | P97.5 (90% CI) | |||||||
| Rebagliato et al., 201014 | Valencia | 8–12 | NA | 131.5 | 530 | Auto-DELFIA | 0.17 | 4.13 | 0.66 | 1.12 | |
| Guipúzcoa | 171 | 106 | 0.05 | 4.33 | 0.65 | 1.06 | |||||
| Sabadell | 97 | 112 | 0.12 | 4.44 | 0.65 | 1.09 | |||||
| Bocos-Terraz et al., 200911 | Aragón | <14 | Negative | 481 | Architect Abbott | 0.94 | 0.41 | 2.63 | 0.84 | 1.38 | |
| Sastre et al., 201515 | Toledo | 9 | Negative | NA | 261 | Architect Abbott | 1.6 | 0.21 (0.14–0.28) | 3.8 (3.73–3.87) | ||
| 12 | Negative | NA | 335 | 1.41 | 0.27 (0.18–0.35) | 3.62 (3.53–3.70) | 0.87 (0.869–0.871) | 1.3 (1.299–1.301) | |||
| Ruiz Ochoa et al., 201716 | Cantabria | 10–12 | Negative | 171.3 | 411 | Architect Abbott | 1.53 | 0.26 | 3.95 | (P10) 0.94 | (P90) 1.22 |
| Santiago et al., 201134 | Jaén | 7–10 | Negative | 109.85 | 305 | Access Beckman | 1.52 | (P3) 0.23 | (P97) 4.18 | 0.6 | 1.06 |
| Damas et al., 201635 | Jaén | <10 | Negative | 148.18a | 996 | Access Beckman | 1.74 | 0.17 | 4.49 | ||
| Álvarez Ballano et al., 201717 | Zaragoza Huesca | 8–12 | Negative | 178.6 | 378 | Dxi Access Beckman | 1.49 | 0.15 | 4.06 | 0.63 | 1.09 |
| 204.3 | Centaur Xp Siemens | 1.31 | 0.12 | 3.42 | 0.89 | 1.38 | |||||
| Vila et al., 201018 | Catalonia | 9 | Negative | 163 | 178 | Centaur Siemens | 1.36 | 0.12 | 4.75 | 0.8 | 1.6 |
| Lombardo Grifol et al., 201112 | El Bierzo | 8–13 | Negative | 118 | 219 | Centaur Xp Siemens | 0.497 (0.415–0.584) | 3.595 (3.298–3.914) | 0.9 (0.88–0.92) | 1.42 (1.39–1.45) | |
| Hospital de Málaga, 2013 | Málaga | 9–10 | Negative | NA | 812 | Centaur Siemens | 1.82 | 0.049 | 4.42 | ||
| Puiggros et al., 201636 | Villafranca del Penedés | 8–12 | Negative | NA | 366 | Centaur Xp Siemens | 0.01 | 4.15 | 0.92 | 1.67 | |
| García de Guadiana et al., 201013 | Cartagena | 11–13 | Negative | NA | 400 | Cobas 6000/E-170 Roche | 1.44 | 0.13 | 3.71 | 0.89 | 1.5 |
| Aller et al., 201319 | Oviedo | 6–12 | Negative | 197b | 264 | Roche | 1.76 | 0.17 | 4.15 | ||
| Díaz-Soto et al., 201431 | Valladolid | 10 | Negative | NA | 1156 | Cobas 6000 Roche | 0.27 (0.159–0.346) | 4.05 (3.973–4.170) | 0.94 (0.92–0.95) | 1.5 (1.47–1.55) | |
| Goñi et al., 201432 | Bilbao | 9–11 | Negative | NA | 1005 | Cobas 8000 Roche | 1.94 | 0.18 | 4.48 | ||
| Ortega Carpio et al., 201733 | Huelva | <11 | Negative | NA | 145 | Cobas 8000 Roche | 1.67 | 0.246 | 4.68 | 0.95 | 1.57 |
| Hospital Virgen del Rocío, 2015 | Seville | 7–8 | Negative | NA | 470 | Cobas 8000 Roche | 0.24 | 0.24 | 3.98 | ||
| Hospital Valme | Seville | 9–11 | Negative | NA | 282 | Cobas Roche | 0.17 | 4.39 | 0.97 | 1.88 | |
| Hospital Puerto Real | Cádiz | <10 | Negative | NA | 828 | Cobas Roche | 1.83 | 0.22 | 3.86 | ||
All studies exclude women with previously known thyroid disease. NA: not available.
All the studies excluded women with previously known thyroid disease. The number of pregnant women included in the studies ranged from 106 to 1156.
Based on the data of all the selected studies, the mean TSH cut-off points defining hypothyroidism (P97.5) according to the immunoassay used were: Abbott 3.5μU/ml (2.63–3.95); Siemens Centaur 4.06μU/ml (3.42–4.75); Roche 4.11μU/ml (3.71–4.68) and Beckman 4.24μU/ml (4.06–4.49).
Taking into account the heterogeneity of the studies, we analyzed the possible differences in TSH value (P97.5) with respect to the global data, based on the following aspects.
Assuming the first trimester of pregnancy to cover the time up to week 12, the studies published by Bocos et al.,11 Lombardo et al.12 and García de Guadiana et al. 13 included pregnant women up to week 13–14. Consequently, strictly speaking the TSH and FT4 values of these women should not be considered as corresponding to the first trimester of pregnancy.
With regard to thyroid autoimmunity, all the studies except that of Rebagliato et al.,14 excluded patients with positive anti-TPO antibodies, though anti-Tg antibodies were only recorded in the studies of Bocos et al.,11 García de Guadiana et al.,13 Sastre et al.,15 Ruiz Ochoa et al.16 and Álvarez Ballano et al.,17 and in the hospitals of Virgen del Rocío and Valme, in Seville. In these studies, the TSH P97.5 value ranged from 2.63 to 4.39μU/ml.
In relation to the influence of iodine nutritional status, ioduria was measured in 8 of the 19 studies included. Only the publications of Vila et al.,18 Aller and Rabal,19 Ruiz Ochoa et al.16 and Álvarez-Ballano et al.17 found pregnant women to have urinary iodine levels >150μg/l, corresponding to the lower limit of the range defining adequate iodine nutritional status according to the World Health Organization (WHO).20
Only the studies of Ruiz-Ochoa et al.16 and Álvarez-Ballano et al.17 met all the abovementioned requirements (up to 12 weeks of pregnancy, negative thyroid autoimmunity defined as negative anti-TPO and anti-Tg antibodies, and adequate iodine nutritional status), though the laboratory test methods used were different.
DiscussionThe measurement methods used have a great influence upon the TSH and particularly the FT4 results obtained.
Measurement of TSH concentrationThe immunoassay methods used for measuring TSH are quite robust and precise; however, there is no reference technique, and so standardization is not possible. A number of options have been proposed in an attempt to reduce the variability of the results obtained with the different methods, such as the expression of the data obtained as multiples of the median (MoM).21 The MoM is calculated by dividing each individual value by the median of the reference population (comprising the TSH values of a significant number of women, revised and adjusted on a periodic basis if necessary). The MoM values are independent of differences between immunoassays, and the cut-off points for the different methods can therefore be more easily generalized. This way of working reduces the differences between different immunoassays and laboratories, so making it possible to take into account the influence of factors making an impact upon TSH and FT4 levels, such as gestational age or maternal weight. However, it is not easy to implement and does not solve the problem of the variability of the reference ranges according to the choice of reference population.
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has created a project for harmonizing the results produced by the different commercial immunoassays. The project is currently at a very advanced stage, and has been shown to reduce the differences in reference ranges among the methods.22 The implementation of this harmonization system requires that the manufacturers adequately calibrate their immunoassays (based on the target values set by the IFCC) and thus produce very similar results.
At present, the differences among immunoassays for measuring TSH are sufficiently significant to make obligatory the use of specific reference ranges for each measurement method.
Measurement of FT4 concentrationThe reliable measurement of FT4 levels during pregnancy is far from simple. The most robust measurement methods are direct techniques based on prior separation of the free hormone phase through dialysis or ultrafiltration, with the subsequent measurement of thyroxine using liquid chromatography and tandem mass spectrometry. These methods are very reliable, and in fact the method proposed by the IFCC as reference for the standardization of FT4 measurement is of this kind.23 However, these methods are inconvenient, very slow and expensive. Consequently, not only are they hardly practical for routine use, but they are also currently inaccessible to most clinical laboratories.
Immunoassays estimate the free hormone levels in serum, in the presence of protein-bound T4. These methods are competitive, but differ considerably. Three large groups can be distinguished according to the design involved: one-step methods, two-step methods and methods using labeled antibodies.
These are the methods used in clinical laboratories, though their main limitation for the measurement of FT4 levels during pregnancy is their potential susceptibility to changes in thyroid hormone transporter protein concentration or affinity.
Samples with high concentrations of thyroxine-binding globulin (TBG) tend to falsely increase the FT4 concentration, and samples with low albumin concentrations tend to yield lower FT4 values. The presence of a high concentration of free fatty acids in serum tends to artificially raise FT4 levels by inhibiting T4 binding to serum proteins.24 Pregnant women have a high concentration of TBG and non-esterified fatty acids, and a low serum albumin concentration. The degree of interference is conditioned by the design and type of antibody or analog used.
This limitation has caused considerable controversy regarding the reliability of immunoassay measurements of FT4 during pregnancy.25 Fortunately, rigorous studies are available,26 in which most immunoassays are shown to yield FT4 levels that correlate to those obtained with the reference method and are therefore suitable for measurement. However, it should be taken into account that the influence of the protein alterations inherent to pregnancy differs greatly depending on the immunoassay used, and that not all methods available on the market produce adequate results.
Pregnancy induces significant changes in thyroid physiology that influence TSH and FT4 levels. These changes in thyroid homeostasis determine a specific pattern in the concentrations of both parameters over the course of pregnancy. This concentration pattern is method-dependent, particularly for FT4, due to the methodological peculiarities involved.
Therefore, the adequate assessment of thyroid function during pregnancy depends on the existence of specific reference ranges stratified by trimesters, the measurement method used, and the population being screened.
On evaluating the data from all the selected studies, the TSH cut-off point for defining hypothyroidism (P97.5) appears to be lower for the Abbott method and somewhat higher for the Siemens Centaur, Beckman Access, and Roche methods. However, there is an overlap between the reference ranges obtained with the different methods, and even within the same method considerable heterogeneity can be seen in the reference ranges obtained in the different studies reviewed. This finding probably reflects the significant differences in the characteristics of the populations chosen as reference, particularly as regards the number of pregnant women included, gestational age, nutritional iodine status, and even the definition of negative thyroid autoimmunity.
One of the limitations found in most of the reviewed studies is the use of a reference population that is too small. In this respect, despite the classical recommendation of the National Academy of Clinical Biochemistry regarding the need to have at least 120 normal euthyroid and rigorously screened volunteers for central 95% calculation (between P2.5 and 97.5), a larger number is desirable, and some authors recommend that because of the high interindividual variability of TSH and to some extent of FT4, a minimum of about 400 individual values are required for adequate calculation of the reference ranges.27
Considering those studies involving over 400 pregnant women, and without taking the methodological differences into account, all except the study by Bocos et al.11 (with P97.5 2.63μU/ml) report TSH P97.5 values that are close together (3.71–4.49μU/ml) and near to 4μU/ml. This agrees with the recommendations regarding TSH levels in the first trimester of pregnancy of the American Thyroid Association (2017).28
The inclusion in some studies of pregnant women between weeks 6 and 9 (before the hCG peak) or up to week 14 may also have influenced the observed variability.
With regard to thyroid autoimmunity, some of the studies define it as being negative when the anti-TPO and anti-Tg titers are below the cut-off point. However, other studies only measure anti-TPO titers, which may condition the inclusion of pregnant women with thyroid autoimmune positivity for anti-Tg antibodies. Unuane et al. reported the presence of anti-Tg antibodies in 5% of pregnant women with negative anti-TPO antibodies, as well as a mean TSH value higher than in women who test negative for both antibodies.29 Considering those articles that excluded pregnant women with negative anti-TPO and anti-Tg antibodies, and again excluding the article published by Bocos et al.11 (P97.5: 2.63μU/ml) and that of Hospital de Valme (P97.5: 4.39μU/ml), all the studies reported TSH P97.5 values ranging from 3.42 to 3.98μU/ml. This is slightly lower than when we consider all the studies globally, though not all of them used the same immunoassay for measuring TSH levels.
As regards the influence of iodine nutritional status in studies where ioduria was >150μg/l, the TSH P97.5 values in the first trimester were close to 4μU/ml (4.75 and 3.42 according to Siemens Centaur; 4.15 according to Roche; 4.06 according to Beckman Access; and 3.95μU/ml according to Abbott, respectively), and clearly higher than the upper limit of normal for TSH (2.5μU/ml) established by the different international guides published up until 2012.4–6 The value of 4.75 obtained by Vila et al.18 – considerably higher than the values of the other studies using this method – could have been influenced by the fact that the pregnant women were assessed at week 9, just before the usual hCG peak.
Considering the results of the only two studies (Ruiz-Ochoa et al.16 and Álvarez-Ballano et al.17) that take into account the fundamental requirements for selecting the reference population (up to 12 weeks of pregnancy, negative thyroid autoimmunity defined as negative anti-TPO and anti-Tg antibodies, and adequate iodine nutritional status), the TSH cut-off points obtained for defining hypothyroidism are between 3.42 and 4.06μU/ml. The lowest cut-off point corresponds to the Siemens Centaur method (3.42μU/ml), with very similar values for Abbott Architech (3.95μU/ml) and Beckman Access (4.06μU/ml).
With the only exception of the study by Bocos et al.11 cited in the 2011 ATA guide,5 with a TSH P97.5 value in the first trimester of 2.63μU/ml, globally all the studies published in Spain to date (Table 1), report TSH P97.5 values in the first trimester of pregnancy that are clearly higher than those established in the international guides, with values close to 4μU/ml in most cases.
Among the limitations of the present review, it should be noted that the individual data of each study and the TSH P97.5 confidence intervals of most of them were not available. This prevented expression of the results as multiples of the median, a method that can reduce the differences between the different immunoassays and allow easier generalization of the cut-off points.21 In addition, the heterogeneity of the populations of the different studies precluded our conducting a meta-analysis with the reviewed data.
Some authors have emphasized the relevance of the discrepancy between the TSH levels defined as normal by the international guides published up until 20124–6 and the reference values inherent to each of the studies. Thus, when using the upper TSH limit of 2.5μU/ml during the first trimester of pregnancy, the prevalence of subclinical hypothyroidism ranges from 19%16 to 40.8%.30 This represents a clear situation of overdiagnosis that has important economic and healthcare consequences. By contrast, the use of proprietary TSH reference values may reduce this prevalence considerably, as reported by Díaz-Soto et al.31 These authors recorded a 38% prevalence of subclinical hypothyroidism with a TSH cut-off point of <2.5μU/ml; however, the prevalence dropped to only 11% when they applied their own reference values to the studied pregnant population. Similar results have been obtained by other authors such as Aller and Rabal19 (28.2–7.2%), Goñi et al.32 (34.5–5.91%), Ruiz-Ochoa et al.16 (19.8–8.52%) and Ortega-Carpio et al.33 (22.7–6.2%).
In the last decade, many international publications have reported reference TSH and FT4 values during pregnancy. Almost 90% documented TSH limits in the first trimester above the cut-off point of 2.5μU/ml recommended by the previous international guides.27 Considering these facts, and as already commented on, the latest ATA guide of 201728 proposes that in the absence of local reference values, a TSH cut-off point of 4μU/ml should be used in the first trimester. This cut-off point represents a decrease of approximately 0.5–1μU/ml with respect to the TSH reference range in the non-pregnant population. However, the studies in Spain exhibit important variations depending on the population studied and the analytical method used.
Many factors influence serum TSH and FT4 levels, and therefore should be taken into account both when reference ranges are established and when interpreting the results obtained in each specific patient. Obtaining an adequate reference population is not simple, though at the very least we should take into account the precise gestational age, classify pregnant women based on their ethnic group, and include only women with optimum iodine nutrition and negative thyroid autoimmunity. The reference ranges proposed by the manufacturers of the different immunoassays do not always meet these requirements, or do not even provide sufficient information for it to be possible to know whether they can be adapted to a specific institution. Each laboratory should therefore establish its own ranges. The scope of the required studies makes this option inaccessible for most centers. It is therefore essential to have published data that reflect the measurement method used and the characteristics of the reference population, with a view to adopting the reference ranges that are best adjusted to the measurement method used and to the population being attended to. Due to the physiological changes that occur during pregnancy, specific reference ranges for TSH and FT4 are required for the correct assessment of maternal thyroid function. The differences between measurement methods make it essential for these ranges to also be specific for each immunoassay. It is therefore understandable that once again the ATA guide of 201728 stresses the need for local reference values allowing for the diagnosis and treatment of thyroid dysfunction during pregnancy.
ConclusionsThe differences found in the reviewed studies do not allow us to establish a unified TSH value in the first trimester of pregnancy. However, despite the observed heterogeneity, we documented a TSH concentration of close to 4μU/ml.
Using a TSH cut-off point of 2.5μU/ml would result in a significant overdiagnosis of subclinical hypothyroidism and thus in a considerable rise in the financial and healthcare costs.
Many factors influence serum TSH levels in pregnancy, and should be taken into account when reference ranges are established.
The differences between measurement methods make it essential that the ranges be specific for each immunoassay. In addition, population-specific reference ranges should be available.
Conflicts of interestThe authors declare that they have no conflicts of interest.
On behalf of the members of the Thyroid Function Committee of the Thyroid Area of the SEEN: M. Pino Alberiche, Orosia Bandrés, Gema Grau, Piedad Santiago, Julia Sastre and Lluís Vila.
Please cite this article as: Donnay Candil S, Oleaga Alday A, Álvarez-García E. Valores de referencia de TSH en población gestante española. ¿Podemos unificar criterios? Endocrinol Diabetes Nutr. 2019;66:124–131.