To evaluate metabolic control and satisfaction with a telemedicine diabetes education programme for the initiation of flash glucose monitoring (FGM) in type 1 diabetes.
Material and methodsProspective study in 48 patients (52.1% women, 22.9% on insulin pump) who started FGM. They were analysed at baseline and 3 months after the beginning of the FGM. The results were compared with an on-site learning cohort matched by age, sex and HbA1c.
ResultsAt the beginning and 3 months after the MFG, HbA1c improvement was observed (7.9±1.4 vs 7.3±1.1%), p<0.01; with a decrease in time below range - TBR - (4.7±4.9 vs 3.5±3.5%), p<0.05 and number of hypoglycaemic events (9.4±8.7 vs 6.9±5.7/15 days), p<0.05, associated with a worsening in time above range - TAR - (33.5±19.9 vs 37.0±20.9%), p<0.05. No significant differences were observed in the TIR 70–180mg/dl (61.7±18.6 vs 59.4±20.0%), glycemic variability or the use of FGM. Patient satisfaction with telemedicine training was 4.8±0.3 out of 5.
No significant differences were observed in the follow-up, either in HbA1c or other glucometer parameters between on-site and online training.
In a multivariate analysis adopting the HbA1c at follow-up as the dependent variable, only the TIR (β=−0.034; p<0.001) and the initial HbA1c (β=0.303; p<0.001) maintained statistical significance, unrelated to the on-site or online training (β=0.136; p=ns).
ConclusionsA telemedicine programme is an adequate tool for training in FGM, with results similar to on-site training, and it was associated with a high degree of satisfaction.
Evaluar el control metabólico y la satisfacción con un programa de educación terapéutica en diabetes mediante telemedicina para el inicio de la monitorización flash de glucosa (MFG) en diabetes tipo 1.
Material y métodosEstudio prospectivo en 48 pacientes (52,1% mujeres, 22,9% en tratamiento con bomba de insulina) que iniciaron MFG. Se analizaron basalmente y a los 3 meses tras el inicio del MFG. Los resultados se compararon con una cohorte de formación presencial pareada por edad, sexo y HbA1c.
ResultadosAl comparar los resultados al inicio y a los 3 meses de la MFG se observó una mejoría en HbA1c (7,9±1,4 vs 7,3%±1,1), p<0,01; tiempo por debajo del rango–TBR-(4,7±4,9 vs 3,5±3,5%), p<0,05 y número de eventos de hipoglucemia(9,4±8,7 vs 6,9±5,7/15días), p<0,05, asociado a un empeoramiento del tiempo por encima del rango–TAR-(33,53±19,9 vs 37,0±20,9%) p<0,05. No se observaron diferencias significativas en el TIR 70–180mg/dl (61,7±18,6 vs 59,4±20,0%), parámetros de variabilidad glucémica o del uso del dispositivo. La satisfacción de los pacientes con la formación por telemedicina fue de 4,8±0,3 sobre 5.
No se observaron diferencias significativas en el seguimiento, ni en HbA1c ni otras glucométricas, entre la formación presencial frente a la telemática.
Al realizar un análisis multivariante adoptando la HbA1c de seguimiento como variable dependiente, exclusivamente el TIR (β=−0,034; p<0,001) y la HbA1c inicial (β=0,303; p<0,001) mantuvieron la significación estadística, sin relación con la formación online o presencial (β=0,136; p=ns).
ConclusionesLa consulta de telemedicina es una herramienta adecuada para la formación en MFG con resultados similares a la consulta presencial y presenta un alto grado de satisfacción.
In recent years, the use of flash glucose monitoring (FGM) has been established as a reliable method for measuring interstitial glucose, which, in addition, provides new tools for glycaemic control in diabetes patients.1
The use of FGM in patients in real life has demonstrated an improvement in different glycaemic variables, such as a reduction in HbA1c, a reduction of time in hypoglycaemia and hyperglycaemia, as well as an increase in time in range.2
Moreover, the recent improvement in the new glycaemic parameters through interstitial glucose monitoring (IGM) has been correlated with a reduced risk in microvascular and macrovascular complications.3
The efficacy of FGM in achieving greater glycaemic control has been shown to be directly related with the number of scans, involvement and the adequate use of the system by the patient.4 In this regard, the establishment of specific diabetes education therapy protocols for learning how to use and interpret the system data have proven to be a necessary strategy.5 Conversely, the use of FGM has been related with an improvement in the perception of quality of life by patients.6
Currently, the funding of FGM by the National Health System is applied fundamentally to persons with type I diabetes (DM1),7 although preliminary data would seem to support the efficacy thereof in certain type II diabetes (DM2) subgroups.8
One of the additional advantages of using FGM Is based on the capacity of these systems to share glycaemic results, remotely and in real time, with the healthcare professionals and family members/caregivers through specific platforms (Libreview®). This tool has facilitated virtual consultations, allowing, In turn, the detailed analysis of the outpatient glucose profile by the diabetes care team and the patient.
As a result of the SARS-CoV-2 pandemic, and thanks partly to these devices, the working methodology in diabetes therapeutic education consultations has been modified, to include teletraining, as a new safe and reliable distance training tool, thus making it possible to maintain close contact with the patient.9
Indeed, telemedicine platforms have been shown to be feasible and effective for providing care to diabetes patients, although it is advisable to take precautions, which include meticulous adaptation to the institution, the physician and the population of patients attended, to ensure that the virtual care has the greatest possible impact.10
The aim of this study was to evaluate the degree of metabolic control and satisfaction in a group of patients with DM1 attended by means of online consultation on diabetes therapeutic education for training in FGM, both at baseline and three months after the implementation thereof, as well as to compare the results obtained with a cohort of DM1 patients with face-to-face training in FGM.
Material and methodsProspective study in all patients with DM1 attended during the period from May 2020 to May 2021, who performed online training for the implementation of FGM (FreeStyle Libre, Abbot Diabetes Care, Witney, United Kingdom). Clinical and anthropometric data (treatment type, gender, age, time course of diabetes, body mass index), along with blood chemistry (HbA1c – Roche Diagnostics, Geneva, Suiza) and glycometric (time in range between 70–180mg/dl [TIR], time above range [TAR], time below range [TBR], coefficient of glycaemic variation [CV], standard deviation [SD] and Glucose management indicator formerly known as estimated HbA1c [GMI])11 and FGM usage (number of scans on the device and percentage of sensor use) data were collected. The variables were analysed 15 days after Commencement of the usage of the device (Baseline situation) and three months after its implementation. Lastly, the patients' satisfaction with the online training received in the diabetes therapeutic education clinic was evaluated by means of a five-point Likert scale comprising 9 questions performed for this end, three months after completing the training (Table 1).
Five-point Likert scale survey to evaluate the online consultation for Therapeutic Education in Flash Glucose Monitoring.
| What is your opinion of the training received from the Diabetes Therapeutic Education Team by means of telemedicine? | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Has this session covered your needs? | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Overall quality of the care received form the Diabetes Therapeutic Education team responsible for the session. | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Was the communication by this medium suitable? | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Did you understand the instructions given to you by means of online consultation correctly? | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Do you feel the concepts were explained to you correctly? | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Were you able to see the image of the healthcare professional on your device (PC, tablet, mobile phone, etc.) correctly? | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Were you able to hear the healthcare professional on your device (PC, tablet, mobile phone, etc.) correctly? | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
| Overall impression of the training via the telemedicine system used. | ||||
| 1□ | 2□ | 3□ | 4□ | 5□ |
The results obtained were also compared with a historical cohort of controls with FGM who had received face-to-face training prior to the SARS-CoV-2 pandemic (March 2019 to March 2020) and whose data had been collected prospectively both at baseline and three months after the implementation of the device. To guarantee the homogeneity and comparability of the sample, the cases were paired for age, sex and initial HbA1c, adhering to a ratio of 1:2. To compare the results after three months of monitoring from the commencement of the FGM between the face-to-face and online groups, the differences in the different glycaemic variables evaluated after three months and at baseline were analysed for each training group
The education protocol used in FGM was identical for both face-to-face and online training. In both cases the training was carried out in group form (3 or 4 patients) and included two initial sessions, separated by one week, each one lasting 90min. The contents included were: Day 1. Technical training on FGM, placement and use of the device. Day 2: Interpretation of trend arrows, outpatient glucose profile, charts, and insulin dose modification. Similarly, three months after the commencement of the FGM, an individual monitoring session was performed with the same online or face-to-face character as the initial consultation. No intermediate consultations were performed in either training modality. Online training was implemented via an online video-conferencing platform (Zoom Video Communications, Inc.) using the same educational material and graphical support in both the face-to-face and online models. Lastly, online training was offered to all patients with computer devices at home compatible with the platform used, with no prior screening of the candidates by the healthcare team.
Statistical analysisThe results are expressed in terms of mean and standard deviation (SD). The normal distribution of the variables was analysed using the Kolmogorov–Smirnov test. Quantitative variables with a normal distribution were analysed with a bilateral Student’s t-test. Non-parametric variables were evaluated using the Mann–Whitney U test. Qualitative variables were expressed in terms of percentages and were analysed using the chi-squared test (with Fisher’s correction when necessary). Lastly, a multivariate analysis model was used which as independent variables included the effect of age, gender, treatment type, baseline HbA1c and TIR level, as well as the implementation of the online or face-to-face consultation, over the levels of the final HbA1c monitoring visit as a dependent variable.
The SPSS statistical software package, version 17.0 (SPSS Inc., Chicago, IL, United States), was used for analysis. The accepted level of statistical significance was 5% (p<0.05). The study was approved by the Clinical Research Ethics Committee of the Hospital Centre PI 19-1390.
ResultsA total of 48 patients received online training in FGM. 52.1% were women, and the mean age was 37.2±12.3 years and 19.7±10.2 years of DM1 development. 22.9% were under treatment with a subcutaneous insulin infuser (SCII). The baseline HbA1c was 7.9±1.4%. In the first download, 14 days after the commencement of FGM, a mean of 11.3±5.2 daily scans were performed with a sensor use percentage of 91.2±10.6%, with the baseline TIR being 61.7±18.6% (Table 2).
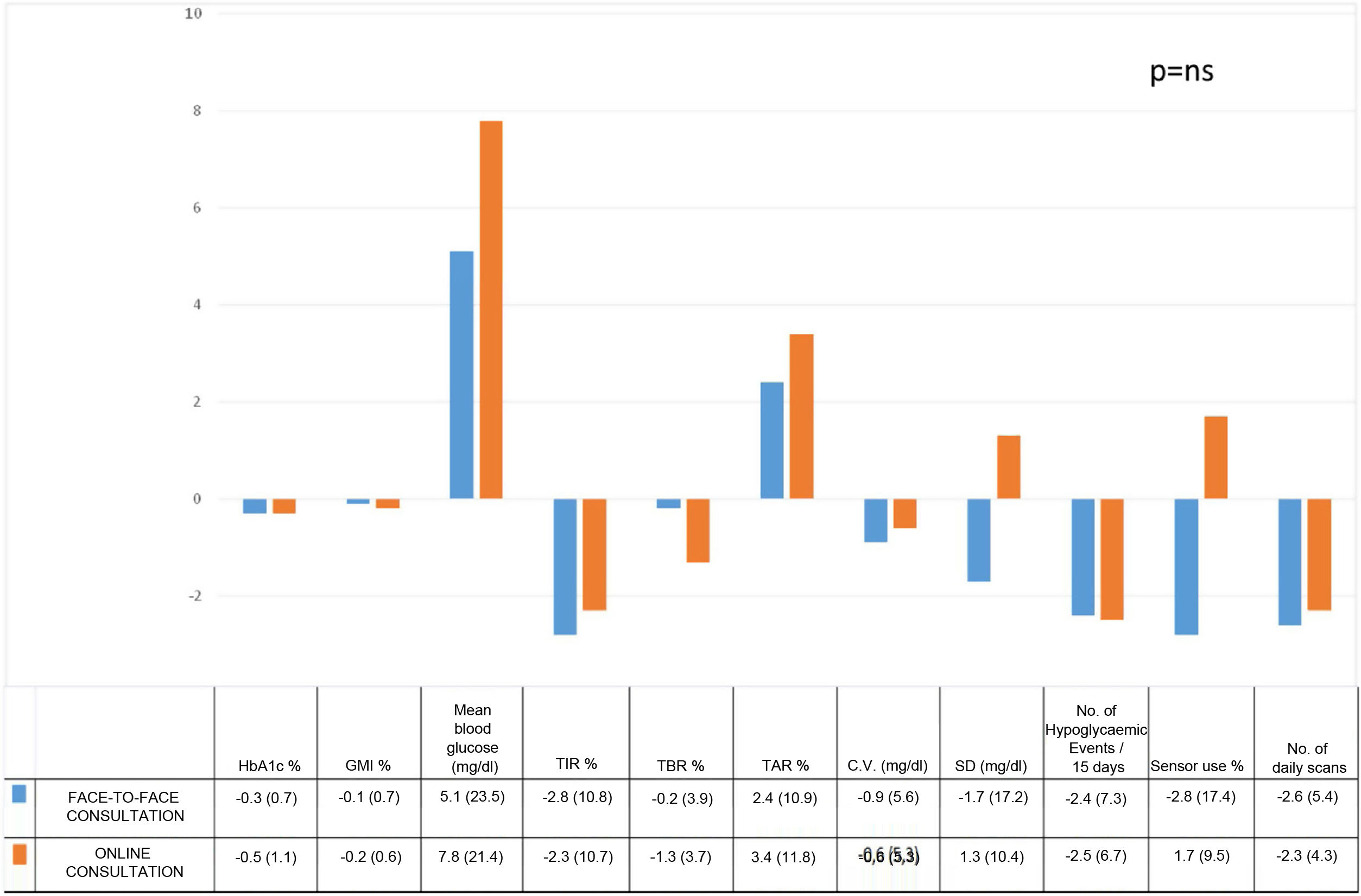
Baseline characteristics and evolution at the start and after three months of training in FGM in online and face-to-face consultation.
| Online consultation | Face-to-face consultation | |||||
|---|---|---|---|---|---|---|
| Patients (n.°) | 48 | 96 | ||||
| CSII (%) | 22.9 | 28.2 | ||||
| Gender (%) | 52.1 women | 55.2 women | ||||
| Mean age (years) | 37.2 (12.3) | 37.5 (12.0) | ||||
| Evolution (years) | 19.7 (10.2) | 19.6 (11.4) | ||||
| BMI (kg/m2) | 24.8 (4.1) | 25.1 (4.13) | ||||
| 0 months | 3 months | p | 0 months | 3 months | p | |
| Mean HbA1c (%) | 7.9 (1.4) | 7.3 (1.1) | <0.01 | 7.8 (1.2) | 7.5 (1.0) | < 0.01 |
| Mean glucose (mg/dl) | 160.0 (32.6) | 167.8 (33.8) | ns | 169.6 (31.3) | 174.7 (37.2) | ns |
| Daily scans (n.°) | 11.3 (5.2) | 9.0 (4.1) | <0.001 | 12.7 (6.3) | 10.2 (6.6) | <0.001 |
| Sensor use (%) | 91.2 (10.6) | 92.9 (5.2) | ns | 92.7 (13.8) | 89.9 (12.8) | ns |
| TIR (%)* | 61.7 (18.6) | 59.4 (20.0) | ns | 54.4 (13.9) | 51.6 (16.3) | ns |
| TAR % (%) | 33.5 (19.9) | 37.0 (20.9) | <0.05 | 38.8 (15.6) | 41.2 (18.0) | <0.05 |
| TBR % (%)* | 4.8 (4.9) | 3.5 (3.5) | <0.05 | 6.7 (5.0) | 6.5 (5.0) | <0.05 |
| CV (%)** | 35.7 (5.9) | 35.1 (5.9) | ns | 41.3 (6.5) | 40.4 (7.1) | ns |
| SD (mg/dl)** | 57.4 (15.2) | 58.7 (15.3) | ns | 70.4 (18.5) | 68.7 (21.9) | ns |
| GMI (%) | 7.4 (1.0) | 7.2 (1.0) | ns | 7.5 (1.4) | 7.4 (1.3) | ns |
| Number of hypoglycaemic events /15 days* | 9.4 (8.7) | 6.9 (5.7) | <0.05 | 13.4 (9.2) | 11.0 (7.1) | <0.05 |
FGM: flash glucose monitoring; BMI: body mass index; TIR: time in range; TAR: time above range; TBR: time below range; CV: coefficient of glycaemic variability; SD: standard deviation; GMI: glucose management indicator.
When evaluating the evolution of patients from baseline to three months after the online training, a statistically significant improvement was observed in the levels of HbA1c (7.9±1.4% vs. 7.3±1.1%) p<0.001, in TBR (4.8±4.9% vs. 3.5±3.5%) p<0.05 and in the number of hypoglycaemic events/14 days (9.4±8.7 vs. 6.9±5.7), p<0.001. Additionally, a significant worsening of the TAR (33.5±19.9% vs. 37.0±20.9%) p<0.001 was observed; a reduction in the number of scans was also observed (11.3±5.2 vs. 9.0±4.1 /day); p<0.001. Nonetheless, no differences were observed in the glycaemic variability parameters (Table 2). When evaluating the satisfaction of patients with the online training received by means of a five-point Likert scale survey, a score of 4.8±0.3 points was obtained.
On the other hand, a cohort prior to the SARS-CoV-2 Pandemic for face-to-face training was evaluated, pairing the controls for age, gender and HbA1c compared to those with online training. Finally, a total of 96 patients in face-to-face training were analysed. The mean age was 37.5±12.0 Years and 19.6±11.4 years of evolution of DM1, with 55.2% being women. 28.2% were under treatment with CSII (Table 2).
When comparing the baseline characteristics of the face-to-face group with the online group, no differences in the baseline clinical anthropometric, device use or plasmatic HbA1c variables were found. However, statistically significant higher values were observed in the online training group in baseline TIR (61.7±18.6 vs. 54.4±13.9%), p<0.05; and lower values for TBR (4.8±4.9 vs. 6.7±5.0%), p<0.01; CV (35.7±5.9 vs. 41.3±6.5%, p<0.01); SD (57.4±15.2 vs. 70.4±18.5mg/dl), p<0,01; and number of hypoglycaemic events/15 days (9.4±8.7 vs. 13.4±9.2), p<0.05 (Table 2).
The evolution of patients in face-to-face training from baseline level to three months was also evaluated. A statistically significant improvement was observed in the glucometric parameters evaluated in parallel to those for online training in levels of HbA1c (7.8±1.2 vs. 7.5±1.0%), p<0.001; in TBR (6.7±5.0 vs. 6.5±5.0%), p<0.05; and in the number of hypoglycaemic events/15 days (13.4±9.2 vs. 1.0±7.1), p<0.001. And a significant worsening was observed in the TAR (38.8±15.6 vs. 41.2±18.0%) p<0.001; as well as a reduction in the number of scans (12.7±6.3 vs. 10.2±6.6%), p<0.001; with no differences being observed in the glycaemic variability, in a similar way to the online group (Table 2).
The results obtained in the online and face-to-face training for FGM were compared three months after the launch of the device. To do so, the differences between the mean parameters at three months and baseline were used for each type of online or face-to-face training. No significant differences were observed in any of the metabolic control variables evaluated (Fig. 1). No acute glycaemic imbalances requiring preferential/urgent attention from the healthcare team were observed in either the face-to-face or online groups. Similarly, no differences were observed when evaluating the results obtained in the CSII or multiple doses of insulin.
Differences at three months after the commencement of sensor use in face-to-face consultation as opposed to online consultation.
BMI: body mass index; TIR: time in range; TAR: time above range; TBR: time below range; CV: coefficient of glycaemic variability; SD: standard deviation; GMI: glucose management indicator.
Lastly, when performing a multivariate analysis taking the HbA1c of monitoring as a dependent variable, only the TIR (β=−0.034; p<0.001) and baseline HbA1χ (β=0.303; p<0.001) maintained statistical significance, with no statistical relationship with online or face-to-face training being observed (β=0.136; p=ns).
DiscussionThe appearance of IGM has completely modified care for diabetes patients, particularly in the case of DM1, providing new glucomentric variables and the possibility of the healthcare team evaluating the glycaemic profile remotely.12 Although the implementation of these systems was prior to the SARS-CoV-2 pandemic, the healthcare crisis has undoubtedly provided the definitive backing for the implementation of telemedicine in diabetes care.13 Indeed, previous studies in Spain calculate the adoption of teleconsultation from 19.5% to 97.8% during the lock-down in 2020.14
It seems clear that online consultations can adequately replace face-to-face consultation in chronic diseases, such as DM1, especially when analysing the outpatient glucose profile obtained by means of FMG in patients already using the device.12 Nonetheless, the implementation of any IGM system requires a diabetes therapeutic education programme which guarantees maximum performance in the use of the device. Indeed, previous experiences have shown how patients who receive specific educational programmes for FGM achieve better results in glucose control parameters.5 Nonetheless, few studies have investigated the efficacy of diabetes therapeutic education programmes conducted entirely by means of telemedicine15 and, to the best of our knowledge, none of these presents a prospective control group which had performed face-to-face consultation.
The results of our study are in line with those published by other groups.16 Patients with online training achieved a significant improvement in plasmatic HbA1c of 0.4%, as well as a reduction in the TBR, from 4.8 to 3.5%, and in the number of hypoglycaemic events/15 days, with a high level of satisfaction in relation to the type of online consultation. In our case, this reduction in the TBR was accompanied by stability in the TIR, and a slight increase in the TAR, in accordance with the criteria of the International Consensus on TIR.11 The stability in the TIR, as opposed to the improvement which would have been desirable, can be justified by the baseline characteristics of the patients in our sample, with a tighter initial control than in other series and, in part, similar to those included in the Impact study (with good diabetes control defined as HbA1c < 7.5%, and high risk of hypoglycaemic episodes).17 In this case, the most suitable initial degree of control would have been able to favour the implementation of avoidance behaviours aimed at reducing hypoglycaemic episodes, and the secondary increase in levels of hyperglycaemia. In the same regard, the high percentage of patients under treatment with CSII in both groups is significant, there being no difference with the results obtained with the patients under treatment with multiple doses of insulin. On the other hand, the ratio of TIR and plasma HbA1c (and even the GMI) is not strictly linear, and could be influenced by other variables, such as glycaaemic variability, which would explain the stability of the TIR.18
In any case, these results are in line with those obtained in a sample paired by age, gender and baseline plasma HbA1c, whose FGM training was performed under the same protocol and face-to-face. Both face-to-face and online consultation achieved HbA1c reductions in the region of 0.3%, presenting stable levels of TIR, as well as decreases in the time and number of hypoglycaemia events associated with minimal increases in TAR, with no significant differences between the groups. That is to say, the results obtained are independent of the type of consultation (online or face-to-face) performed. Indeed, the multivariate analysis supports this hypothesis, as the type of consultation does not affect the level of HbA1c in the monitoring. Nonetheless, the final HbA1c was directly related with the baseline levels of HbA1c and TIR, a ratio described recently.18
The differences between the baseline characteristics of the online and face-to-face training groups are worthy of separate mention. Despite the online group being paired with the face-to-face group for age gender and baseline HbA1c to ensure the comparability of the analysis, those patients in face-to-face consultation presented a greater degree of glycaemic variability and time in hypoglycaemia and, consequently, a lower TIR. In our view, these results do not go against both groups being comparable, rather they demonstrate the complicated interrelation between the different glucometrics faced with the same level of HbA1c, and the importance of glycaemic variability when analysing this ratio.18 What is more, the intragroup differences in the TIR are at the limit of clinical significance (5%).19 In either case, and despite the face-to-face training group presenting greater variability and lower TIR, both groups achieved similar changes in the different glucometrics, irrespective of the training model employed.
This study has certain limitations, the principal one being that the online training group commenced the FGM during the SARS-CoV-2 pandemic; however, the face-to-face control training group was prior to the healthcare crisis. The situation is undoubtedly a reflection of the situation experienced globally at that time.14 On the other hand, there are numerous studies, at times with conflicting results, which have described the influence of the pandemic on glycaemic control in diabetes patients.12 Although we cannot rule out the effect of the pandemic on the results obtained, by conducting the study outside the months of strict lock-down in Spain, and presenting the results between both groups, we believe that if there were any effect, this would be mitigated. Moreover, the evaluation of the therapeutic education programme in the long term would have been desirable; nonetheless, we believe that a period of three months after completing the implementation of FGM Is sufficient to be able to evaluate the usefulness of the education programme in its two modalities after having demonstrated the commencement of benefit in FGM In the first days of using the device.6 Similarly, the results of the diabetes therapeutic education programme in longer evaluation periods could have been affected by interim educational reinforcement, regardless of the strict training for the use of the device, including through communication channels other than the initial one. Lastly, even though all the patients during the period from May 2020 to May 2021 were offered online training in FGM without prior screening, certain patients may have rejected this training as they lacked the minimum necessary IT support or a different socio-economic or cultural level, data which were not collected in this study
As strong points, we would certainly highlight the prospective nature of the study in both the face-to-face and online groups. Furthermore, to the best of our knowledge, this study is the first to evaluate online training in FGM prospectively and with a paired control group. Both groups received the same type of training (number of sessions, duration, documentary and graphic support, etc.) with the only variation being that of attendance.
By way of conclusion, telemedicine is an adequate tool for training in FGM, with results similar to face-to-face consultation three months after starting the use of the device, and has a high degree of satisfaction among users. These online training systems have proven to be especially useful during the SARS-CoV-2 pandemic, facilitating the patient's accessibility to the healthcare team.
Collaboration of authorsMONM and GDS have contributed equally to this manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.