This position statement is intended to clearly establish obesity as a disease, one of the most prevalent and underestimated (and less diagnosed and treated) diseases in history.1
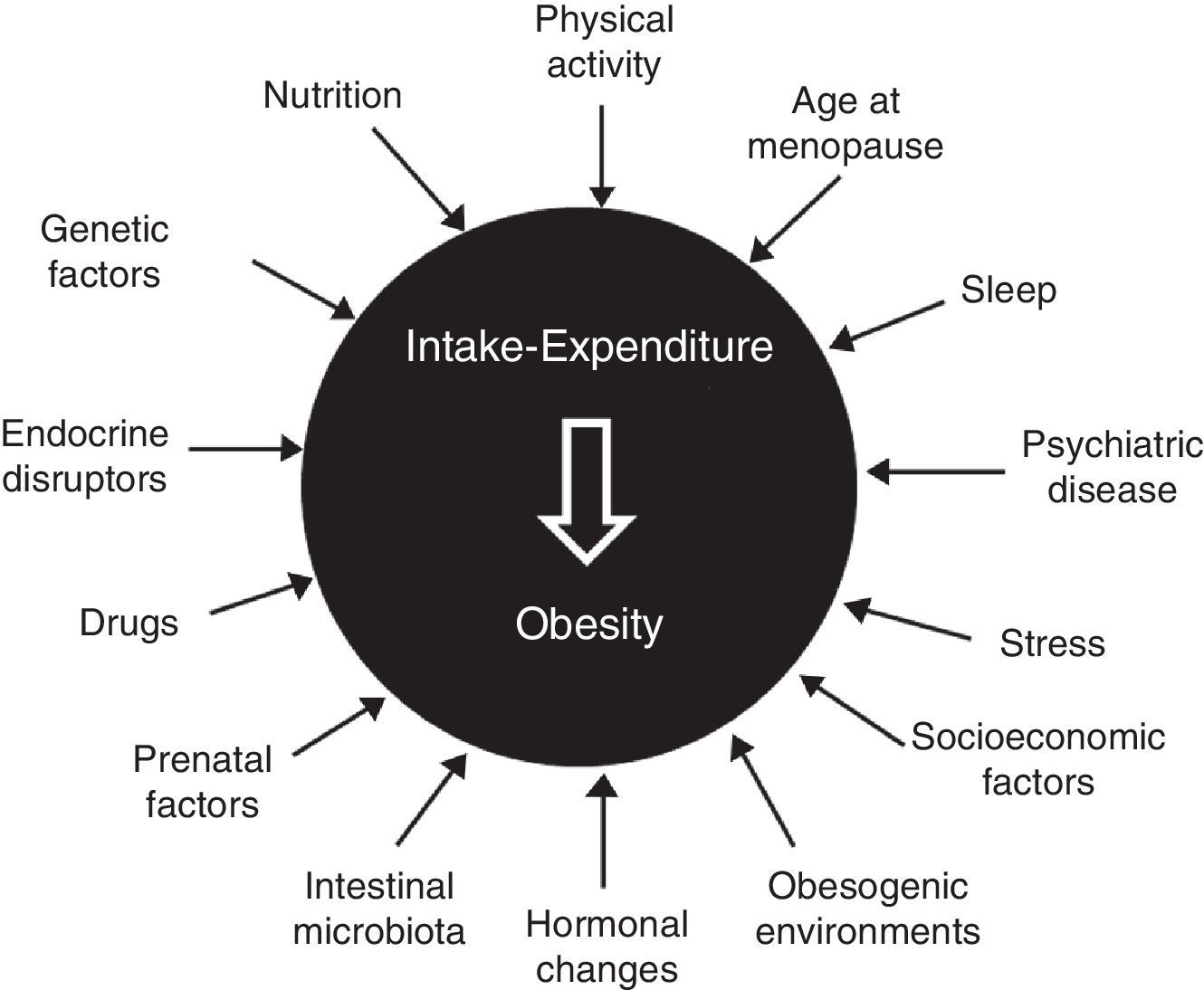
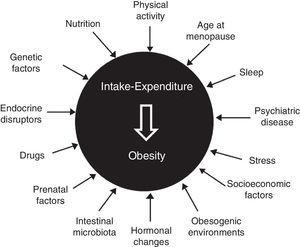
Etiopathogenesis: classical and new elementsClassical elements include age (sarcopenia and greater fat mass in subjects over 65 years of age), sex (decline in levels of anabolic hormones and estrogens after menopause), genetics (multiple gene variants involved, but with an isolated influence), sedentary lifestyle, nutritional behavior (interaction of biological and emotional factors), certain drugs (antidiabetics, contraceptives, antihistamines, and psychotropics), hypothalamic-pituitary dysfunction, and some endocrine diseases2–5 (Fig. 1).
The complexity of the disease is also shown by participation in its development of chronodisruption (circadian desynchrony, changes in the sleep-wake cycle, and sleep deprivation), epigenetics and fetal programming (malnutrition and maternal overfeeding, even before conception), psychiatric disease (irrespective of treatment), stress (both chronic and uncontrolled), intestinal microbiota (predominance of firmicutes over bacteroidetes, a poorly diverse microbiota), endocrine disruptors (bisphenol A, phthalates, pesticides, and insecticides), feeding pattern (excess fat and refined sugars, inadequate fruit and vegetable consumption), an undeprivileged socioeconomic status, and an obesogenic environment (urban dispersion, food availability…).6–9
How and when is obesity diagnosed?Obesity is defined as a proportion of fat mass (FM) greater than 25% in males and 33% in females. When FM cannot be measured, BMI (mild of class I obesity [30–34.9kg/m2], moderate or class II obesity [35–39.9kg/m2], and severe, morbid, or class III obesity [≥40kg/m2]) or waist circumference (WC) (abdominal obesity [≥102cm in males, ≥88cm in females while standing and on the iliac crest]) are used. BMI does not inform about body fat distribution, does not differentiate between lean mass (LM) and FM, and is a poor indicator in subjects of short stature or advanced age, with great muscle mass and water and salt retention, or pregnant.10 Measurement of WC is not considered useful when BMI is ≥35kg/m2.
The SEEDO promotes use of classifications of obesity that combine anthropometric and clinical descriptors,11 as well as mathematical formulas developed in the Spanish population to estimate percent FM (Clínica Universidad de Navarra-Body Adiposity Estimator).12
Epidemiology in SpainBased on BMI, prevalence is 21.6% (22.8% in males, 20.5% in females), increases with age, and is greater in females from 50 years of age. It is greater in Asturias (25.7%) and lower in the Balearic Islands (10.5%).13 According to WC values, the abdominal obesity rate is 36%, and increases up to 62% in subjects over 65 years of age.14
Use of technologyBody composition analysis should be used for diagnosis, clinical evaluation, and monitoring of obesity Bioelectrical impedance is simple and noninvasive, and estimates fat-free mass and, indirectly, total body fat. There is no adequate validation with BMI values >35kg/m2. Dual-energy X-ray densitometry is the gold standard for assessing total body fat and regional fat distribution.15 Both computerized tomography and magnetic resonance imaging are standard procedures to estimate the visceral and subcutaneous fat area at L4-L5, and intrahepatic fat at T12-L1.
Classical comorbidities, what's new?The high prevalence of metabolic syndrome in obesity suggests that the different components share lipotoxicity as etiopathogenic mechanism. High blood pressure (HBP) is 25–40% more common in the obese than in the general population, implicating in its development the greater sympathetic activation and activation of the renin-angiotensin-aldosterone system. Obesity accounts for 44% of the burden of type 2 diabetes mellitus (T2DM), in which obesity is two times more prevalent than in the general population.
Obesity and new comorbiditiesObesity is a preventable cause of colorectal cancer, breast cancer in postmenopausal women, and endometrial, kidney, esophagus, and pancreatic tumors.16 It is the main risk factor for sleep apnea-hypopnea syndrome. In addition, obesity increases the chance of mood disorders and anxiety by 25%. Prevalence of non-alcoholic fat liver disease reaches 100% in subjects with morbid obesity.17 Obesity is also associated to osteoarthritis of the hip and knee, but also in non-weight-bearing joints such as the hands.
Benefits of weight lossWhen combined with lifestyle changes, a 5–10% weight loss decreases by up to 1.0% HbA1c levels and drug requirements for diabetes, as well as systolic and diastolic blood pressure and use of antihypertensive treatment.18 Increases the feeling of well-being and functional capacity. Moderate 3–5% weight loss will already have benefits. Losing from 2.5 to 5.5kg of weight after 2 years decreases risk of T2DM by 30–60%.
After bariatric surgery, a decrease occurs in fatal and non-fatal cardiovascular events, with favorable effects on overall mortality, diabetes, cardiovascular risk factors, cancer, sleep apnea-hypopnea syndrome, joint pain, and quality of life.19
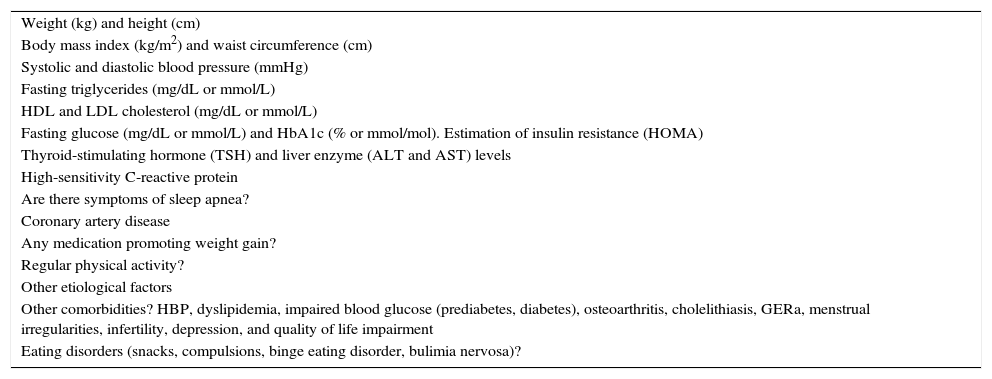
Risk associated to obesityTable 1 lists the minimal data to be recorded in the clinical history of subjects with obesity. The Edmonton classification system used 5 categories based on morbidity and risk profile of the disease, and is able to predict increased mortality.20 Although no healthy obesity exists, subjects who have one or no cardiometabolic abnormality associated to obesity are defined as metabolically healthy.
What are the minimal clinical and laboratory data required for adequate assessment of patients with obesity?
| Weight (kg) and height (cm) |
| Body mass index (kg/m2) and waist circumference (cm) |
| Systolic and diastolic blood pressure (mmHg) |
| Fasting triglycerides (mg/dL or mmol/L) |
| HDL and LDL cholesterol (mg/dL or mmol/L) |
| Fasting glucose (mg/dL or mmol/L) and HbA1c (% or mmol/mol). Estimation of insulin resistance (HOMA) |
| Thyroid-stimulating hormone (TSH) and liver enzyme (ALT and AST) levels |
| High-sensitivity C-reactive protein |
| Are there symptoms of sleep apnea? |
| Coronary artery disease |
| Any medication promoting weight gain? |
| Regular physical activity? |
| Other etiological factors |
| Other comorbidities? HBP, dyslipidemia, impaired blood glucose (prediabetes, diabetes), osteoarthritis, cholelithiasis, GERa, menstrual irregularities, infertility, depression, and quality of life impairment |
| Eating disorders (snacks, compulsions, binge eating disorder, bulimia nervosa)? |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL, high density lipoprotein cholesterol; HOMA, homeostasis model assessment; HBP, high blood pressure; LDL, low density lipoprotein cholesterol; GER, gastroesophageal reflux.
Every 5kg/m2 increase in BMI significantly increases mortality of T2DM (HR 2.16), chronic kidney disease (HR 1.59), ischemic heart disease (HR 1.39), stroke (HR 1.39), respiratory disease (HR 1.20), and cancer (HR 1.10).21
Modest weight increases (≥5kg) after 18 years in females (after 20 years in males) increase the risk of heat disease and T2DM, regardless of initial BMI.
In older obese subjects, comorbidity is more prevalent and sever, although BMI is associated to lower relative mortality.22
Obese child, obese adult?In childhood, diagnosis is made based on BMI percentiles. In children aged 6–9 years, prevalence in Spain is 18.3% (our country ranks second in boys and third in girls in Europe).23 Prevalence of comorbidities is lower than in adults, and if projected to older ages, with critical times in its development: the first year of life, the period from 4 to 6 years, and adolescence.24 Treatment is based on three mainstays: reorganization of dietary habits, promotion of physical activity, and child motivation with the help of the family and social environment.
Dietary planObesity prevention and treatment require achievement and maintenance of a healthy lifestyle (diet, exercise, social, geopolitical, and environmental determinants). Includes both quantitative (decreased portions and energy provision) and qualitative changes (varying the proportion of different nutrients).
A healthy pattern includes greater vegetable and fruit consumption followed, although with a lower grade of evidence, by whole grain cereals, low fat dairy products, fish, pulses, and nuts. A decreased meat intake, including processed meat and sugary foods, is also characteristic of this pattern.25
The low-calorie Mediterranean diet is the model supported by the SEEDO because it best represents this balanced and healthy approach, with low intake of saturated and trans fatty acids and added sugars, and high intake of vegetable fiber and monounsaturated fatty acids. Its benefits on health, including mortality, have been clearly established.26
As regards modified macronutrient diets, no studies showing its advantages are available. Because of this, together with their widespread use, lack of strict medical control, and presence of commercial interests, the SEEDO does not recommend their use. Use of functional foods is not supported by scientific evidence either.
The risks of an inadequate diet include malnutrition or micronutrient deficiency, worsening of cardiovascular risk, promotion of eating disorders, transmission of wrong nutritional concepts, or promotion of a feeling of frustration.
The SEEDO stands against any diet with no scientific support. It is essential to emphasize the need for a varied, healthy, and balanced diet in the context of the Mediterranean diet, and for regular physical exercise.
Physical activity: Not everything is the samePhysical activity (PA) represents daily muscle movement. Physical exercise (PE) is structured, planned, and repetitive PA. Sports activity is PE practiced according to some rules, usually with competitive purposes. Fitness is the set of benefits achieved from the practice of PE.
PA is measured using the resting metabolic rate (MET), corresponding to 3.5mL O2/kg/min, the minimum oxygen consumption to maintain vital signs. Reading, driving, work in a sitting position, and house chores spend 1–3 METs. Moderate PA (brisk walking, quiet cycling) is performed at 3–6 METs, and intense PA (running, jumping, aerobic exercise) from 6 METs.
Weight loss is not clinically relevant when PE is only performed, although there is a great individual variability. However, a PE program is important, almost indispensable, to prevent recovery of the weight lost.27 The psychological improvement provided by exercise contributes to decrease energy intake for emotional reasons, and is most useful in people with stress hyperphagia.
How should a physical exercise program be prescribed?At least 30min daily of moderate or intense exercise five or more days per week (150min/week; 300min/week to prevent weight gain) and restriction of physical inactivity are recommended.28
Combined aerobic (walking, jogging, dancing, skiing, pedaling, etc.) and anaerobic PE achieves better results than either PE alone.29 Because of its effect on muscle mass, anaerobic exercise is particularly indicated for elderly people.
Exercise prescription should be personalized, considering the preferences and skills, physical condition, cardiorespiratory and orthopedic status, medication, and disabilities of each person (Table 2). Adverse effects to be considered include overload of weight-bearing joints and respiratory compromise.
Advice to start prescription of a physical exercise program in standard clinical practice.
| Advice 1 | Measure the activity/inactivity level. A podometer may be used to classify obese subjects as sedentary (<5000steps/day), moderately active (5000–10,000 steps/day), or active and healthy (>10,000steps/day). An alternative is the short version of the International Physical Activity Questionnaire (PAQ), validated in Spanish, which classifies physical activity level as low (not done), moderate, or intense |
| Advice 2 | Establish as a goal to change and increase activity level depending on the condition of each person |
| Advice 3 | Perform at least 30min daily of moderate to highly intense exercise 5 or more days per week (150min/week); all weekly exercise may be concentrated in only 2 sessions. Choose any activity that is pleasant, accepted by the patient, and easy to do. They may be daily activities (such as walking or cycling), supervised programmed exercises (such as classes at a gym), or other activities such as swimming, climbing stairs, etc. |
| Advice 4 | Prior warm-up, including joint movement, for approximately 10min is advisable to prevent muscle lesions |
| Advice 5 | Indicate frequency and intensity with the greatest possible flexibility, using both variables to achieve the same goal. Increases in intensity of the activity provide improvements in fitness. A training modality of high intensity and short duration is high intensive interval training, which achieves cardiorespiratory improvements |
| Advice 6 | Contact with some friend or perform in group. Use music to set the pace |
| Advice 7 | Maintain adequate hydration, drinking approximately 1.5L of water before, during, and after exercise. Avoid drinking more than 200mL/15min (maximum bowel absorption rate), because it would cause intestinal discomfort (the typical flank pain) |
| Advice 8 | Wearing adequate clothes and shoes makes exercise more pleasant and prevents injuries |
| Advice 9 | After exercise, cool down for some 5min (for example, walking slowly) to allow for disappearance of muscle lactate |
Drugs must be used in subjects with BMI >30kg/m2, or >27kg/m2 associated to major comorbidities, when they do not lose >5% of initial weight after 3–6 months in a structured program. If program is well tolerated and weight loss is greater than 5% of initial weight, program should be continued while the indication persists.
After orlistat, the European Medicines Agency approved in 2015 two new drugs: liraglutide 3.0mg (Saxenda®) and the combination of bupropion (360mg) and naltrexone (16 or 32mg) (Mysimba®). Liraglutide is a GLP-1 receptor agonists for daily subcutaneous administration. After 56 weeks of treatment, liraglutide decreases weight by 8.0±6.7% (8.4±7.3kg).30 Bupropion/naltrexone is administered orally, and achieves weight losses of 5.4–8.1%. This combination may be useful in patients with depressive symptoms.31 Side effects include nausea and vomiting with liraglutide, and headache, dry mouth, nausea, and dizziness with bupropion-naltrexone. Both drug treatments are contraindicated in pregnancy. Side effects are transient and are not a major cause of discontinuation.
Liraglutide contributes to decrease blood pressure and improve cardiovascular risk parameters, and decreases cardiovascular mortality. It is the drug of choice in patients with T2DM or prediabetes.32 Bupropion/naltrexone improves blood pressure and lipid profile.
Prevention of obesityThe goal is to decrease development of overweight and obesity in subjects with normal weight and overweight respectively by preventing weight recovery. We recommend identification of and action on the groups at greater risk (Table 3).33
Risk groups that should be identified to apply selective prevention measures intended to avoid weight gain.
| Overweight subjects |
| Central body fat distribution |
| Obese people who have lost weight |
| Cyclical weight changes |
| Diseases predisposing to obesity (genetic, orthopedic, endocrine, etc.) |
| Patients receiving certain treatments (corticoids, antihistamines, anxiolytics) |
| Familial predisposition to obesity and sedentary lifestyles |
| Environmental risk factors (low sociocultural level, fruit and vegetables not available) |
| Inadequate dietary habits (increased intake of calorie and fat, high-calorie foods and sugary drinks, etc.) |
| Life periods critical for obesity (pregnancy, from 5 to 7 years, adolescence, and menopause) |
| Former smokers |
| Subjects highly motivated to prevent weight increase |
Implication should be sought of the food industry (nutritional quality and labeling), communication media (true, verified information), legislative power and authorities (regulatory and coercive measures for industry, economic incentives to use of some food items), local administration (town planning), educational institutions (school canteens, restriction of vending machines with sugary drinks), workplaces (remove lifts and mechanical means, provide leisure areas), and scientific societies. These should lead the fight against obesity, warn of its consequences, and foster prevention strategies.
Treatment of “diabesity”, is there anything new?Weight reduction is the first step, and is an essential part of all phases of treatment, together with exercise.34 The time of diagnosis is critical to prescribe weight loss.
Drug treatment for diabetes is conditioned by the weight gain associated to sulfonylureas, thiazolidinediones, and insulin. However, GLP-1 receptor agonists are associated to 2.9kg weight reductions as compared to placebo, oral antidiabetics, or insulin.35 Similarly, SGLT2 inhibitors have been reported to cause weight losses ranging from 1.8 to 2.3kg versus placebo.36
T2DM remission rate after gastrointestinal surgery is 72% at two years and 30.4% at 15 years.37 A reduction has also been reported in the incidence of microvascular and macrovascular complications, and better metabolic control is achieved as compared to standard treatment.38,39 Prognostic factors associated to remission include younger age, shorter disease duration, lower HbA1c values, higher C-peptide levels, and no need for insulin therapy.40 In Spain, the indication for subjects with BMI <35kg/m2 only applies to participation in clinical trials.
Bariatric surgeryThis is indicated for subjects with BMI≥40kg/m2, or less severe forms of obesity (BMI 35–40kg/m2) with severe associated comorbidities, aged 18–60 years (individualizing adolescents and elderly subjects). Dietary pattern, BMI, associated comorbidities, and surgical should be considered when a surgical procedure is decided.
Patient expectations should be modulated before surgery. The goal of surgery is to achieve a weight loss that improves comorbid conditions and quality of life. Although Roux-en-Y gastric bypass (RYGBP) is considered as the reference procedure, vertical sleeve gastrectomy is increasingly used because of its simplicity and effectiveness.41 Several studies have reported an initial weight loss similar to that achieved by RYGBP, but long-term results should be assessed.42 A laparoscopic approach should be used, and intraoperative mortality should be <0.5%. The main complications include infection, bleeding, and suture failures.41
Long-term multidisciplinary follow-up is required to ensure adequate weight loss and adherence to healthy lifestyles. It should not be forgotten that surgery may modify absorption and bioavailability of some drugs.43
While modification of the gastrointestinal tract may be a therapeutic alternative for the components of metabolic syndrome, generalized use of metabolic surgery cannot be recommended.44
After surgery, effective contraceptive methods not dependent on intestinal absorption should be used, and pregnancy should be avoided until 12–18 months have elapsed.45
Where should obese patients be treated?A single protocol for assessment and treatment of obesity, stating the criteria for referral between the primary and specialized care settings should be prepared, and communication between professionals should be facilitated. Continued training activities and coordinated development of research lines should be a constant.46
FundingNovo Nordisk and AstraZeneca provided unconditioned support to the Spanish Society for the Study of Obesity (SEEDO).
Conflicts of interestA. Lecube received honoraria as scientific advisor (Novo Nordisk, AstraZeneca, Janssen) and for sponsored lectures (Novo Nordisk, AstraZeneca, Sanofi, Boehringher-Lilly). D. Bellido received honoraria as scientific advisor (Novo Nordisk, Boehringher-Lilly, Sanofi) and for sponsored lectures (Novo Nordisk, Boehringher-Lilly, Sanofi, AstraZeneca, Janssen, Almirall, Novartis, MSD). P.P. García-Luna received honoraria as scientific advisor (Novo Nordisk, Vegenat) and for sponsored lectures (Novo Nordisk, Vegenat, Nestlé, MSD, Janssen). F.F. Casanueva received research grants and fees for consultancy or lectures from Novo Nordisk, Lilly, Pfizer, AstraZeneca, Boheringer Manheim, Novartis y Janssen.
S. Monereo, M.A. Rubio, P. Martínez-de-Icaya, A. Martí, J. Salvador, L. Masmiquel, A. Goday, E. Lurbe, J.M. García-Almeida, F.J. Tinahones, E. Palacio, M. Gargallo, I. Bretón, S. Morales-Conde, A. Caixàs, E. Menéndez, and M. Puig-Domingo have no conflicts of interest related to their participation in this study.
Equal first authors.
Please cite this article as: Lecube A, Monereo S, Rubio MÁ, Martínez-de-Icaya P, Martí A, Salvador J, et al. Prevención, diagnóstico y tratamiento de la obesidad. Posicionamiento de la Sociedad Española para el Estudio de la Obesidad de 2016. Endocrinol Nutr. 2017;64:15–22.
This document has been approved by the board of directors of the Spanish Society of Endocrinology and Nutrition and the Spanish Diabetes Society. The complete version may be found in the web site of both societies (www.see.es; www.sediabetes.org), an in the web site of the Spanish Society for the Study of Obesity (www.seedo.es).