Activating G-protein alpha-subunit (GNAS) mutations are responsible for McCune-Albright syndrome (MAS) and have been identified in several benign conditions, like toxic nodular goiter (TNG),1 gastric heterotopia (GH), adrenocortical, liver and pancreatic lesions, as well as in myelodysplastic syndromes2 and sporadic tumors arising in different organs, including pituitary, thyroid, adrenals, kidney and colorectum.3 They are also able to promote intestinal tumorigenesis. However, recent studies suggest that GNAS activating mutations affecting codon 201 are rare in most human tumors,4 and up to this date there are only 3 reported cases of GNAS activating mutations in ovarian Leydig cell tumors (LCT), all consisting in R201C.5 We here report two unrelated women with virilizing ovarian LCT harboring this mutation, one of whom had been partially described before.6
Case 1A 72-year-old woman consulted at La Paz University Hospital (Madrid) because of a 1-year history of progressively worsening signs of virilization. Extremely high serum testosterone levels were confirmed (12,038 and 30,000pg/ml, NR: 0.2–2pg/ml). After administration of a gonadotropin-releasing hormone agonist (gonadorelin 3.5mg i.m.) serum LH and FSH levels decreased to 1IU/L and testosterone and androstenedione levels by 30% from baseline. Ultrasound revealed a 10-mm tumor in the left ovary. Histopathological study after total hysterectomy and bilateral oophorectomy described a well-encapsulated left 10-mm LCT, with several plump rod-shaped intracytoplasmic Reinke crystals. Tumor cells showed intense and diffuse cytoplasmic immunoreactivity for α-inhibin. Postoperatively, testosterone levels instantly dropped into the normal range, and virilization slowly regressed.6 She was a heterozygous carrier of the hemochromatosis H63D mutation and showed a nodular enlargement of her left adrenal gland and a deletion in CYP21A1P pseudogene. Eight years after surgery the patient remained asymptomatic and cured of her LCT. However, one year later, she presented at the emergency room with abdominal pain and died 2 days later. An advanced occult metastatic gastric cancer was suspected on the basis of the last CT images, but no autopsy was done to confirm this diagnosis.
Case 2A 64-year-old female with past medical history of type 2 diabetes, hypertension, hyperlipidemia, peptic ulcer disease, low back pain, cataracts and tobacco abuse, initially presented to the Endocrinology Department at Cooper University Hospital (New Jersey) for TNG. Upon questioning, she reported two years of progressive facial hirsutism, voice deepening, clitoromegaly, and increased libido. Laboratory testing revealed normal FSH, LH, DHEA-S, IGF-1, and 8 a.m. cortisol. Total testosterone was elevated (443ng/dl and 515ng/dl, 10 times the normal value). The ovaries were not well-identified in the ovarian ultrasound. Subsequent CT of abdomen/pelvis revealed normal-appearing adrenals and ovaries with an incidental 2.9cm right renal lesion. Follow-up MRI showed similar findings. Patient was referred to urology and gynecology and underwent partial right nephrectomy and bilateral oophorectomy to rule out the ovaries as the source of abnormal testosterone production. Pathology demonstrated a right papillary renal cell cancer (PRC) and a benign 1.5cm right ovarian LCT. Only 4 days after the surgery, the patients’ total testosterone had dropped to <1ng/dl. She also had GH at duodenal mucosa, gastric eosinophils and remained well controlled on 5mg methimazole daily for her subclinical hyperthyroidism.
Three five-micrometer-tissue sections (tumoral and peritumoral for patient 1 and tumoral for patient 2) from formalin-fixed, paraffin-embedded tissue blocks were used for mutation analysis. Genomic DNA was isolated from tissue samples using the QIAamp DNA FFPE Tissue Kit (Qiagen, Düren, Germany) according to the manufacturer's instructions. For both patients, mutational analysis was initially undertaken by direct DNA sequencing of PCR-amplified target sequence of the GNAS gene. Patient's 2 DNA was re-analyzed by nested PCR. In both cases the R201C mutation was detected in the tumor tissue (and absent in the peritumoral section and leukocyte DNA in case 1). Results were confirmed by an independent PCR and subsequent enzymatic digest-based technique, modified from Yoshimoto et al.7
LCT account for less than 0.2% of all ovarian tumors, cause hyperandrogenism in about 75% of patients and are typically small unilateral lesions more frequently diagnosed in postmenopausal women.5 Their exact etiology is unknown, but it has been demonstrated that amino-acid substitution at GNAS codon 201 or 227 leads to permanent activation of Gsα subunit and accelerated cAMP production in the absence of stimulatory hormone, mimicking gonadotropin binding to G-protein-coupled LH and FSH receptors. Therefore, GNAS mutations may stimulate both gonadal steroid production and hyperplastic growth of Leydig cells, transforming them into autonomous hormone-producing tumors, as they do with pituitary somatotrophs and thyroid cells.3,4 Additionally, since Gsα can also activate pro-inflammatory pathways, a potential utility as premalignant markers has been proposed for GNAS mutations, as their postzygotic occurrence generates a mosaic distribution of mutant cells resulting in varying degrees of tissue involvement that range from a single site to a widespread distribution.8,9
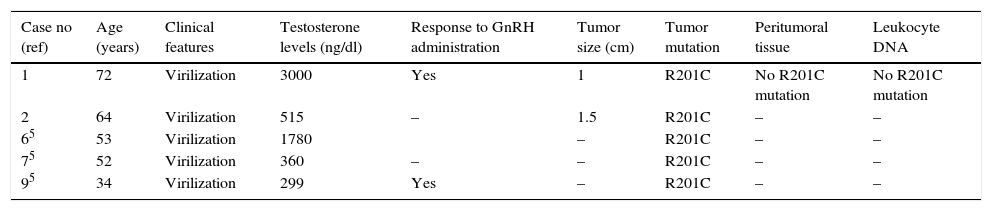
In contrast to the two patients presented here, the three patients reported before.5 with virilizing ovarian LCT and the same activating GNAS R201C mutation were all younger than 53, and only the youngest showed a response to GnRH-analog. The joint study of these five cases also reveals that testosterone levels seem to correlate with age (Table 1). The same mutation was present in the tumoral tissue of a 29-year-old male with bilateral Tanner V gynecomastia, low levels of testosterone (with slightly elevated estradiol levels), azoospermia and a testicular LCT,5 a 68-year-old male patient with a left testicular LCT10 – and absent in normal peritumoral tissue and leukocyte DNA –, and a 23-year-old woman with MAS and a left virilizing ovarian sclerosing-stromal tumor.8 It was also distributed across many tissues (including testicles) of a male patient with MAS.9 Although this mutation has been described in other conditions diagnosed in our patient 2 (PRC, TNG1 and GH) we couldn’t genotype the affected tissues and rule out a low level of GNAS mosaicism.
Clinical, pathological and hormonal data, and G protein gene analysis in women with Leydig cell tumors and GNAS mutations.
| Case no (ref) | Age (years) | Clinical features | Testosterone levels (ng/dl) | Response to GnRH administration | Tumor size (cm) | Tumor mutation | Peritumoral tissue | Leukocyte DNA |
|---|---|---|---|---|---|---|---|---|
| 1 | 72 | Virilization | 3000 | Yes | 1 | R201C | No R201C mutation | No R201C mutation |
| 2 | 64 | Virilization | 515 | – | 1.5 | R201C | – | – |
| 65 | 53 | Virilization | 1780 | – | R201C | – | – | |
| 75 | 52 | Virilization | 360 | – | – | R201C | – | – |
| 95 | 34 | Virilization | 299 | Yes | – | R201C | – | – |
In conclusion, the study of these cases suggests that the R201C GNAS mutation and subsequent cAMP increase may play a significant role in the pathogenesis of virilizing LCT through the stimulation of androgen production and tumor development, retaining in some cases their responsiveness to gonadotropins. Testosterone levels seem to correlate with age. An active search for this mutation in LCT patients might help to unravel why, when and how it triggers tumorigenesis, and improve patients’ long-term outcomes.