To establish whether fasting glucose levels in the first trimester (FGFT) of pregnancy ≥92mg/dl (5.1mmol/l) (FGFT) anticipate the occurrence of maternal-fetal complications of gestational diabetes mellitus. To assess whether FGFT can replace diagnosis of GDM using the classical two-step oral glucose tolerance test (OGTT).
MethodsA retrospective study of 1425 pregnancies with FGFT and O'Sullivan Test (OST) and/or OGTT according to OST results in the second trimester. FGFT sensitivity and specificity were assessed as compared to the conventional diagnosis of GDM. The relationship between maternal-fetal complications and FGFT was assessed in the total group and after excluding mothers who received specific medical treatment for GDM.
ResultsSensitivity and specificity of FGFT levels ≥92mg/dl were 46.4% and 88.8% as compared to diagnosis of GDM using Carpenter and Coustan criteria. In the total group, a statistically significant relationship was found between FGFT levels ≥92mg/dl and newborn weight (3228±86 versus 3123±31g; p<0.05), as well as a higher rate of macrosomia (6.9% versus 3.5%; p<0.05). This association persisted after excluding patients diagnosed with and treated for GDM (weight: 3235±98 versus 3128±31g; p<0.05; percentage of macrosomia: 7.2% versus 3.4%; p<0.05).
ConclusionsFGFT is not a good substitute for conventional diagnosis of GDM in the second trimester. Pregnant women with FGFT levels ≥92mg/dl, even with no subsequent diagnosis of GDM, are a risk group for fetal macrosomia and could benefit from dietary measures and physical exercise.
1) Determinar si una glucemia basal en el primer trimestre (GBPT) del embarazo ≥ 92mg/dl anticipa la aparición de complicaciones materno-fetales de diabetes mellitus gestacional (DMG). 2) Valorar si la GBPT puede sustituir al diagnóstico clásico de DMG mediante sobrecarga oral de glucosa (SOG).
MétodosEstudio retrospectivo de 1.425 embarazos con GBPT y test de O'Sullivan (TOS) en el segundo trimestre más SOG según resultado del TOS. Valoración de la sensibilidad y especificidad de la GBPT respecto al diagnóstico clásico de DMG. Relación de las complicaciones materno-fetales con la GBPT en el grupo total y tras excluir a las madres que realizaron tratamiento médico específico de DMG.
ResultadosLa sensibilidad y la especificidad de la GBPT≥92mg/dl respecto al diagnóstico de DMG en el segundo trimestre, usando los criterios clásicos basados en la SOG de Carpenter y Coustan, fueron respectivamente del 46,4 y el 88,8%. Respecto a las gestantes con GBPT<92mg/dl, las gestantes con GBPT≥92mg/dl tienen mayor peso del recién nacido (3.228±86 versus 3.123±31g; p<0,05) y mayor porcentaje de macrosomía (6,9% versus 3,5%; p<0,05). Esta relación se mantuvo tras excluir a las pacientes diagnosticadas y tratadas por DMG (peso: 3.235±98 versus 3.128±31g; p<0,05; porcentaje de macrosomía: 7,2% versus 3,4%; p<0,05).
Conclusiones1) La GBPT no es un buen sustituto del diagnóstico clásico de DMG en el segundo trimestre. 2) Las gestantes con GBPT≥92mg/dl, aun sin diagnóstico posterior de DMG, constituyen un grupo de riesgo de macrosomía fetal y podrían beneficiarse de la instauración de tratamiento nutricional y ejercicio físico.
Gestational diabetes mellitus (GDM) is diabetes diagnosed in pregnancy and without evidence of prior diabetes.1 When in addition to a pregestational predisposition to diabetes (genetic factors, obesity and other causes of insulin resistance)2 a patient develops contrainsular effects inherent to the physiological adaptation to pregnancy,3 we observe the pathological alterations of carbohydrate metabolism referred to as GDM. In most cases GDM is a mild and self-limiting condition, though even so it significantly increases the risk of obstetric, fetal and perinatal complications that can be avoided with appropriate medical treatment.4–6
In general, the association of the physiological effects of pregnancy with susceptibility to type 2 diabetes does not give rise to an all-or-nothing situation, but rather to a continuous variation in the before and after oral glucose glycemia values. In some cases, a pregestational predisposition mainly gives rise to insulin resistance with a relative increase in fasting blood glucose versus postprandial blood glucose. In other cases, a reduced beta-cell response to glucose overload occurs, with normal fasting and high postprandial glycemia values. In some instances both alterations occur together. These two different types of physiopathological behavior condition the diagnostic efficacy of the criteria based on fasting blood glucose values or glycemia after an oral glucose tolerance test (OGTT). In this regard, the HAPO study7 showed both fasting blood glucose and glycemia after an oral glucose tolerance test to be linearly related to obstetric and neonatal adverse effects, with no clear cut-off point.
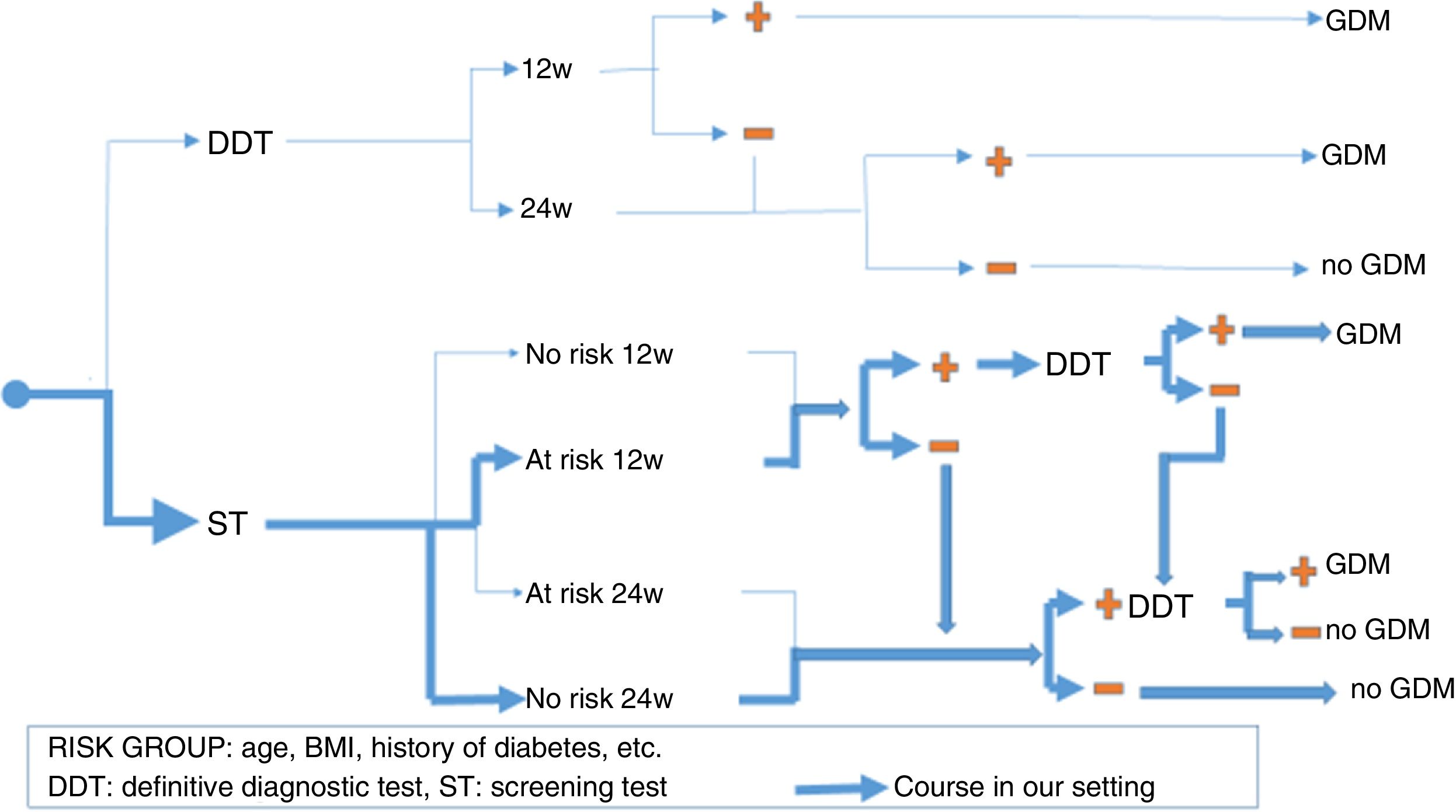
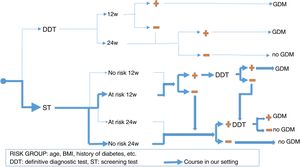
There is no prior gold standard for estimating either the true prevalence of GDM or the sensitivity and specificity of each diagnostic criterion. This is why it is so important to seek consensus criteria when selecting pregnant women who should receive full medical care for GDM, thereby maximizing its benefits without needlessly incrementing the costs. Furthermore, since not all pregnant women are at the same risk of developing GDM, we also have to take the strategic decision as to whether to perform a definitive diagnostic test in all pregnant women (“one-step criterion”), or rather perform screening tests and limit definitive tests to cases with a positive screening result (“two-step criterion”). Even within the context of screening, there are two alternative options: the O'Sullivan test (OST) or fasting blood glucose (FG). In addition, there has been controversy regarding timing, over whether there should be universal screening in the first trimester; screening in the first trimester only if there are risk factors for GDM; or no screening in the first trimester, thus leaving the entire diagnostic process for the second trimester. Fig. 1 shows the different diagnostic strategies for GDM currently used and the strategy employed in our setting.
With regard to the diagnostic criterion, and apart from the classical criteria of Carpenter and Coustan (CC) based on OGTT8 (OGTT 100g and a diagnosis of GDM if values of over 95, 180, 155 and 140mg/dl are obtained after 0, 60, 120 and 180min) and the National Diabetes Data Group (NDDG)9 (OGTT 100g with two or more values above 105, 190, 165 and 145mg/dl after 0, 60, 120 and 180min), the criterion of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) has been added,10 established from the HAPO study (OGTT 75g and a diagnosis of GDM if values of over 92, 180 and 153mg/dl are obtained at one or more points after 0, 60 and 120min). Of course, this latter diagnostic criterion significantly raises the prevalence of GDM (16.1%)7 with respect to the classical CC (11.6%) and NDDG (8.8%) criteria.11,12 However, the most relevant change is that a single high blood glucose value in OGTT suffices to establish the diagnosis according to the IADPSG; accordingly, a fasting blood glucose value of ≥92mg/dl is diagnostic of GDM without the need for OGTT.
In any case, apart from the classical CC and NDDG criteria and the novel approach of the IADPSG, there are many other consensuses referring to the diagnostic orientation of diabetes in pregnancy. In this respect, the Endocrine Society (USA),13 the World Health Organization (WHO)14 and the Australasian Diabetes Society (ADIPS)15 recommend the “one-step criterion”. The American College of Obstetricians and Gynecologists (ACOG)16 in turn advises the “two-step criterion”. The American Diabetes Association (ADA)17 advocates either option. As regards the time of the first screening, the WHO and the Endocrine Society recommend universal screening in the first trimester, while the others only recommend screening in the first trimester if there are risk factors for GDM. Lastly, the National Institute for Health and Care Excellence (NICE)18 does not support the universal screening model recommended by the IADPSG.
In our healthcare setting, laboratory tests are performed as prenatal screening of all pregnant women in the first trimester. The use of fasting blood glucose in such tests for the screening or diagnosis of GDM has clear cost advantages over OGTT. However, there are doubts regarding the suitability of fasting blood glucose in the first trimester (FGFT) as a screening test, and even more so as a definitive diagnostic test.
The present study was designed, on the one hand, to estimate the sensitivity and specificity of FGFT as a screening and/or diagnostic test for GDM and, on the other hand, to assess the incidence of maternal and fetal complications related to diabetes among the pregnant women in our setting who met the criterion FGFT≥92mg/dl, regardless of whether they were finally diagnosed and treated as having GDM or not.
Material and methodsA retrospective study was made of the 1425 women with no previous diagnosis of diabetes mellitus (DM) who underwent prenatal screening in the first trimester of pregnancy (week 10.2±2.6) during a calendar year and who were monitored throughout pregnancy and until delivery by the Obstetrics Department of Hospital Severo Ochoa (Madrid, Spain). Patients with prior DM and the 59 women who underwent prenatal screening but suffered subsequent miscarriage were excluded. Blood samples were drawn into sodium fluoride/potassium oxalate tubes and centrifuged within 30min. In our hospital, the O'Sullivan test (OST) is performed under fasting conditions, with the determination of glycemia at 0 and 60min. Glucose was measured based on an enzymatic method using hexokinase with a Cobas 8000 autoanalyzer® (Roche Diagnostics GmbH).
In addition to the laboratory test data from the Biochemistry Department, information was obtained on the mother, pregnancy, delivery and the newborn infant from the primary care electronic records and obstetric discharge reports.
Biochemical data included FGFT and OST in weeks 24–28. In the OST-positive patients the OGTT 100g results were also compiled. The CC criterion was used for the diagnosis of GDM.
The documented maternal data included age, weight, height, the body mass index (BMI) in the first trimester stratified as normal (<25kg/m2), overweight (25–29.9kg/m2) or obese (≥30kg/m2), and any family history of diabetes. The obstetric data and complications recorded included gestational hypertension, preeclampsia, polyhydramnios, preterm delivery (defined as delivery before week 37), and delivery to term (spontaneous, induced, eutocic, instrumental) or cesarean section. The recorded complications in the newborn infant comprised the following: macrosomia (weight >4000g), hyperbilirubinemia, polyglobulia, hypoglycemia, intrauterine fetal death, malformations, trauma to the newborn resulting from labor, respiratory distress and admission to the Neonatal Intensive Care Unit.
We evaluated the sensitivity and specificity of FGFT≥92mg/dl with respect to positive OST and the diagnosis of GDM (OGTT 100g according to CC criterion). The relationship between FGFT and the obstetric and fetal complications was investigated. The same variables were subsequently related to the glycemia groups <92 and ≥92mg/dl, after the patients diagnosed with GDM in the second trimester were excluded, and who therefore received specific medical treatment.
The chi-squared test was used to compare two categorical variables, while the Student t-test was used for the comparison of means, in this case for checking the normal distribution of the variables and the homogeneity of variance. The Student t-test for paired groups in turn was used to compare mean glycemia in the first trimester with mean glycemia at time 0 of the OST. Binary logistic regression analysis was used to correlate binary dependent variables to different exposure variables. The SPSS version 19 statistical package was used for data analysis, and statistical significance was considered for p<0.05.
The study was approved by the Ethics Committee of our hospital.
ResultsThe mean age of the pregnant women was 32.6±5.0 years, with a mean BMI of 24.9±4.6kg/m2. A total of 263 (18.5%) had a family history of diabetes, 24 (1.7%) had pregestational arterial hypertension, and 211 (14.7%) were smokers. In turn, 606 (42.5%) had had one or more previous pregnancies, and 405 (28.4%) had suffered one or more previous miscarriages. Twenty-four (4.0%) of the 606 patients with previous pregnancies had experienced GDM.
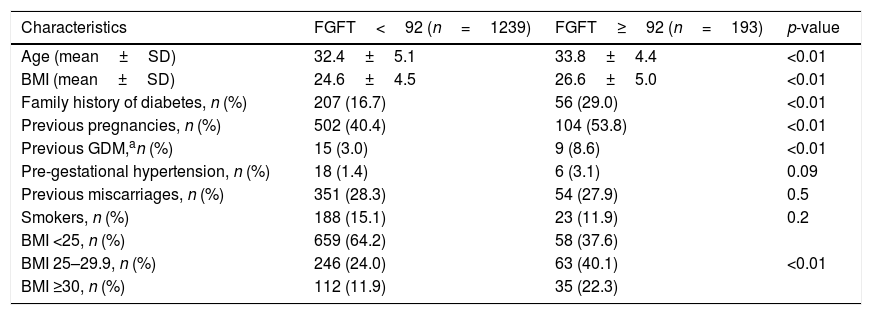
Table 1 shows the patient characteristics according to FGFT above or below 92mg/dl.
Patient characteristics according to fasting glucose in the first trimester (FGFT) ≥92mg/dl and <92mg/dl.
| Characteristics | FGFT<92 (n=1239) | FGFT≥92 (n=193) | p-value |
|---|---|---|---|
| Age (mean±SD) | 32.4±5.1 | 33.8±4.4 | <0.01 |
| BMI (mean±SD) | 24.6±4.5 | 26.6±5.0 | <0.01 |
| Family history of diabetes, n (%) | 207 (16.7) | 56 (29.0) | <0.01 |
| Previous pregnancies, n (%) | 502 (40.4) | 104 (53.8) | <0.01 |
| Previous GDM,an (%) | 15 (3.0) | 9 (8.6) | <0.01 |
| Pre-gestational hypertension, n (%) | 18 (1.4) | 6 (3.1) | 0.09 |
| Previous miscarriages, n (%) | 351 (28.3) | 54 (27.9) | 0.5 |
| Smokers, n (%) | 188 (15.1) | 23 (11.9) | 0.2 |
| BMI <25, n (%) | 659 (64.2) | 58 (37.6) | |
| BMI 25–29.9, n (%) | 246 (24.0) | 63 (40.1) | <0.01 |
| BMI ≥30, n (%) | 112 (11.9) | 35 (22.3) |
The pregnant women with FGFT≥92mg/dl were older (33.8±4.4 versus 32.4±5.1 years; p<0.01), with a greater BMI (26.6±5.0 versus 24.6±4.5kg/m2; p<0.01), and with a more frequent family history of diabetes (29.0% versus 16.7%; p<0.01) compared with the pregnant women with FGFT<92mg/dl. Furthermore, the women with FGFT≥92mg/dl had had comparatively more previous pregnancies (53.8% versus 40.4%; p<0.01) and more prior GDM (8.6% versus 3.0%; p<0.01).
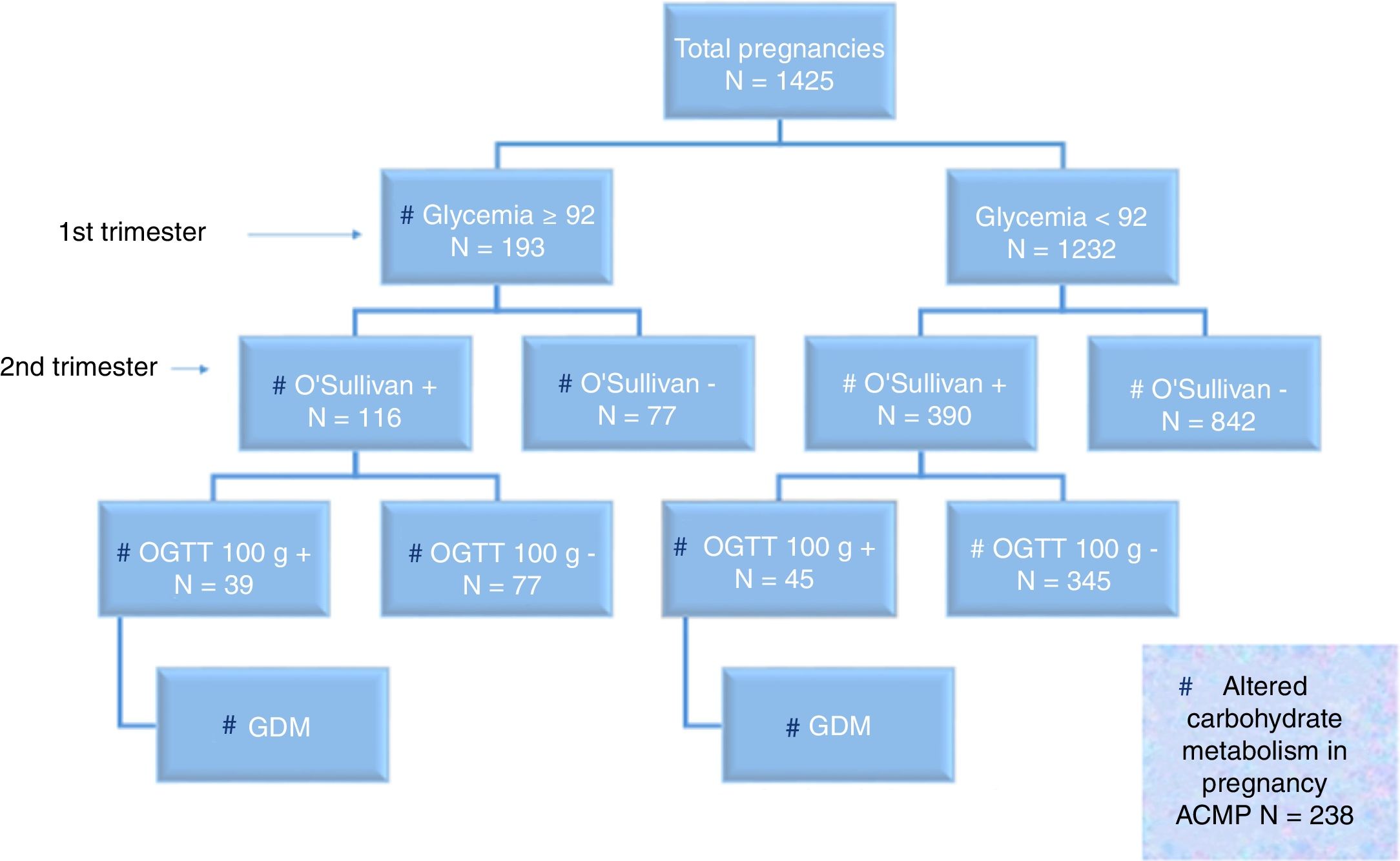
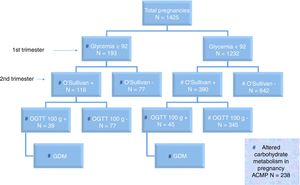
Fig. 2 shows the distribution of the screening and diagnostic test results.
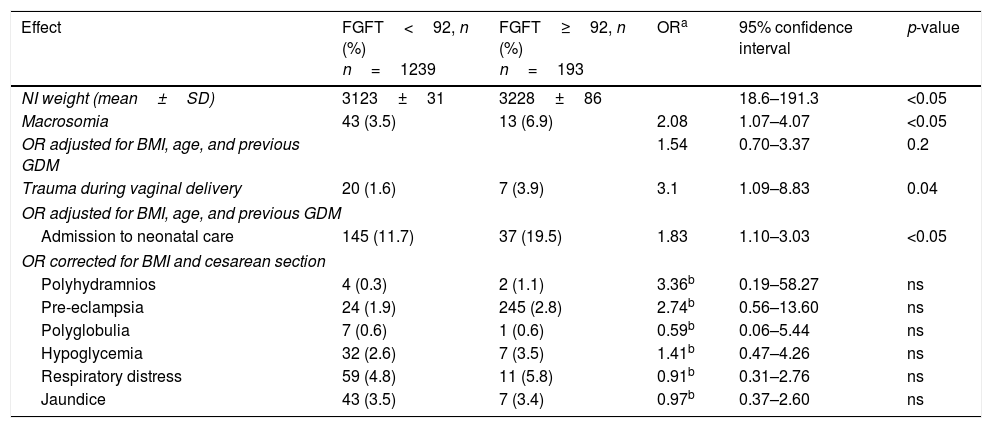
On jointly using the term “altered carbohydrate metabolism in pregnancy” (ACMP) in reference to FGFT≥92mg/dl and/or positive OGTT, we found 238 of the 1425 pregnant women to have ACMP (16.7%). Of these 238 cases with ACMP, 39 had FGFT≥92mg/dl and positive OGTT (16.4%); 45 had FGFT<92mg/dl and positive OGTT (18.9%); and 154 had FGFT≥92mg/dl and negative OST or OGTT (64.7%). Excluding miscarriages (47/1279 [3.7%] in pregnant women with FGFT<92mg/dl and 12/205 [5.9%] in pregnant women with FGFT≥92mg/dl; p=0.3), the obstetric and perinatal adverse effects according to FGFT <92 or ≥92mg/dl are shown in Table 2.
Results referring to obstetric and fetal complications according to fasting glucose in the first trimester (FGFT) ≥92 and <92mg/dl.
| Effect | FGFT<92, n (%) n=1239 | FGFT≥92, n (%) n=193 | ORa | 95% confidence interval | p-value |
|---|---|---|---|---|---|
| NI weight (mean±SD) | 3123±31 | 3228±86 | 18.6–191.3 | <0.05 | |
| Macrosomia | 43 (3.5) | 13 (6.9) | 2.08 | 1.07–4.07 | <0.05 |
| OR adjusted for BMI, age, and previous GDM | 1.54 | 0.70–3.37 | 0.2 | ||
| Trauma during vaginal delivery | 20 (1.6) | 7 (3.9) | 3.1 | 1.09–8.83 | 0.04 |
| OR adjusted for BMI, age, and previous GDM | |||||
| Admission to neonatal care | 145 (11.7) | 37 (19.5) | 1.83 | 1.10–3.03 | <0.05 |
| OR corrected for BMI and cesarean section | |||||
| Polyhydramnios | 4 (0.3) | 2 (1.1) | 3.36b | 0.19–58.27 | ns |
| Pre-eclampsia | 24 (1.9) | 245 (2.8) | 2.74b | 0.56–13.60 | ns |
| Polyglobulia | 7 (0.6) | 1 (0.6) | 0.59b | 0.06–5.44 | ns |
| Hypoglycemia | 32 (2.6) | 7 (3.5) | 1.41b | 0.47–4.26 | ns |
| Respiratory distress | 59 (4.8) | 11 (5.8) | 0.91b | 0.31–2.76 | ns |
| Jaundice | 43 (3.5) | 7 (3.4) | 0.97b | 0.37–2.60 | ns |
SD: standard deviation; GDM: gestational diabetes mellitus; BMI: body mass index; n: number of patients; ns: nonsignificant; OR: odds ratio; NI: newborn infant.
The infants of mothers with FGFT≥92mg/dl had greater weight (3228±86 versus 3123±31g; p=0.02) and a higher percentage of macrosomia (6.9% versus 3.5%; p=0.02). After adjusting the odds ratio (OR) for the BMI, age and previous gestational diabetes, a greater proportion of macrosomia was seen to persist among the infants of mothers with FGFT≥92mg/dl (OR 1.54; 95% confidence interval [95%CI] 0.70–3.37), though the difference was no longer statistically significant. In addition, we recorded no statistically significant differences in neonatal adverse effects in mothers with FGFT≥92mg/dl, except as regards admission to neonatal care (OR 1.83; 95%CI: 1.10–3.04; p<0.05) and trauma in vaginal delivery (OR 3.1; 95%CI 1.09–8.83; p<0.04).
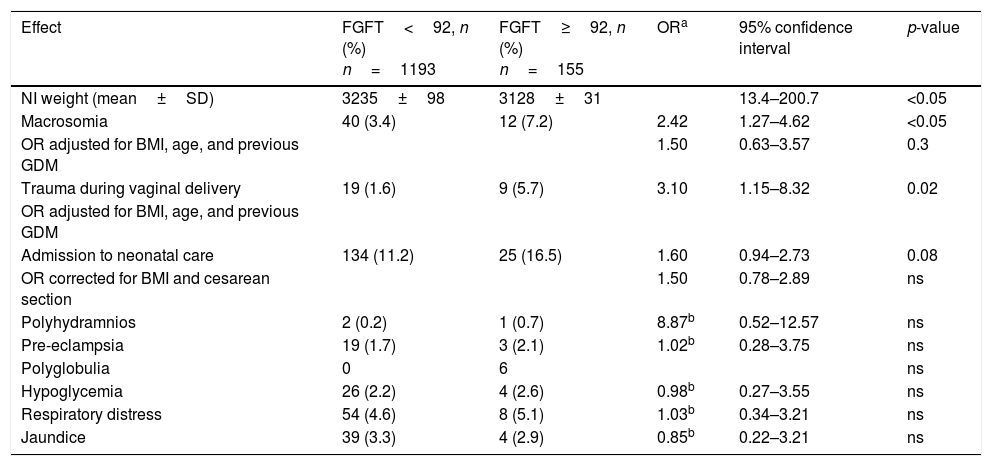
The recorded obstetric and perinatal adverse effects according to FGFT <92 or ≥92mg/dl, excluding the 84 women diagnosed and treated for GDM, are reported in Table 3.
Results referring to obstetric and fetal complications according to fasting glucose in the first trimester (FGFT) ≥92 and <92mg/dlin pregnant women not diagnosed with diabetes.
| Effect | FGFT<92, n (%) n=1193 | FGFT≥92, n (%) n=155 | ORa | 95% confidence interval | p-value |
|---|---|---|---|---|---|
| NI weight (mean±SD) | 3235±98 | 3128±31 | 13.4–200.7 | <0.05 | |
| Macrosomia | 40 (3.4) | 12 (7.2) | 2.42 | 1.27–4.62 | <0.05 |
| OR adjusted for BMI, age, and previous GDM | 1.50 | 0.63–3.57 | 0.3 | ||
| Trauma during vaginal delivery | 19 (1.6) | 9 (5.7) | 3.10 | 1.15–8.32 | 0.02 |
| OR adjusted for BMI, age, and previous GDM | |||||
| Admission to neonatal care | 134 (11.2) | 25 (16.5) | 1.60 | 0.94–2.73 | 0.08 |
| OR corrected for BMI and cesarean section | 1.50 | 0.78–2.89 | ns | ||
| Polyhydramnios | 2 (0.2) | 1 (0.7) | 8.87b | 0.52–12.57 | ns |
| Pre-eclampsia | 19 (1.7) | 3 (2.1) | 1.02b | 0.28–3.75 | ns |
| Polyglobulia | 0 | 6 | ns | ||
| Hypoglycemia | 26 (2.2) | 4 (2.6) | 0.98b | 0.27–3.55 | ns |
| Respiratory distress | 54 (4.6) | 8 (5.1) | 1.03b | 0.34–3.21 | ns |
| Jaundice | 39 (3.3) | 4 (2.9) | 0.85b | 0.22–3.21 | ns |
SD: standard deviation; GDM: gestational diabetes mellitus; BMI: body mass index; n: number of patients; ns: nonsignificant; OR: odds ratio; NI: newborn infant.
On excluding the patients treated for GDM, the infants of mothers with FGFT≥92mg/dl continued to register greater weight (3235±98 versus 3128±31g; p<0.05), a higher percentage of macrosomia (7.2% versus 3.4%; p<0.05) and a greater proportion of trauma at delivery (OR 3.10; 95%CI 1.15–8.32; p=0.02). After adjusting OR for the BMI, age and prior gestational diabetes, a greater proportion of macrosomia was seen to persist in the infants of mothers with FGFT≥92mg/dl (OR 1.50; 95%CI 0.63–3.57), though the difference was no longer statistically significant. Moreover, the differences found in percentage macrosomia, admission to neonatal care and polyhydramnios likewise failed to reach statistical significance. The remaining adverse effects were similar between the groups. In this case, statistical significance was also not modified by age or a history of prior gestational diabetes.
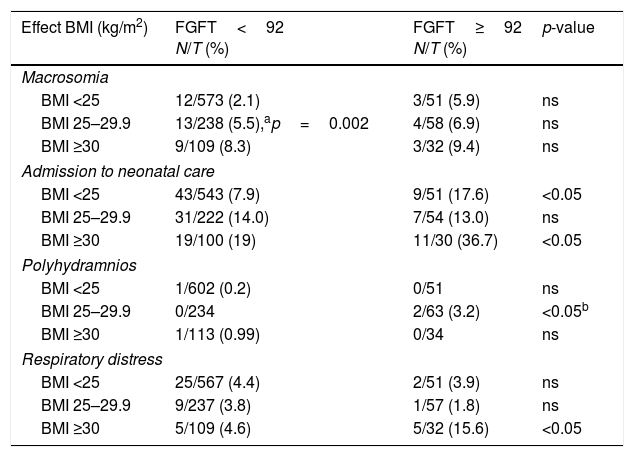
In order to determine whether the effects seen with FGFT≥92mg/dl were due only to the overweight and obesity found to be more prevalent in this group of pregnant women, we studied the distribution of the most significant neonatal complications according to FGFT and the maternal BMI groups (Table 4).
Effects of pregnancy in women with glycemia ≥92 and <92mg/dl in the first trimester (FGFT) according to the BMI.
| Effect BMI (kg/m2) | FGFT<92 N/T (%) | FGFT≥92 N/T (%) | p-value |
|---|---|---|---|
| Macrosomia | |||
| BMI <25 | 12/573 (2.1) | 3/51 (5.9) | ns |
| BMI 25–29.9 | 13/238 (5.5),ap=0.002 | 4/58 (6.9) | ns |
| BMI ≥30 | 9/109 (8.3) | 3/32 (9.4) | ns |
| Admission to neonatal care | |||
| BMI <25 | 43/543 (7.9) | 9/51 (17.6) | <0.05 |
| BMI 25–29.9 | 31/222 (14.0) | 7/54 (13.0) | ns |
| BMI ≥30 | 19/100 (19) | 11/30 (36.7) | <0.05 |
| Polyhydramnios | |||
| BMI <25 | 1/602 (0.2) | 0/51 | ns |
| BMI 25–29.9 | 0/234 | 2/63 (3.2) | <0.05b |
| BMI ≥30 | 1/113 (0.99) | 0/34 | ns |
| Respiratory distress | |||
| BMI <25 | 25/567 (4.4) | 2/51 (3.9) | ns |
| BMI 25–29.9 | 9/237 (3.8) | 1/57 (1.8) | ns |
| BMI ≥30 | 5/109 (4.6) | 5/32 (15.6) | <0.05 |
BMI: weight/height2; N: number of events; T: total number.
The infants of pregnant women with FGFT≥92mg/dl had a higher prevalence of macrosomia in all the BMI groups. Furthermore, the group with FGFT<92mg/dl showed a higher proportion of macrosomia with an increasing BMI (p<0.05). We also recorded a high proportion of respiratory distress in obese mothers only in the case of FGFT≥92mg/dl. In relation to the remaining complications, the proportions tended to be higher with an increasing maternal BMI, though statistical significance was not reached.
Fasting blood glucose in the first trimester (≥92mg/dl) exhibited a sensitivity of 22.9% and a specificity of 91.6% with respect to positive OST in the second trimester, and a sensitivity of 46.4% and a specificity of 88.8% with respect to the diagnosis of GDM based on the CC criterion. The prevalence of GDM with the CC criterion in pregnancies in our setting was 5.9% (2.4% with the NDDG criterion and 14.7% with the IADPSG criterion).
The mean FGFT was 84.4±0.2mg/dl, with a mean fasting glucose level at time 0 of the OST of 81.53±0.2mg/dl (confidence interval for the difference of means: 2.5–3.3; p<0.001).
DiscussionIn our study, the prevalence of GDM was found to be 5.9% with the CC criteria and 14.7% with the IADPSG criteria. In 2006, the Spanish Diabetes and Pregnancy Group (Grupo Español de Diabetes y Embarazo [GEDE]) estimated the prevalence of GDM according to the CC criteria to be 12%,12,19 versus 16.1% based on the IADPSG criteria.7,12 Other Spanish national publications have reported prevalence rates of between 1 and 12%.19 As can be seen, even when using the same criteria, the prevalence of GDM differs significantly between studies, and this cannot be attributed solely to the different characteristics of the populations or the diagnostic strategies used. For example, in the study published by Duran et al.,20 likewise conducted in the Community of Madrid, with a population of pregnant women presenting a mean age and a BMI similar to those of our own series, the prevalence of GDM with the CC criteria was 10.6% versus 35.5% with the IADPSG criteria. Altered carbohydrate metabolism in pregnancy constitutes a continuum on which the cut-off points for diagnosing GDM are decided. Therefore, with the same cut off points, a minor variability in the serum glucose measurements between different laboratories can have a very significant impact upon the proportion of pregnant women diagnosed with GDM.
Assuming these underlying limitations, the first objective of our study was to determine whether a simple measurement such as FGFT is able to replace the currently used screening (OST) and diagnostic tests (OGTT 100g) referring to GDM. In this regard, the poor sensitivity of FGFT in relation to OST (22.9%) and OGTT (46.4%) shows that FGFT cannot be used as a substitute for oral glucose testing, because doing so would leave a considerable number of cases of GDM undiagnosed. In addition, the comparative analysis of the tests revealed a significant difference between the fasting glucose values of the first (84.4±0.2mg/dl) and second trimesters (81.53±0.2mg/dl). This difference should possibly be taken into account when one is considering the limiting values of the different diagnostic tests according to the trimester of pregnancy.
In fact, the only partial overlap of the groups selected on pooling the women with FGFT≥92mg/dl or the women with positive OST and OGTT reflects the difference in information obtained with the two types of tests. While FGFT detects more pregnant women with insulin resistance, OGTT is mainly aimed at detecting pregnant women with postprandial carbohydrate intolerance. In our study, among the pregnant women with altered carbohydrate metabolism in pregnancy (ACMP), this partial overlap gives rise to three different groups. Firstly, we have the group with high FGFT and negative OST or OGTT 100g (64.7%), corresponding to women with insulin resistance. Secondly, we have the group with normal FGFT and positive OGTT (18.9%), corresponding to women with postprandial carbohydrate intolerance. Thirdly, we have the group with high FGFT and positive OGTT (16.4%), which pools the women with both carbohydrate metabolic alterations.
Fasting glucose in the first trimester is therefore not useful as a replacement for the classical screening and diagnostic tests for GDM. Could FGFT nevertheless play a role in the medical approach to pregnancy? Our study has shown that pregnant women with high FGFT exhibit a statistically significant increase in fetal weight and macrosomia. Moreover, even accepting that the small number of complications limits the statistical significance of the findings, many studies of diabetes in pregnancy appear to reflect a relationship between high FGFT and other maternal-fetal complications attributable to GDM.21–25 Most importantly, these relationships are seen to persist when pregnant women diagnosed with GDM are excluded. In other words, women with ACMP but meeting no full criteria of GDM (the first and the most numerous group) have a carbohydrate metabolism that is sufficiently altered to cause an increased risk of complications of GDM.
It seems clear that most fetal complications attributable to GDM are a result of fetal overfeeding, particularly on taking into account that evolution-based physiology is intended to cope with nutritional shortage rather than excess. Such overfeeding is mainly attributable to the maternal hyperglycemia caused by diabetes, though it is also due in part to maternal obesity and overfeeding in the course of pregnancy. In our study, the infants of women with FGFT≥92mg/dl had a higher proportion of macrosomia in all BMI categories and, in turn, there was a higher proportion of macrosomia according to the BMI in both the FGFT<92mg/dl group and the FGFT≥92mg/dl group. Although these differences were not statistically significant in all comparisons (possibly because of the small number of events observed on stratifying according to the BMI), the observed trend appears to indicate that both high FGFT and overweight and obesity are associated factors in the generation of macrosomia.
The management of GDM, aimed at controlling these fetal overfeeding mechanisms, comprises two conceptually related but strategically separable components. The first component – formal diet, physical exercise and contained weight gain – comes at little cost to the healthcare system.26 However, the second component – monitoring of the condition through glycemia self-control, medical supervision, and eventual drug treatment with insulin or other drugs – can result in a very significant healthcare burden, depending on the number of pregnant women finally diagnosed with GDM and referred to the endocrinology and nutrition departments.
If FGFT>92mg/dl is used to screen for ACMP, we can ensure the early identification of women not only presenting an increased risk of GDM in the second trimester, but most importantly with already existing metabolic disorders that can potentially give rise to macrosomia and neonatal complications. Almost certainly, this group of women would benefit from the aforementioned first component of management for GDM (healthy eating habits and regular physical exercise), and which could be prescribed in the obstetric care setting from the first trimester and throughout the duration of pregnancy, with referral to the endocrinology clinic being reserved for those patients subsequently diagnosed with GDM on the basis of positive OST and OGTT results.
Furthermore, it seems logical to assume that an increase in FGFT is only the continuation of an increase in pregestational fasting glucose, as an expression of insulin resistance present before pregnancy. Accordingly, as in the first trimester, it would make sense to prescribe dietary measures and physical exercise to all women with fasting glucose ≥92mg/dl and a wish to become pregnant (somewhat analogous to the pregestational administration of folic acid). In fact, in a recent study, women with GDM diagnosed before week 12 of pregnancy27 presented the same adverse effects as patients with DM before pregnancy.
Lastly, in relation to women with FGFT<92mg/dl and normal OST results, and bearing in mind that the complications attributable to GDM also occur (albeit less frequently) in women with normal glucose tolerance as a result of overfeeding in the gestational period, would it not be reasonable to make universal diet and exercise recommendations for all pregnant women?
The present study has two main limitations: its retrospective design and the fact that although the number of cases allowed us to demonstrate the relationship between FGFT and the weight of the newborn infant, as well as intuit a correlation between FGFT and the maternal-fetal complications of GDM, the sample size was too small to clearly confirm the existence of such an association. In this respect, a larger sample would be needed to increase the statistical power of the trial. Furthermore, it would be advisable to conduct an interventional study in a cohort of women with FGFT>92mg/dl in order to determine whether a protocolized formal diet and physical exercise program is able to influence newborn infant weight and possible maternal-fetal complications.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: López del Val T, Alcázar Lázaro V, García Lacalle C, Torres Moreno B, Castillo Carbajal G, Alameda Fernandez B. Glucemia basal en el primer trimestre como acercamiento inicial al diagnóstico de la diabetes en el embarazo. Endocrinol Diabetes Nutr. 2019;66:11–18.