Although discouraged in previous Kidney Disease Outcomes Quality Initiative (K-DOQI) and European best practice guidelines (EPBG), central venous catheters as access for chronic hemodialysis are being increasingly used in hemodialysis units. Tunnelled, cuffed, double-lumen catheters are currently preferred for dialysis patients. The advantage of central venous catheters is that these devices can be quickly and easily inserted and provide immediate access for hemodialysis The most common complications are delayed complications, consisting of catheter dysfunction secondary to thrombotic events and catheter migration, catheter-related bacteraemia (CRB) and central venous stenosis. Intrinsic catheter-related thrombosis is the principal complication associated with hemodialysis catheters and is the main cause of catheter loss. Intraluminal lytic enzyme therapy must be tried. If this therapy is unsuccessful in restoring blood flow, the catheter must be changed after previously obliterating the fibrin sheath. Central venous stenosis is usually asymptomatic. In symptomatic lesions, the treatment of choice is percutaneous angioplasty. There are few data on the benefits of stents in hemodialysis.
CRB is a complication that carries high morbidity and mortality. Various preventive measures have been advocated to diminish the infection rate. The adoption of a strict aseptic protocol alone significantly reduces the incidence of CRB. Early treatment is the most effective measure to prevent the fatal outcome that sometimes occurs.
El catéter como acceso para hemodiálisis, aunque no está recomendado por las guías K-DOQI y EPBG, cada vez tiene una mayor prevalencia en las salas de hemodiálisis. En la actualidad los catéteres tunelizados, con cuff de doble luz son de elección en la población en diálisis. La ventaja fundamental es la posibilidad de ser insertados con facilidad y permitir un acceso de uso inmediato. Las complicaciones más frecuentes son las tardías y son la disfunción del catéter secundaria a procesos trombóticos o migración del catéter, la estenosis venosa central y la bacteriemia relacionada con el catéter (CRB). La trombosis intrínseca representa la principal complicación y la causa fundamental de pérdida del catéter. Debe intentarse tratamiento intraluminal con enzimas líticas y si son incapaces de restaurar el flujo se debe cambiar el catéter previa destrucción de la vaina de fibrina. La estenosis venosa central normalmente es asintomática. En las lesiones sintomáticas la angioplastia percutánea es el tratamiento de elección. Hay pocos datos respecto al beneficio del stent en hemodiálisis.
La CRB es una complicación de gran morbimortalidad. Se han desarrollado distintas medidas preventivas para disminuir la tasa de infección. La adopción de un protocolo de asepsia estricto reduce significativamente la incidencia de CRB. El tratamiento precoz es la herramienta más efectiva para prevenir el desenlace fatal que a veces ocurre.
The advantage of central venous catheter is their ability to be inserted quickly, easily and that allow immediate access for haemodialysis. The disadvantages, however, are significantly more frequent complications with great morbid-mortality rate. Immediate complications (bleeding, arterial puncture, arrhythmia, air embolism, pneumothorax…) are generally derived from insertion of catheter and diagnosis could be immediately made1. An earlier dysfunction of the catheter is secondary to tip malposition, a wrong vessel catheterization and a new catheter placed without previous disruption of a fibrin sheath2.
The most common complications are delayed complications and they are catheter dysfunction (impossibility to achieve a flow of 300ml/h) secondary to thrombotic events and catheter migration, catheter related bacteraemia3 and central venous stenoses.
Intravenous catheter causes endothelial trauma and inflammation, which can lead to venous thrombosis. Risk factors for thrombotic events are those related with the uremic medium that favour prothrombotic states but the most important ones are catheter related factors such as size of the catheter, catheter malposition (catheter tip must be in atrium), and catheter infection.4
It is unclear whether there is a reliable effective means for prevention thrombosis; good controlled studies have not been reported. Some studies advocated from benefits with anticoagulation at therapeutics levels but others report benefit with the maintenance of target INR in the range of 1.5–2. Systematic anticoagulation could be a good option in patients at high risk for thrombosis.5 Heparin coated catheter were associated with a decrease risk of thrombosis, however studies are made with catheter placed for a short time (less than 3 weeks). Evidence suggest that locking with 4% citrate or heparin may be both effective in reducing thrombosis.6
The extrinsic catheter related thrombosis (CRT) may be venous, mural or intraatrial trombo.
Vein thrombosis generally is asymptomatic and it is suspected by the presence of dilated chest vein and dysfunction. When patient is symptomatic uni or bilateral edema usually appears if cava vein is compromised. Signs and symptoms of embolitation are unusual. Dupplex ultrasonography and angio-TAC have emerged as the study of choice. Catheter should be removed if possible and it is recommended that the patient receive anticoagulation for a minimum period of three months. If there is no contraindication to their use thrombolytic therapy may be considered for patients with extensive thrombosis; however there is a lack of experience in hemodialysis.
Movement of the catheter tip may cause damage to the wall of the vessel or the atrium resulting in mural thrombous formation. The tip of the catheter generally becomes embedded in this thrombus formation causing catheter dysfunction.
The diagnosis is usually made with venography performed through the catheter or transesophagical echocardiography. Large mural thrombi may be symptomatic and can result in pulmonary embolitation. Removal of the catheter is indicated and large thrombi should be anticoagulated.
Intraatrial thrombus of less than 2cm is not considered to be of clinical importance, it is necessary to pull the catheter and systemic anticoagulation even for a few weeks prior to catheter removal. If thrombus is greater than 2cm and symptom and signs occur (pulmonary emboli, endocarditis or syncope) catheter removal combined with simultaneous surgical thrombectomy should be considered. If the only abnormality is catheter dysfunction and the patient is asymptomatic catheter removal with prolonged anticoagulation is a reasonable approach.7
Intrinsic CRT represent the principal complication associated with hemodialysis catheter and is the major cause of catheter loss. To prevent intraluminal and catheter tip thrombosis preventive measures must be taken including flushing both lumen of the catheter with saline to completely clear them of all blood and filling with an anticoagulant solution for the whole volume of both lumens.
Fibrin sheaths thrombous is the most common cause of catheter dysfunction.
Treatment of intrinsic CRT consists in forcefull saline flush into the catheter. If this is not effective intraluminal lytic enzyme must be tried and if this is still unable to restore blood flow or the duration of the effectiveness is less than two weeks catheter should be changed. The change over a guide ware is effective but it is important to obliterate a fibrin sheath before. The fibrin sheath can be stripped using a snare catheter.8
Central vein stenoses is usually asymptomatic, when symptomatic the most usual symptom is ipsilateral arm edema. Indirect evidence of the presence of central vein stenoses are dilated venous collaterals and can be diagnosed with Doppler ultrasound. Asymptomatic lesions, even if greater than 50% stenosis, do not require treatment, and is better managed by simple observation. Percutaneous angioplasty is the treatment of choice. In most patients, long-term patency can be maintained with repeated angioplasty. However, for symptomatic lesions that respond poorly to balloon angioplasty, stent deployment has been used. The 2006 KDOQI guidelines recommend that stent placement should be considered if acute elastic recoil of the vein (>50%) after angioplasty or recurrent stenosis within a three month period post-angioplasty. There are little data to demonstrate the benefit of stents for central vein stenoses in hemodialysis patients. Surgical therapy may be warranted in instances in which endovascular therapy is unsuccessful.9
Catheter related bacteraemia (CRB)It is a complication with a high morbid-mortality. The frequency of CRB in several large case series has ranged between 0.4 and 4.5 episodes per 1000 catheter-days in tunnelled catheters.10 The frequency is relatively less in tunnelled versus non-tunnelled catheters.
CRB should be suspected in any dialysis patient with a hemodialysis (HD) catheter and signs and/or symptom of a bloodstream infection, particularly when there is no clinical evidence for an alternate source of infection. Bacteraemia can be present even without any signs and symptoms. Although non-specific fever or chills are the more sensitive clinical manifestation on CRB. The great majority of cases occur in absence of evidence of an exit site infection. Less common clinical manifestation may include hemodinamic instability, catheter dysfunction and signs of sepsis. Metastasic infection such as osteomyelitis, endocarditis, septic arthritis or epidural abscess may occur weeks or even months after the initial bacteraemia event. The definitive diagnosis of CRB requires one of the following:
- -
Simultaneous quantitative cultures of blood samples from CVC and peripheral vein with a ratio of >5:1(CVC versus peripheral), or differential time to positivity (a positivity result of culture from a CVC is obtained at least 2h earlier than is a positive result of culture from peripheral blood).
- -
Culture of the same organism from the catheter tip and at least one percutaneus blood culture (semiquantitative >15cfu per catheter segment) or quantitative (>100cfu per catheter segment).11
Since many of the fever episodes requiring blood culture sampling occur during dialysis with high blood flows through the catheter it is likely that blood cultures collected at that moment will offer similar result as peripheral blood and also among many dialysis patient a peripheral blood sample cannot be obtained then peripheral samples can be omitted. In these cases a quantitative culture of blood from the CVC that yields at least 100ufc/ml of a credible pathogen in a patient with signs and symptoms of bacteraemia but without evidence of an alternate source of infection may be diagnostic without a companion culture of peripheral blood samples (Fig. 1).
The K/DOQI guidelines suggest that the incidence of tunnelled dialysis CRI should be less than 10 and 50% at 3 and 12 months, respectively.
Different preventive measures have been advocated to diminish the rate of infections. Whether all these strategies would have a similar preventive impact in unit with a low baseline incidence of CRB (and presumably stricter hygienic measures) remain to be demonstrated. The adoption of a strict aseptic protocol alone significantly reduced the incidence of CRB. Education based program consisting in education of the personnel in the care of intravascular access emphasizing general measures with strict observation of the basic rules of hygiene is recommended as first-line target.
Perioperative systemic antimicrobial administration has not been found to be beneficial.
The elimination of S. aureus carriage through the use of intranasal muporicin is associated with a decrease of S. aureus bacteraemia rates in haemodialysis patients.
Some trials demonstrated the benefit of antiseptic or antimicrobial impregnated catheters with significant reductions in both catheter colonization and bacteraemia.12 However these catheters have potential risk of anaphylactoide reactions and the emergence of resistant organisms; moreover, there is a lack of available studies assessing CRB rate in central venous catheter inserted for longer than 12 days.
Antimicrobial locks are associated with decreased rates of CRB. The studies were of relatively short duration with most of them lasting <1 year and concerns appear about emergence of antimicrobial resistance, systemic toxicity and superinfection with yeast. This method could be reserved to patients at high risk of infection (such as carriers of femoral catheters or with history of recurrent CRB).13
Chlorexidine based solutions appear to be superior to povidonaíodine.14 None of the current guidelines relating to the use of intravascular devices provide consistent guidelines with regard to the use of antimicrobial interventions apart from the use of chlorexidine skin cleansing. A good approach to the care of exit site may be cleansing with saline and application of chlorexidine and or otic ciprofloxacino. Only in haemodialysis patients with central venous catheter the use of topical antimicrobial ointments significantly reduced CRB. Muporicin must be avoided because the development of resistances has been documented after prolonged use and adversely affects the integrity of polyurethane catheters.15
There is no preference between transparent vs gauze dressings for CVC.16
The clot prevention strategies are effective reducing the rate of CRB.17 A first study has evaluated LMWH for hemodialysis catheter lock (tinazaparin) and suggests that could be a potential alternative18 but more studies are needed to better define the role of LMWH for hemodialysis catheter lock. Citrato is also used as anticoagulant with extra antimicrobial or biofilm removing properties, in contrast to heparin which even tend to antagonize the bactericidal properties of certain antibiotics and promotes biofilm formation.19 Progressively lower concentration of citrate has been applied (from 46.7% to 4%) with still significantly better result, even in the later case than those obtained with heparin locks. Lower concentration diminishes the potential risk of arrytmias.20
Future technological advances in catheter design will likely reduce catheter related complications including infections, thrombosis, malfunction and associated vascular accesss.21
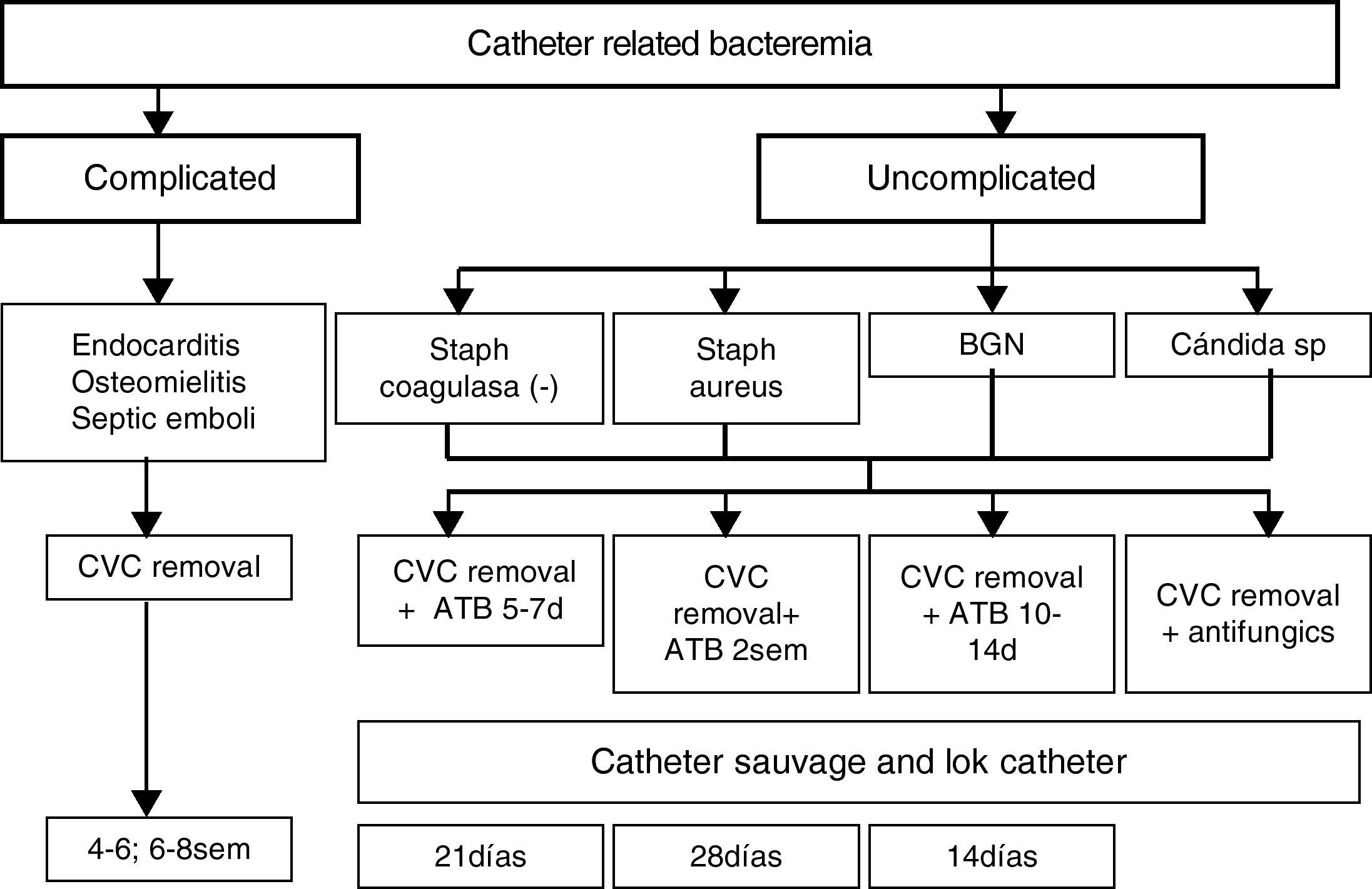
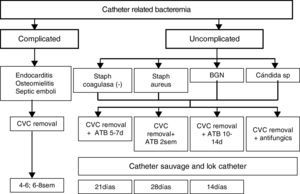
Treatment of infectionExit site infection: If it is a non-tunnelled catheter should be removed. In case of tunnelled catheter uncomplicated exit site infections may be managed with topical antibiotic agents. If it is not resolve and/or is accompanied by purulent drainage should prompt administration of systemic antibiotics for <7 days. The failure to respond may necessitate exchange with the creation of a new exit site or removal if that is feasible.12
Tunnel infection: a no tunnelled catheter should be removed and it is also the best option with tunnelled catheter, but it could be salvaged if there are no bacteraemia and vascular access is lacking. Systemic antibiotic should be administered during 2 weeks in those cases and catheter should be removed if there is no response to antibiotics or Pseudomona, Candida or S. aureus is confirmed.
Catheter related bacteraemiaIndications for catheter removal:
- -
Persistent bacteraemia after 72h of antimicrobial therapy to with the organism is susceptible.
- -
Metastasic infection.
- -
Hemodynamic instability.
- -
CRB due to S. aureus, P. aeruginosa, fungi.
- -
Previous guidewire exchange and significative colonization.
- -
Recurrence of infection once antibiotic therapy is finished.
A temporary non-tunnelled catheter is typically placed and once blood cultures with negative results are obtained a tunnelled catheter can be inserted.
Guidewire exchange: Delayed exchange over a guidewire with a new catheter in afebrile patients after 48h of antibiotics therapy and no evidence of tunnel tract involvement may be effective and a good solution to cases where catheritation at the novo site cannot be achieved. It is not necessary to routinely confirm negative culture results before catheter exchange as long as the patient is asymptomatic. Retrospective studies suggest a cure rate similar to that observed with catheter removal and delayed replacement.22
In cases of lack of vascular access, no existence of tunnel or metastasic infection, hemodynamic stability and microorganism easy to errradicate savage of the catheter may be attempted but systemic antibiotics must be associated to antibiotic lock.
Both in the cases of guidewire assisted replacement and catheter savage close follow-up by assessment of clinical status and repetitive blood cultures after 1 week completion treatment is imperative and if persistent clinical signs of infection and bacteraemia are found after 48–72h the catheter should be removed.
Blood cultures should be drawn after treatment is finished to demonstrate clearance of bacteraemia. Repeatedly positive blood cultures and/or persistent symptoms 72h after catheter removal with appropriate antibiotic therapy should prompt evaluate for metastasic infection such as endocarditis, osteomyelitis, and epidural abscess.
Empiric therapy should quickly administered. Gram positive microorganisms are responsible for most of dialysis catheter infections. Coagulasa negative Staphilococo and S. aureus together account for 40–80% of cases in most studies. Additional coverage for Gram negative should be made. Broad spectrum coverage should consist of vancomycin plus either gentamicin or 3a or 4a generation of cephalosporine. However, nowadays the number of organism with resistance or tolerance to vancomycin is increasing and even with a low minimal inhibitory concentration (<1.5) there is a 20% S. areus that are no responsive to vancomycin. When a meticilin sensible S. aureus (MSSA) is confirmed therapy should be changed to cloxaciline. Treatment failure defined as death or recurrence infection is more common with vancomycin in MMSA CRB.23 If intolerance or allergy to daptomicin is a good option, as well as in case of allergy to vancomycin and MRSA, MIC equal or superior to 1.5, persistent positive cultures after 72h of treatment. Antibiotic lock therapy should be used as an adjunctive therapy together with systemic antibiotic therapy whenever savage of the catheter has been attempted. The premise is to achieve sufficient therapeutic concentrations to kill microbes growing in a biofilm. Antibiotic heparin combination that remain stable without precipitation includes vancomycin, cefazoline and ceftazidime. Gentamicin must be combined with citrate.
Conflicts of interestThe authors declare no conflicts of interest.