Ultrasound has become a diagnostic and therapeutic tool for critical situations. This article reviews the development of ultrasound with respect to critical events and its impact on reducing morbidity and mortality from abdominal and chest trauma, on the recognition of reversible causes of pulseless electrical activity, on decision-making in acute respiratory failure, and on predicting survival and reducing complications associated with invasive procedures. We revised how ultrasounds performed by non-experts with a minimum of training and focused on recognizing specific situations have a good degree of correlation with expert conducted ultrasounds. Some protocols of ultrasound in resuscitation described in the literature are reviewed and a description is made of the most relevant variables in critical situations, including left ventricular function, volume responsiveness, cardiac tamponade, right ventricular dilatation, and pulmonary evaluation.
El ultrasonido se ha convertido en una herramienta diagnóstica y terapéutica en situaciones críticas. Este artículo revisa la evolución del ultrasonido en eventos críticos y su impacto a través de la disminución de morbimortalidad en trauma abdominal y torácico, en el reconocimiento de causas reversibles en actividad eléctrica sin pulso, en la toma de decisiones en falla ventilatoria aguda, en predicción de supervivencia y en la disminución de complicaciones en procedimientos invasivos. Se revisa cómo el ultrasonido realizado por no expertos con mínimo entrenamiento enfocado al reconocimiento de situaciones específicas tiene una adecuada correlación con el experto. Se revisan algunos protocolos de ultrasonido en reanimación descritos en la literatura y se hace una descripción de las variables más relevantes en situaciones críticas como la función ventricular izquierda, respuesta a volumen, taponamiento cardíaco, dilatación del ventrículo derecho y la evaluación pulmonar.
Ultrasound technology has become one of the most useful diagnostic and therapeutic tools of our time. Since leaving the exclusive domain of radiologists and being used by Emergency and Intensive Care Unit (ICU) departments, the ultrasound has arrived in the operating room and is now a tool for perioperative care, regional anesthetics, and vascular access.
In the 1970s, the use of echocardiography in the ICU was limited to evaluating systolic volume and cardiac output.1 In the 1980s and 1990s, it quickly developed to aid in the identification of acute events like cardiac tamponade,2 complications from myocardial infarction,3 hemodynamic assessments in cases of hypotension,4 sepsis,5 and the detection of ruptured aortic aneurisms.6
In trauma, the use of the ultrasound began in 1980 in Europe and Japan.7 In 1992, it was used in the USA to detect hemoperitoneum in cases of closed abdominal trauma.8 Rozycki and collaborators demonstrated the effectiveness of ultrasonography in detecting pericardial effusion and intraperitoneal fluid with 81% sensitivity and 99% specificity. They described it with the acronym “FAST” (Focused Abdominal Sonography for Trauma) for evaluating abdominal trauma.9 In 1997, through international consensus, the ‘A’ was changed from “Abdominal” to “Assessment” and it was included in ATLS.10 In addition, there are a great quantity of studies on the utility of ultrasonography in other scenarios such as pneumothorax,11 hemothorax,12 and vascular accesses.13–15
Thanks to these descriptions, many algorithms for the use of ultrasounds in resuscitation have been published (FATE, CAUSE, RUSH), and, in 2004, the American College of Emergency Physicians (ACEP) considered that bedside ultrasonography should be integrated into routine practice. In 2010, the American Heart Association guides for advanced life support recommend echocardiography for diagnosing treatable causes of cardiac arrest where defibrillation is impossible and to orient treatment.16
What has been the impact of ultrasonography in resuscitation?Its impact can be seen in the reduction of morbidity and mortality from trauma, the recognition of potentially reversible causes of non-shockable cardiac arrest and shock, the prediction of survival, in decision-making in cases of acute respiratory failure, and in the reduction of complications from invasive procedures.
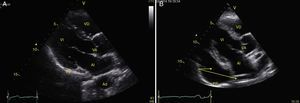
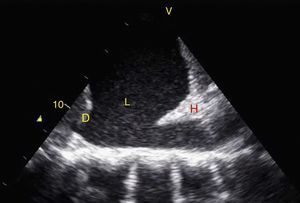
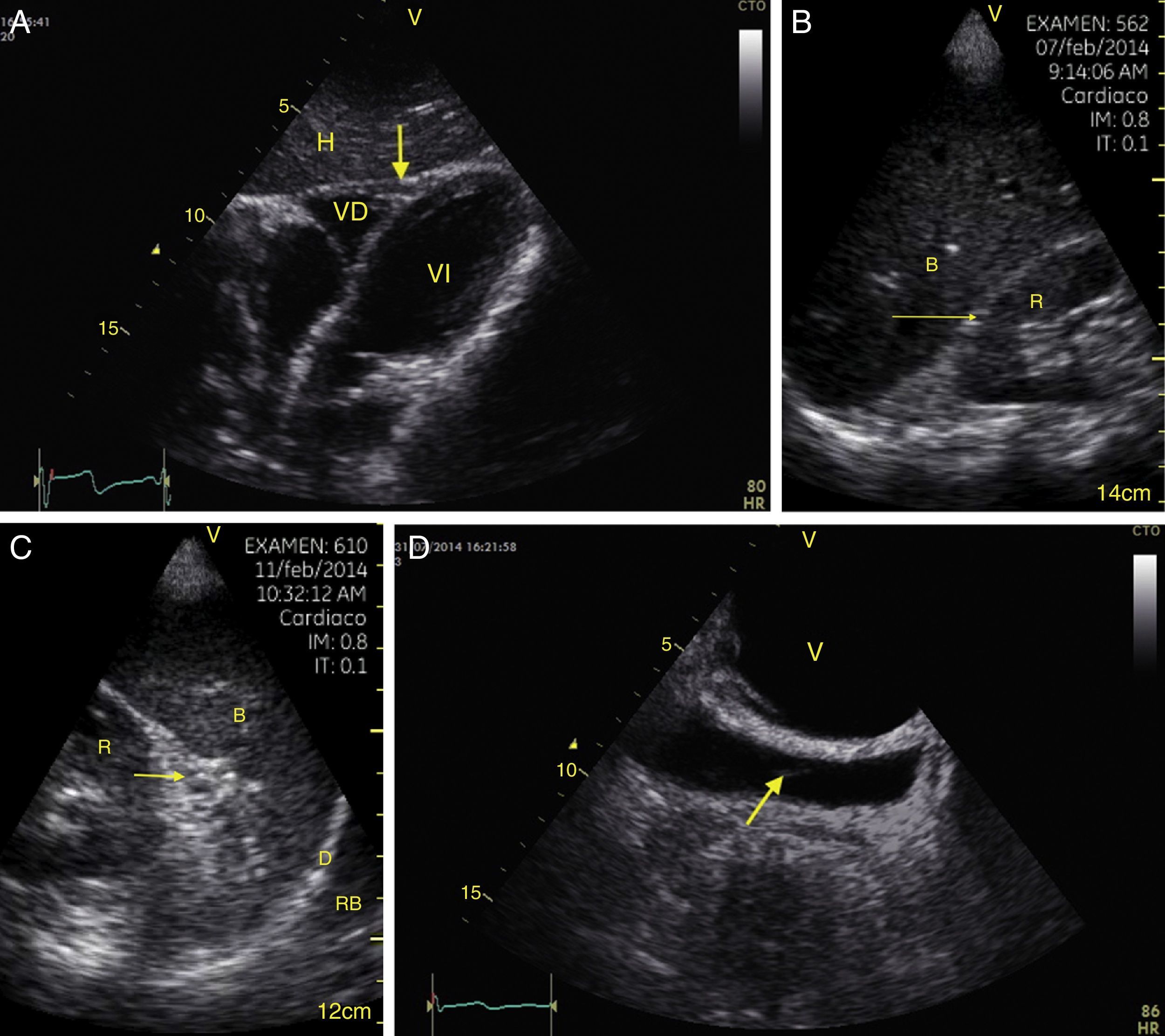
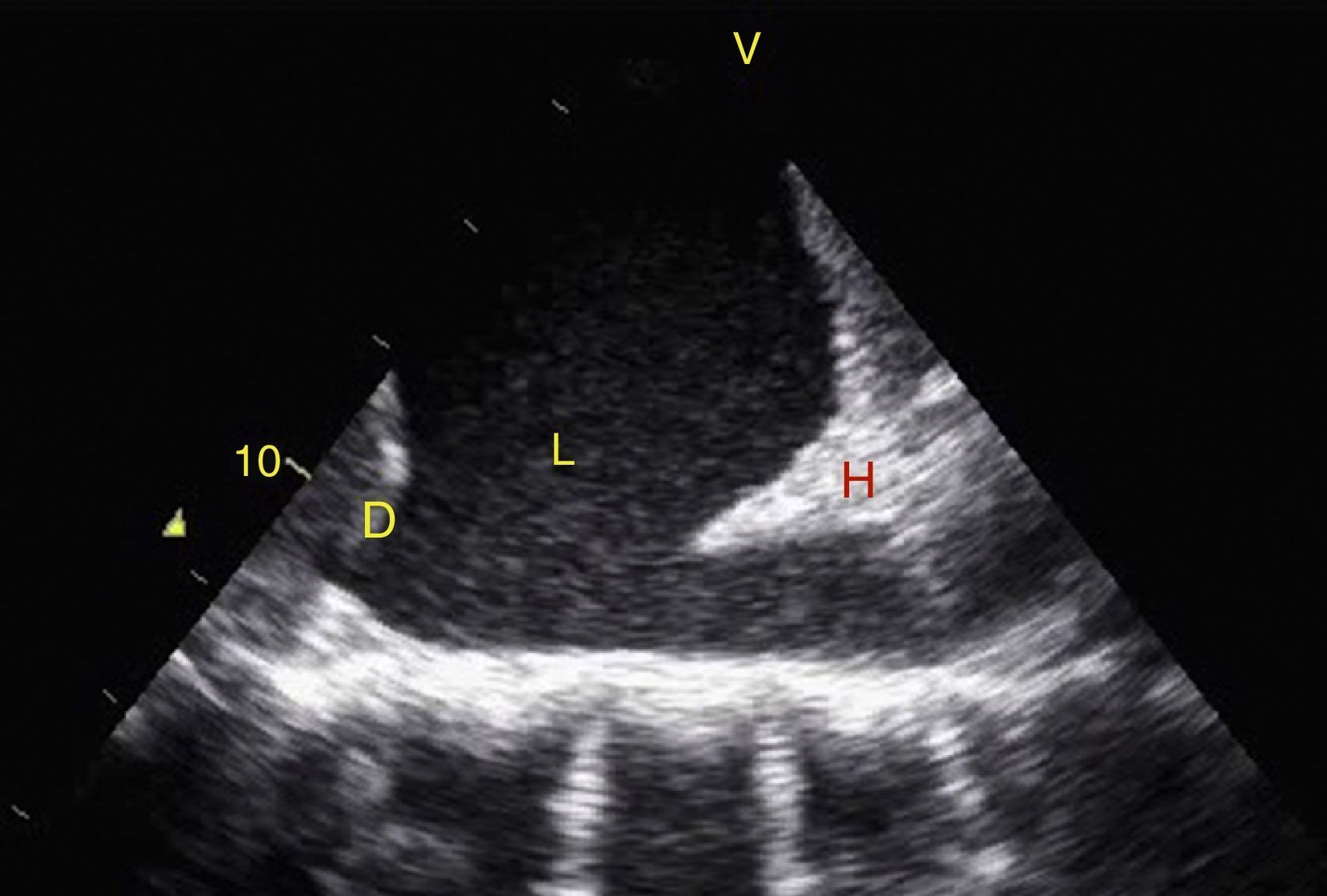
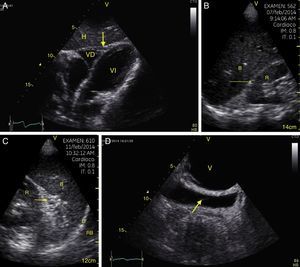
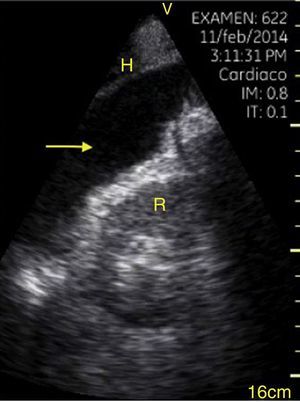
TraumaFAST consists of the evaluation of four points (pericardial, perihepatic, perisplenic, and pelvic (Fig. 1)) to detect hypoechoic images related to free pericardial and intra-abdominal fluids of up to 100ml (Fig. 2) with a sensitivity of 50–88%. Its application has managed to reduce mortality from cardiac and abdominal trauma.17 Its extended application to the thorax (EFAST) for detecting pneumothorax and hemothorax has been very important.18 Firstly this is because it is more sensitive than radiography techniques for diagnosing pneumothorax (48% vs. 20%),17 a pathology that is calculated to be hidden in 5% of all traumas19 and in up to 55% of severe traumas.20 Secondly, echography may detect fluid with a volume of 20ml while radiography detects 200ml.21 Thus, echography has a superior sensitivity and specificity when it comes to detecting hemothorax.22
View of the 4 Ps of FAST. A, Pericardial in the subxiphoid 4 chamber window. Observe that there is no fluid between H, the anterior blade of the pericardium (arrow) and the right ventricle. B, Perihepatic in the upper right quadrant of the abdomen. Observe the Morrison pouch (arrow) between H and R with an absence of fluid. C, Perisplenic in the upper left quandrant of the abdomen. Observe the absence of fluid (arrow) between B and R. Also observe the normal reflection of the spleen above the diaphragm (RB). D, Pelvic. Observe the most anterior hypoechoic image that corresponds to the bladder (V) and the more posterior free fluid (arrow). VI: left ventricle, VD: right ventricle, H: liver, D: diaphragm, B: spleen, R: kidney.
Survival for Pulseless Electric Activity (PEA) and asystole is much less than for other cardiac arrest rhythms. This is probably due to the fact that they depend on the correct identification and rapid treatment of underlying causes.16 Of these causes, only hypoxemia, hypothermia, and hypo/hyperkalemia are easily diagnosed.23 Furthermore, only 45% of physicians correctly diagnose a lack of pulse in cardiac arrest without differentiating between PEA and pseudo PEA. This may lead to physicians not treating a reversible cause.23
In shock, morbidity and mortality also depend on the duration and the rapid treatment of the cause. However, the clinical differentiation between hypovolemic, distributive, cardiogenic, or obstructive shock cannot always be correctly performed24 since the physical examination detects only 57% of cardiac anomalies.25
Ultrasonography plays an important role in these non-shockable cardiac arrest and shock scenarios since it allows physicians to rapidly exclude potentially reversible causes of cardiogenic shock, hypovolemia, cardiac tamponade, pneumothorax, and hemothorax.23–25 Moreover, it increases the exactness of the cardiac physical examination by 60–90% for pericardial effusion, left ventricular function, and cardiomegaly.26 It also helps to differentiate a pseudo PEA from a true one so that behavior can be changed in up to 78% of cases.27
Predicting survivalUltrasonography has been suggested as a tool for ceasing resuscitation since, when there is no evidence of myocardial contractility, the probability of return of spontaneous circulation is 3% in cases of PEA28 and the probability of survival is 2% in cases of trauma.29
Acute respiratory failureProper decision-making in this scenario was documented in 2008 with the BLUE protocol, an observational study that evaluated criteria like pleural sliding and the consolidation and presence of A or B lines in 3 zones of the thorax called: zone 1 (anterior), zone 2 (lateral), and zone 3 (postero-lateral). Each of these zones is halved to create a total of 6 investigation areas. Based on these findings, 6 profiles were established (A, A′, B, B′, AB, C) that, compared to the final diagnosis, had a sensitivity and specificity of greater than 80% and 90% respectively for detecting COPD, asthma, pneumothorax, pulmonary edema, pneumonia, and pulmonary thromboembolism.30
In addition, echography has a high concordance with radiography in various acute pulmonary pathologies (effusions, consolidation, edema) and can be performed in less time.31
Invasive proceduresWhen inserting a central venous catheter (CVC), ultrasonography reduced mechanical complications and insertion time, especially in the internal jugular.32 Furthermore, it determines the correct placement of the point of the CVC with a sensitivity of 70% and a specificity of 100% with the saline flush test.33 In cases of thoracentesis, ultrasound increases the probability of success and reduces the risk of organ puncture.34 For pericardiocentesis, the incidence of complications drops 50–4.7%.35
What does a non-expert need to train themselves and how reliable is it?The American Society of Echocardiography (ASE) determines that the main use of portable ultrasound is to extend the exactitude of the physical examination. It should be objective-guided. For this to take place, at least a basic level of training, including performing 75 examinations and interpreting 150, is required.36
The Council of Emergency Medicine Residency Directors recommends that Emergency training programs include 2weeks/80h of training and 150 evaluations in critical situations, including 40 FAST examinations, 30 deep vein thrombosis (DVT) examinations, and 10 procedure examinations.37
Intensive care programs recommend training in general concepts that include the pleura, thorax, vascular system, and abdomen in addition to basic echocardiography to recognize blood volume, biventricular function, cardiac tamponade, and severe acute valve failure.38 10h of theoretical training in each module and 30 trans-thoracic echocardiograms are recommended.39
Several studies have shown the correlation of non-expert personnel and expert personnel in specific situations. Niendorff et al.40 evaluated residents that received training in the subcostal window for recognizing cardiac tamponade, pulmonary embolism, hypovolemia, and reduction in contractility with a concordance in 80% over 7min in PEA scenarios. Another study, performed on residents and involving 100 cases found a strong correlation in terms of the evaluation of left ventricular function, and pleural, pericardial and moderate effusion to quantify vena cava and valve failure in 6min.41
How to perform and evaluation with echography in resuscitation?There are many protocols described in the literature that include echocardiography, EFAST, the lung, the aorta, the vena cava, DVT, and ectopic pregnancy, etc. For example, the consensus from the ACEP on echocardiograms at the patient's bedside is focused on pericardial effusion, systolic function, right ventricular growth, intravascular volume, and on confirming transvenous pacemakers.42 The FATE protocol (Focus Assessed Transthoracic Echocardiographic) focuses its evaluation on pericardial effusion, the thickness and dimensions of the heart chambers, contractility, and on the pleura.43 The RUSH (Rapid Ultrasound in Shock) protocol establishes a standpoint for differentiating between hypovolemic, cardiogenic, distributive, and obstructive shock based on 3 variables: (1) The pump (pericardial effusion, left ventricular contractility, and dilation of the right ventricle), (2) the tank (inferior vena cava for hypovolemia, EFAST or pulmonary edema to evaluate leaks and pneumothorax for compression), and (3) the pipes (aortic aneurism and DVT).24
Of these variables we must choose the most relevant ones for evaluating critical events in operating rooms such as shock with unclear etiology, cardiorespiratory arrest with non-shockable rhythms, and hypoxemia. The evaluation should be organized and directed depending on the clinical situation and should include: left ventricular function to rule out myocardial dysfunction, volume responsiveness to evaluate hypovolemia, pericardial effusion for cardiac tamponade, dilation of the right ventricle for pulmonary embolism, and pulmonary ultrasound to rule out pneumothorax, hemothorax, and pulmonary edema.
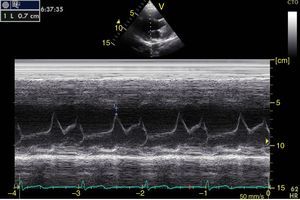
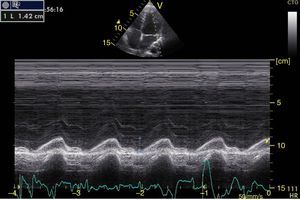
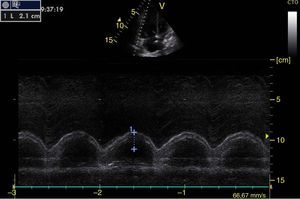
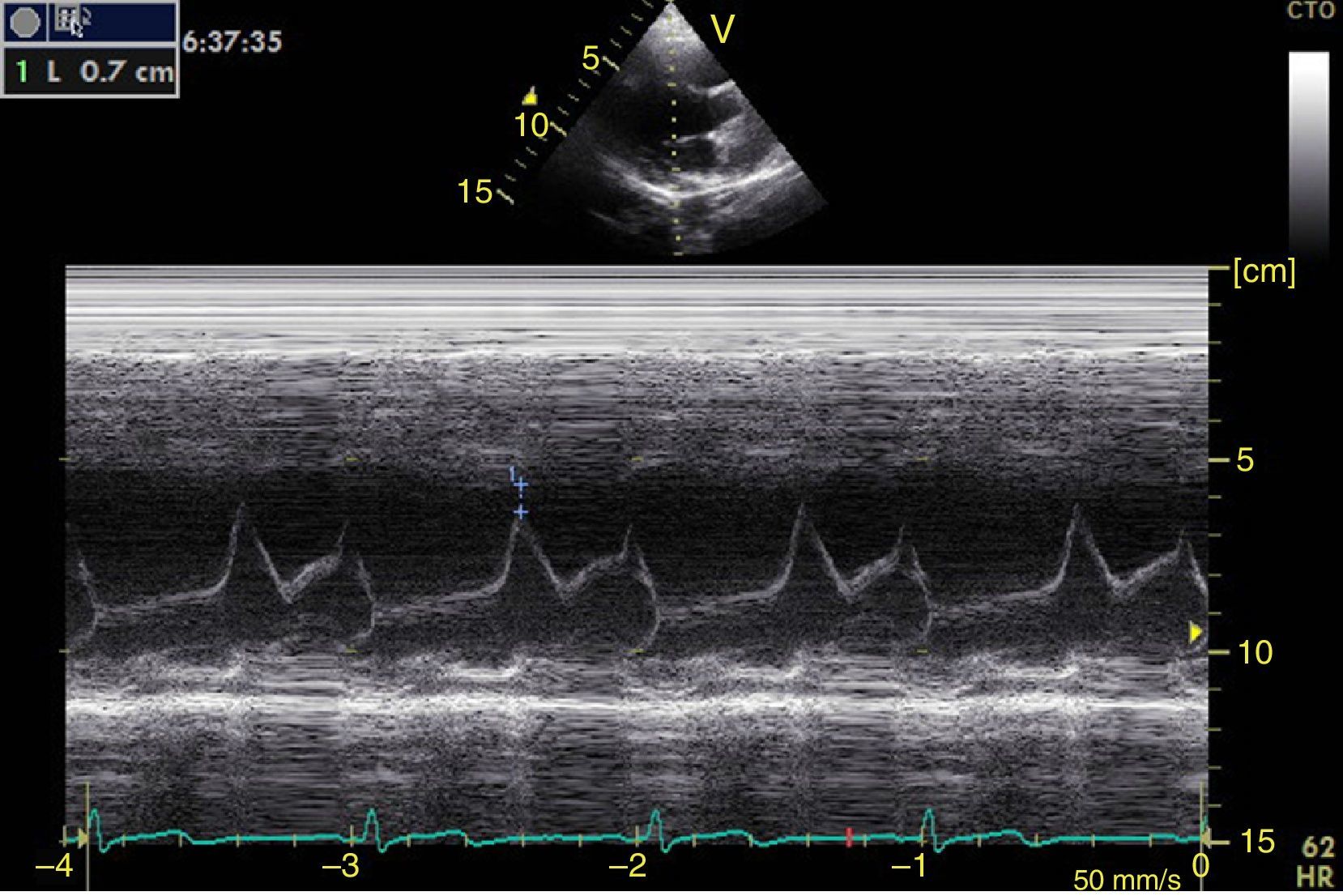
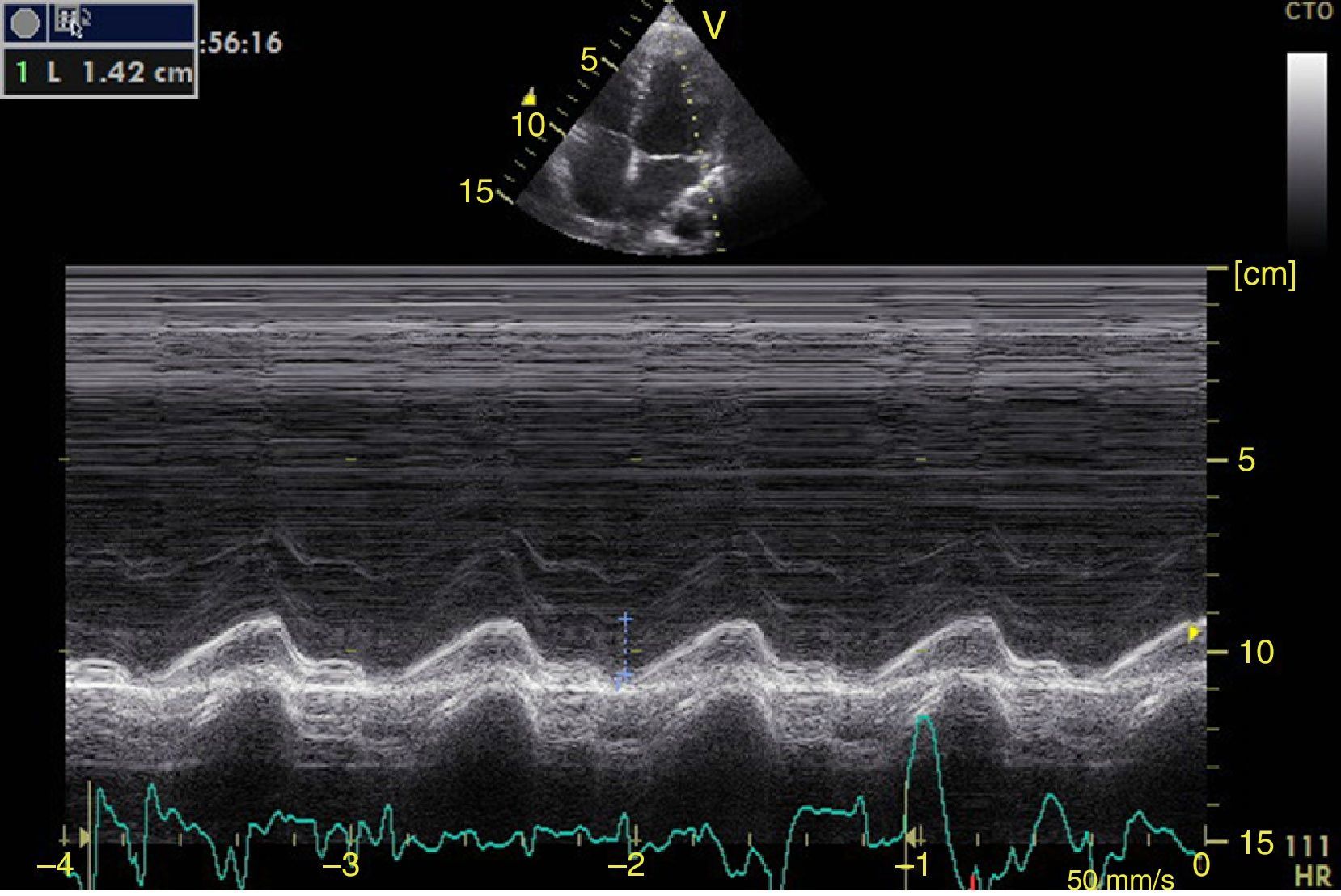
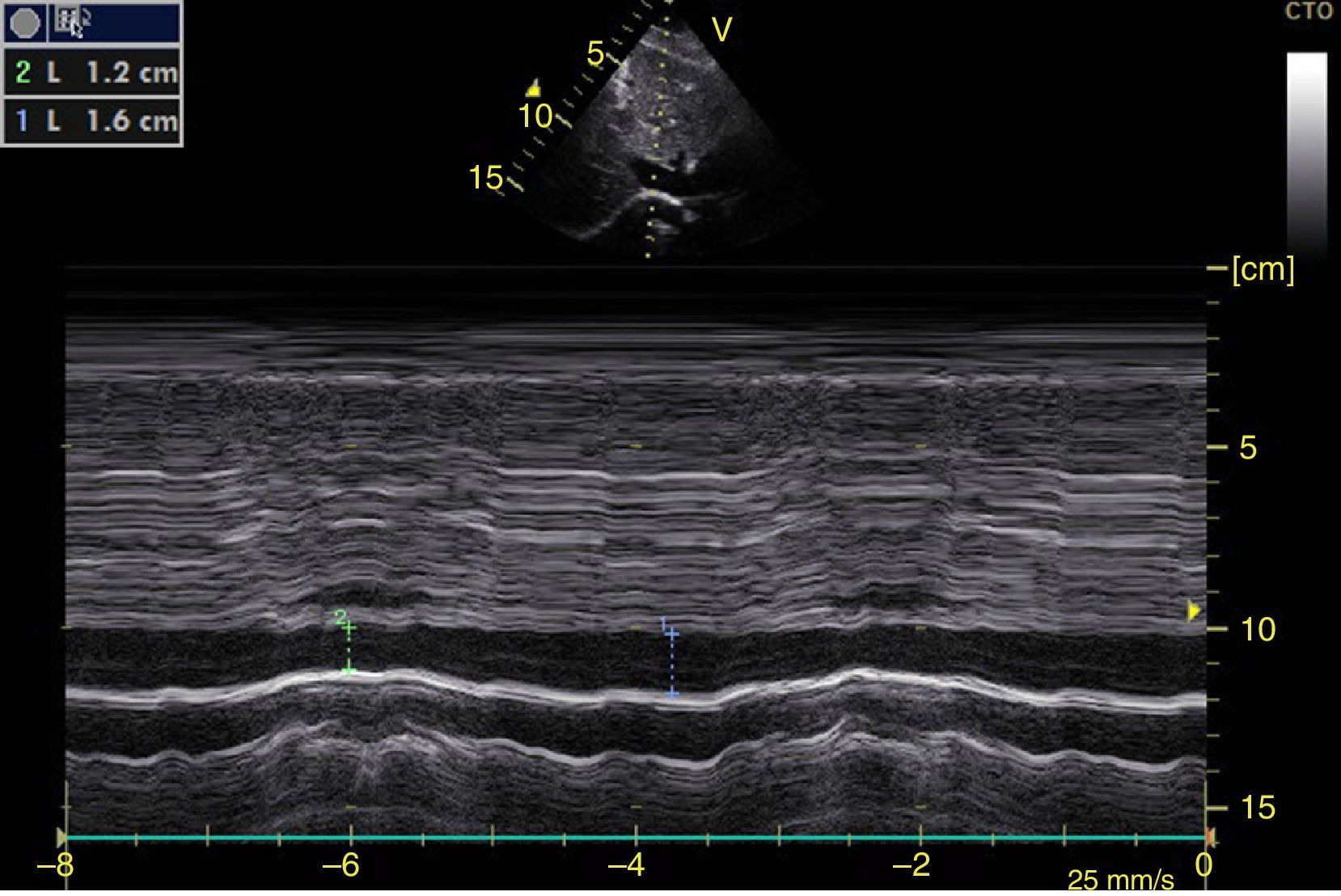
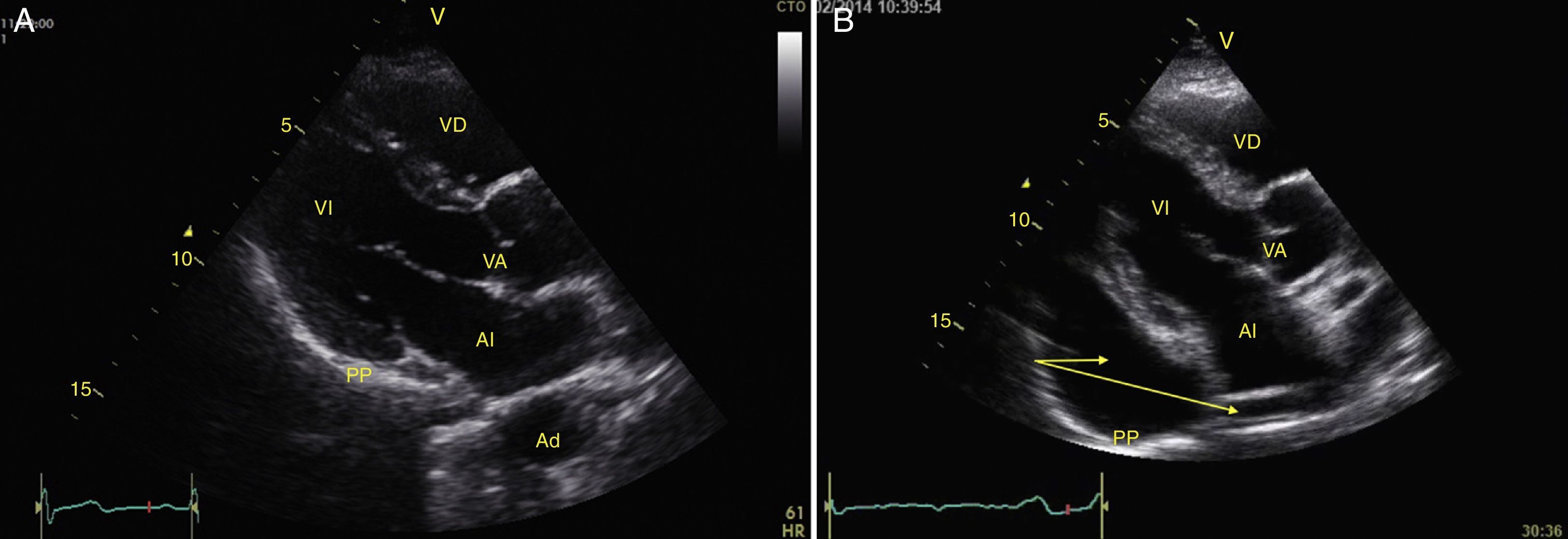
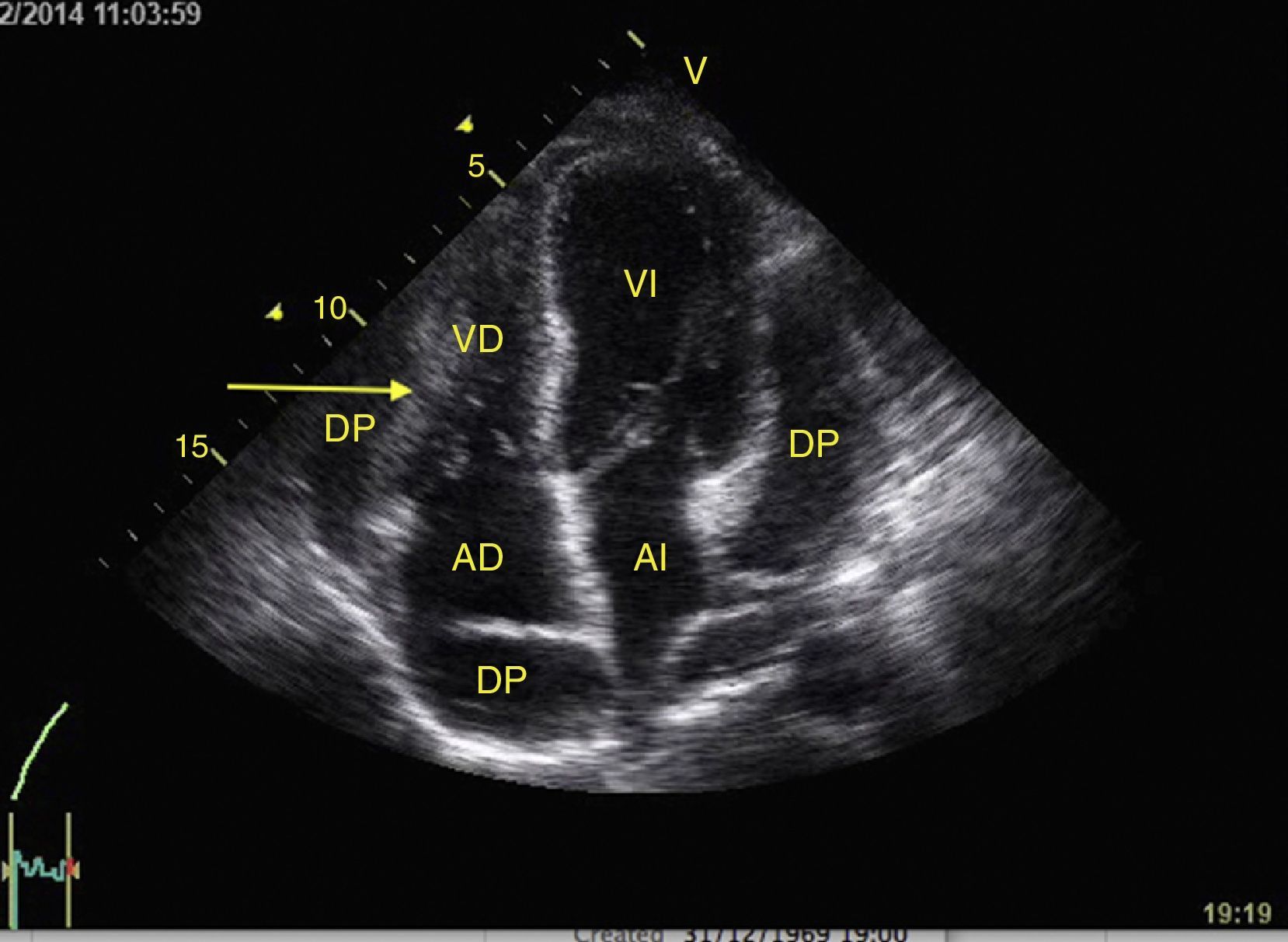
Left ventricular systolic functionContractility can be evaluated qualitatively and quickly through the thickening of the endocardium to differentiate between normal and severe dysfunction. This approach is useful for determining the presence of cardiogenic shock and for guiding the use of inotropic/vasopressor medications or intravenous fluids.44 The standard quantitative evaluation is the calculation of the ejection fraction using Simpson's method. However, this requires 2 planes (apical 4-chamber and 2-chamber) and an advanced calculation that is not always available in these scenarios.45 The M-mode (movement in time) is a more simple method used in the FATE protocol. It allows for the calculation of the shortening fraction (normal greater than 25%) (Fig. 3) and for the approximation of the distance from the anterior mitral valve to the interventricular septum (normal less than 1cm) in parasternal long axis (Fig. 4).43 This method should not be considered appropriate in alterations of segmentary contractility.45 The M-mode evaluates systolic function with mitral annular plane systolic excursion (MAPSE) in apical 4-chamber view (normal greater than 15mm) (Fig. 5).43 Another method is the calculation of systolic volume (normal 45±13ml) with the Doppler mode and the formula Pi×R2×VTI (velocity time integral) from the left ventricle outflow tract (LVOT), where R is the radius of the LVOT. The VTI is also indicative of systolic function with 18–20cm being normal and less than 12cm considered shock.45
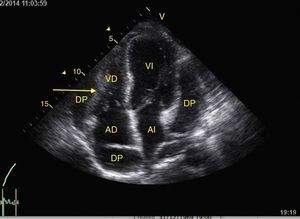
The systolic obliteration of the left ventricle (kissing papillar muscle sign) can be related to hypovolemia, although other parameters have shown better predictions of volume responsiveness, such as respiratory changes in the diameter of the vena cava (vena cava index, VCI) (Fig. 6) and in the maximum velocity of the systolic volume. En patients with invasive mechanical ventilation, inferior VCI greater than 15%46 and a superior VCI greater than 36% is considered to be a volume responder.47 In cases of spontaneous respiration, an adequate correlation with volume responsiveness has not been achieved.48 It has, however, been achieved with values of central venous pressure (CVP), given that inferior VCI>50% with a diameter>2.1cm correlates with CVP<5cm H2O, if it is <50% with diameter>2.1cm it correlates with CVP>10cm H20, and if it does not comply with either, 5–10cm H20.49
Subxiphoid window of inferior vena cava in M-mode. Inferior vena cava index=(DM−Dm/DM)×100. DM: Greatest Diameter (blue line), DM: Smallest Diameter (green line). During mechanical ventilation, a value greater than 15% is considered to be a volume responder. In spontaneous ventilation, a value >50% with diameter<2.1cm correlates with CVP<5cm H2O. If it is <50% with diameter>2.1cm, it correlates with CVP>10cm H20. If it has diameter<2.1cm with value <50%, or diameter>2.1cm with value >50%, it correlates with CVP between 5 and 10cmH2O.
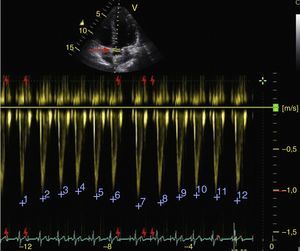
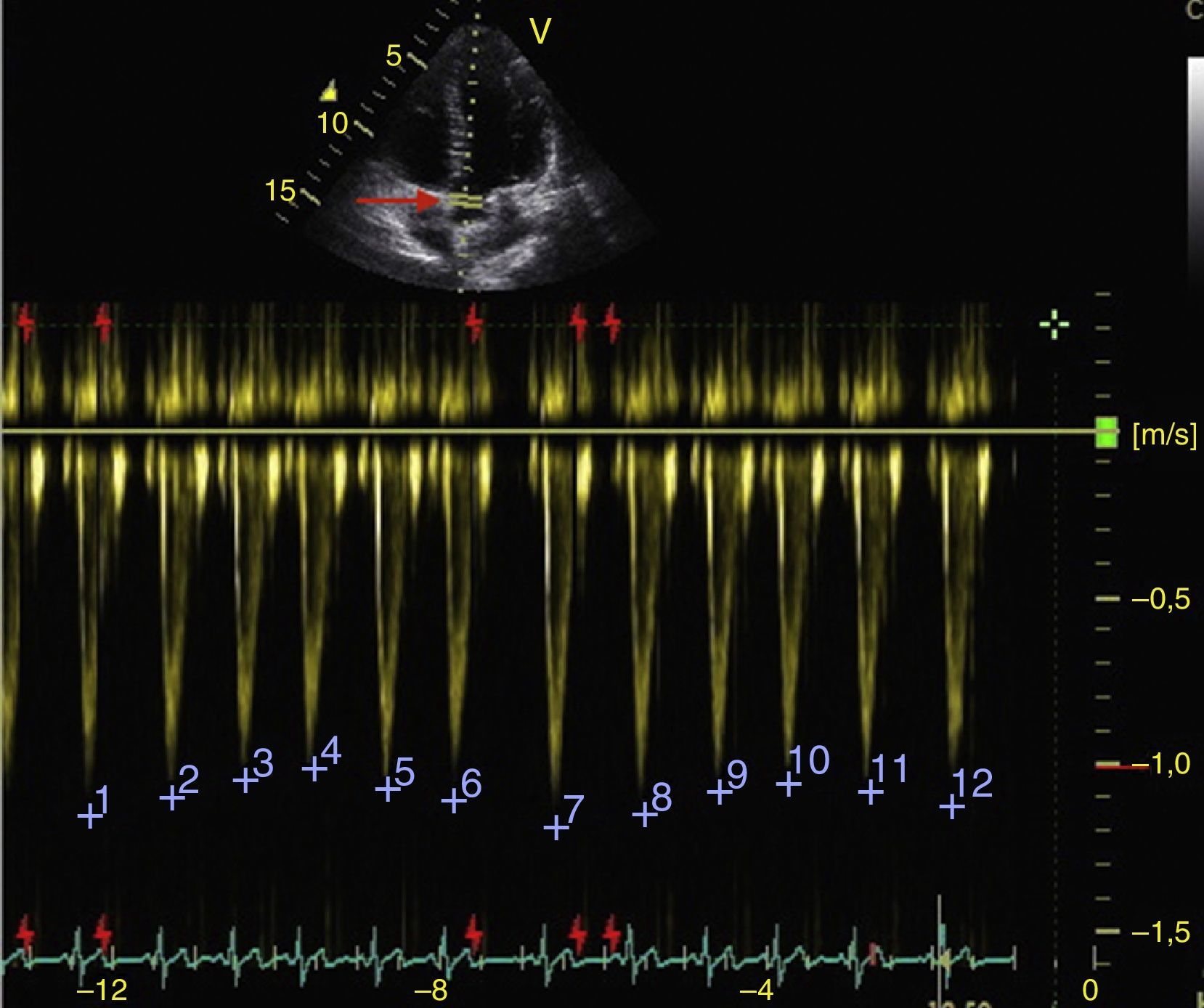
Respiratory changes in the maximum velocity of systolic volume in the LVOT should be greater than 12% to respond to volume (Fig. 7).50
Cardiac tamponadePericardial effusion is identified as a hypoechoic image between the hyperechoic pericardial blades (Fig. 8) and later it is determined whether it contributes to the patient's instability. As cardiac tamponade is produced when the pressure inside the pericardium impedes filling during the relaxation phase, diastolic collapse should be searched for initially in the right cavities since they have lower pressure (Fig. 9).24,51 The spectrum of presentation of the tamponade can range from an inward deviation of the atrium to a complete compression of the chamber in diastole.51 In addition, a distended inferior vena cava can be seen as part of the diagnosis.24
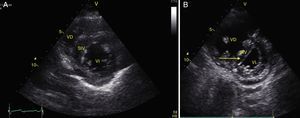
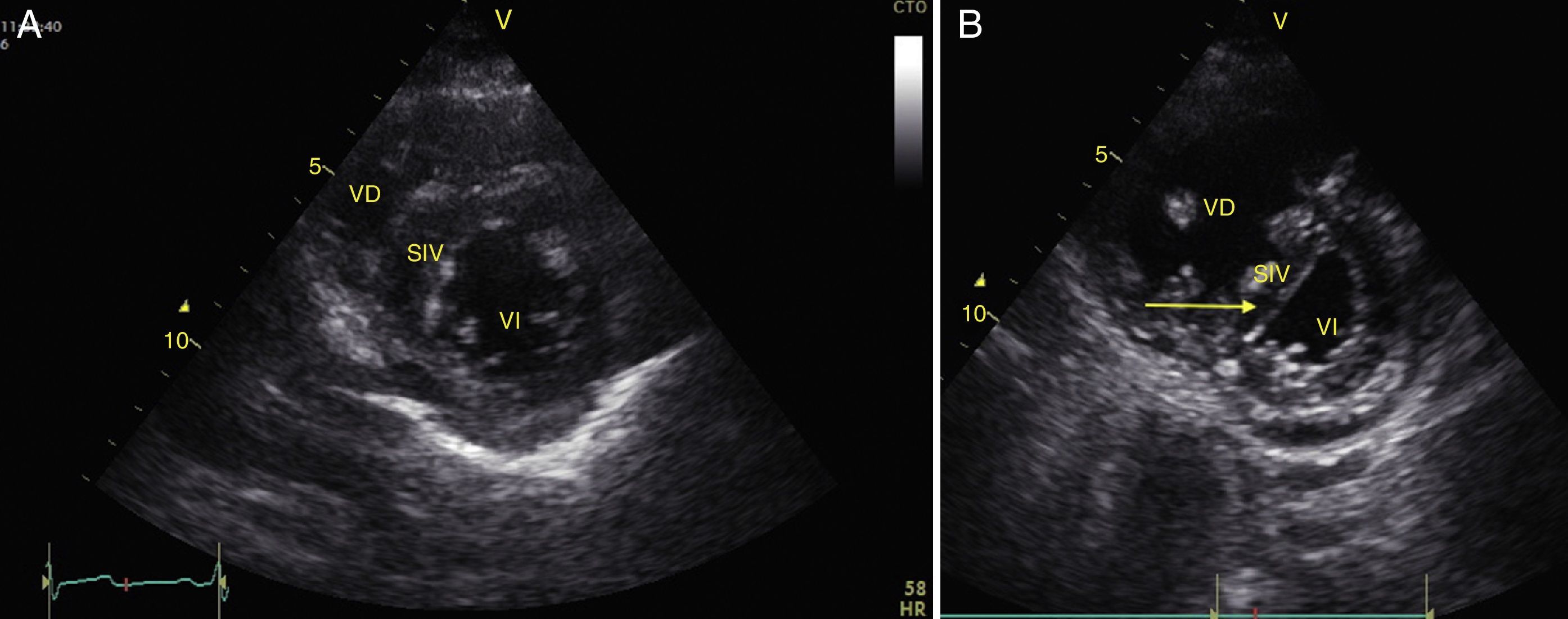
Any condition that suddenly increases pulmonary vascular resistance can result in the acute dilation of the RV. The main cause of this condition is pulmonary embolism.24 This event can be observed through the deformation and dilation of the RV, whose normal relationship with the left ventricle is 66%, and this is better observed in apical 4 chamber or parasternal short axis windows (Fig. 10).49 Another sign that is indicative of an increase in pressure in the RV is the paradoxical movement of the interventricular septum (Fig. 11).24 Some researchers have reported the sensitivity and specificity of the ultrasound for detecting pulmonary embolism as 55% and 69% respectively52 while in the BLUE protocol, a normal pulmonary examination with evidence of DVT indicates PTE with a sensitivity of 81% and a specificity of 99%.28
(A) Parasternal short axis window. Observe the noral relationship between the VD and the VI; (B) Flattening of SIV (arrow) during systole or paradoxical movement makes the VI take the form of D and indicates pressure overload. Also observe the increase in the size of the VD as compared to the VI. VD: Right Ventricle, VI: Left Ventricle, SIV: Interventricular Septum (IVS).
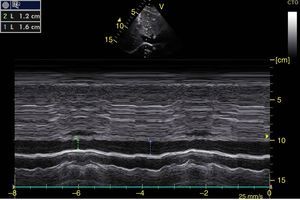
Right ventricular function can also be assessed in M-mode in the apical 4 chamber window with the tricuspid annular plane systolic excursion (TAPSE) that should be greater than 15mm (Fig. 12).49 The change on the fractional area (area end diastole – area end systole/area end diastole×100) greater than 35% is indicative of normal systolic function.49
LungTo rule out pneumothorax, pulmonary edema, consolidation, and pleural effusion in the patient with hypoxemia or shock, we must search for pleural sliding and the A or B lines in the 6 quadrants described in the BLUE protocol as well as signs of pleural effusion.28
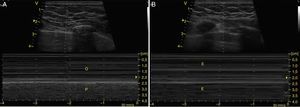
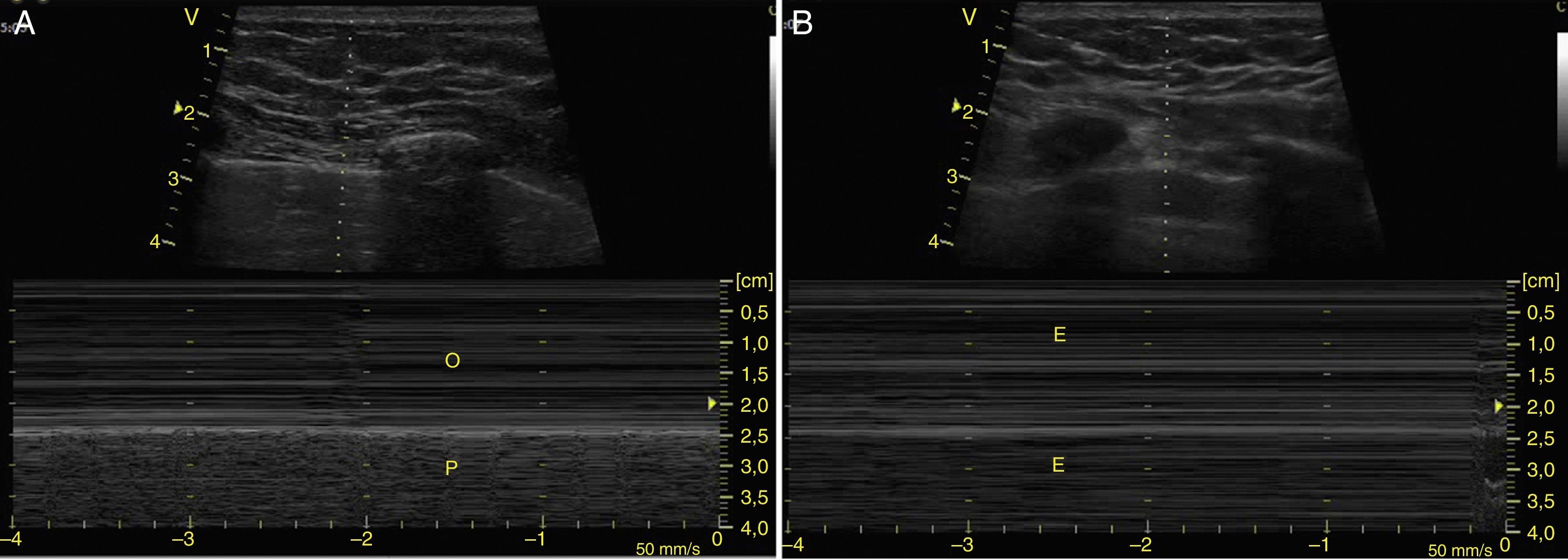
To determine the presence of pneumothorax, with the patient in supine, the pleural line is located (the hyperechogenic line between the ribs) between intercostal spaces 3–5 and movement of the 2 blades (pleural sliding) should be absent.24 Sliding should also be observed in M-mode as the “waves on a beach” sign (Figs. 13 and 14). In addition, the A lines, which represent horizontal artifacts and are the reflection of the air/tissue interface that causes reverberation between the transductor and the lung, should be searched for. It is key that the A lines be accompanied by an absence of sliding (Profile A′) for a diagnosis of pneumothorax, because these lines can be found in healthy individuals or in patients with COPD or asthma (Profile A).28,53,54
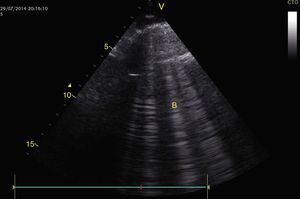
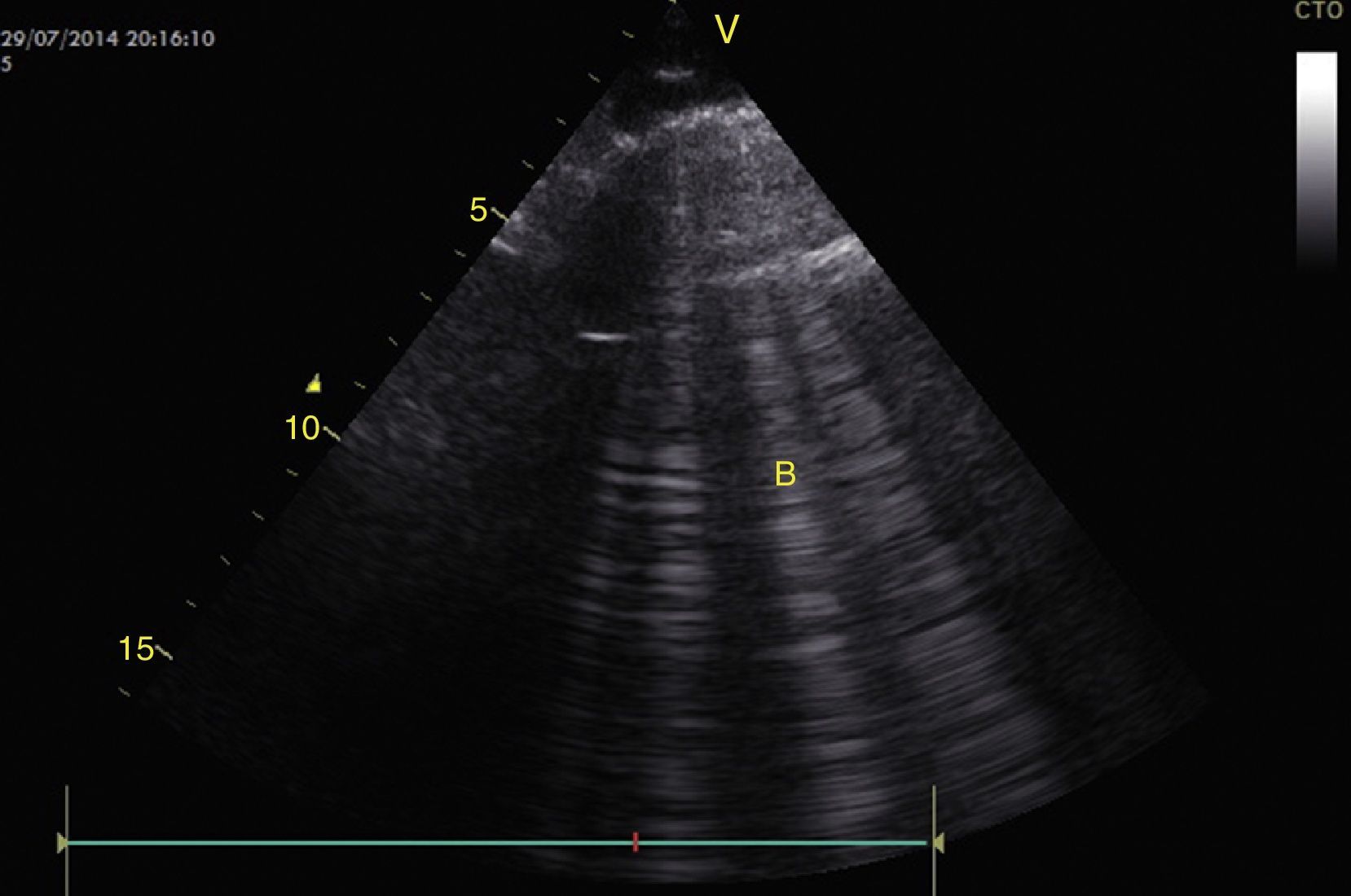
To establish the presence of pulmonary edema, B lines should be identified. These are vertical artifacts of reverberation within the lung that initiate in the pleura toward the bottom of the screen without disappearing and they move with pleural sliding. These B lines with pleural sliding form the interstitial syndrome (Profile B) and are associated with an increase in interstitial water (Fig. 15).28,53,54 When no pleural sliding is present, the B lines are associated with pneumonia, atelactasis, or pulmonary contusions (Profile B′).28,53,54
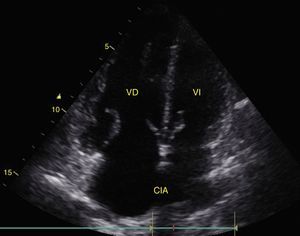
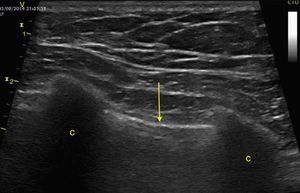
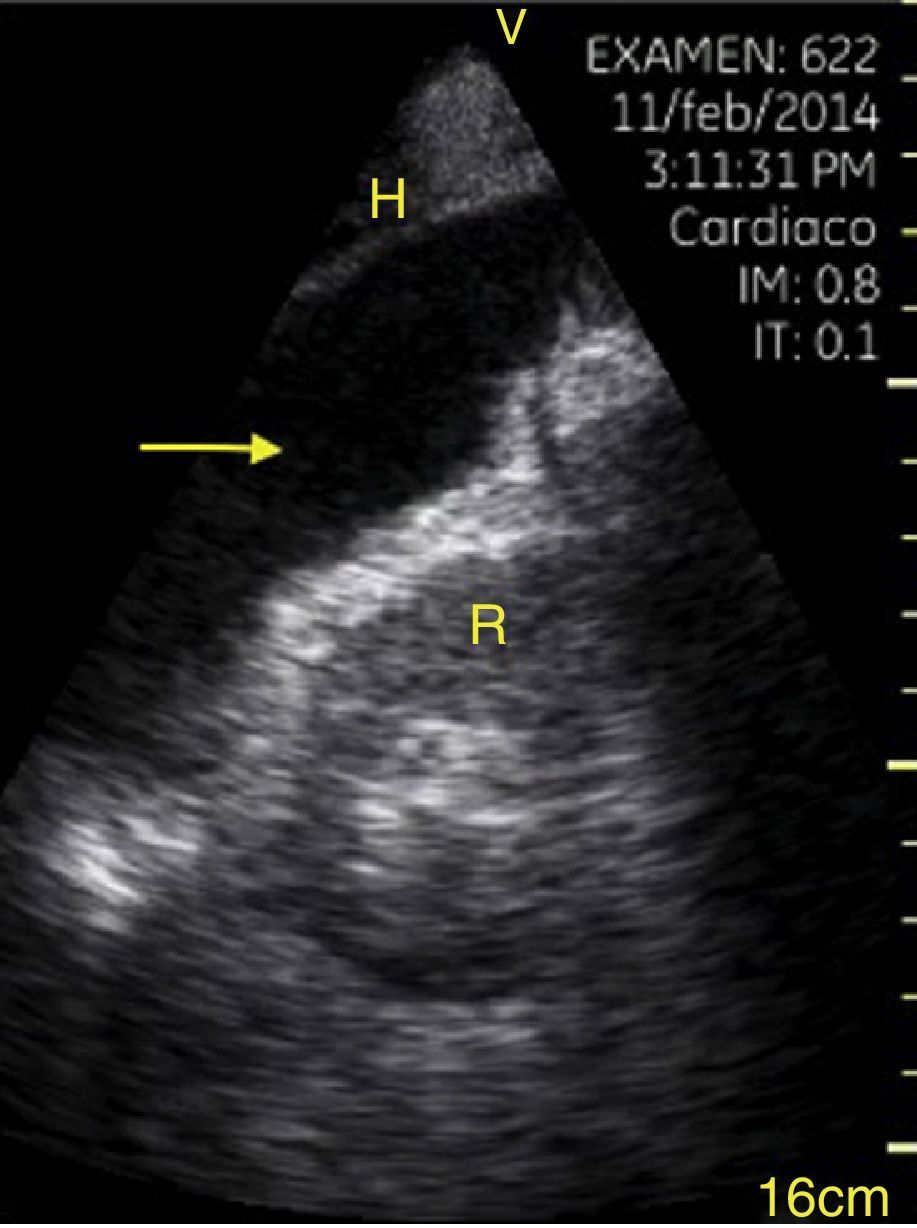
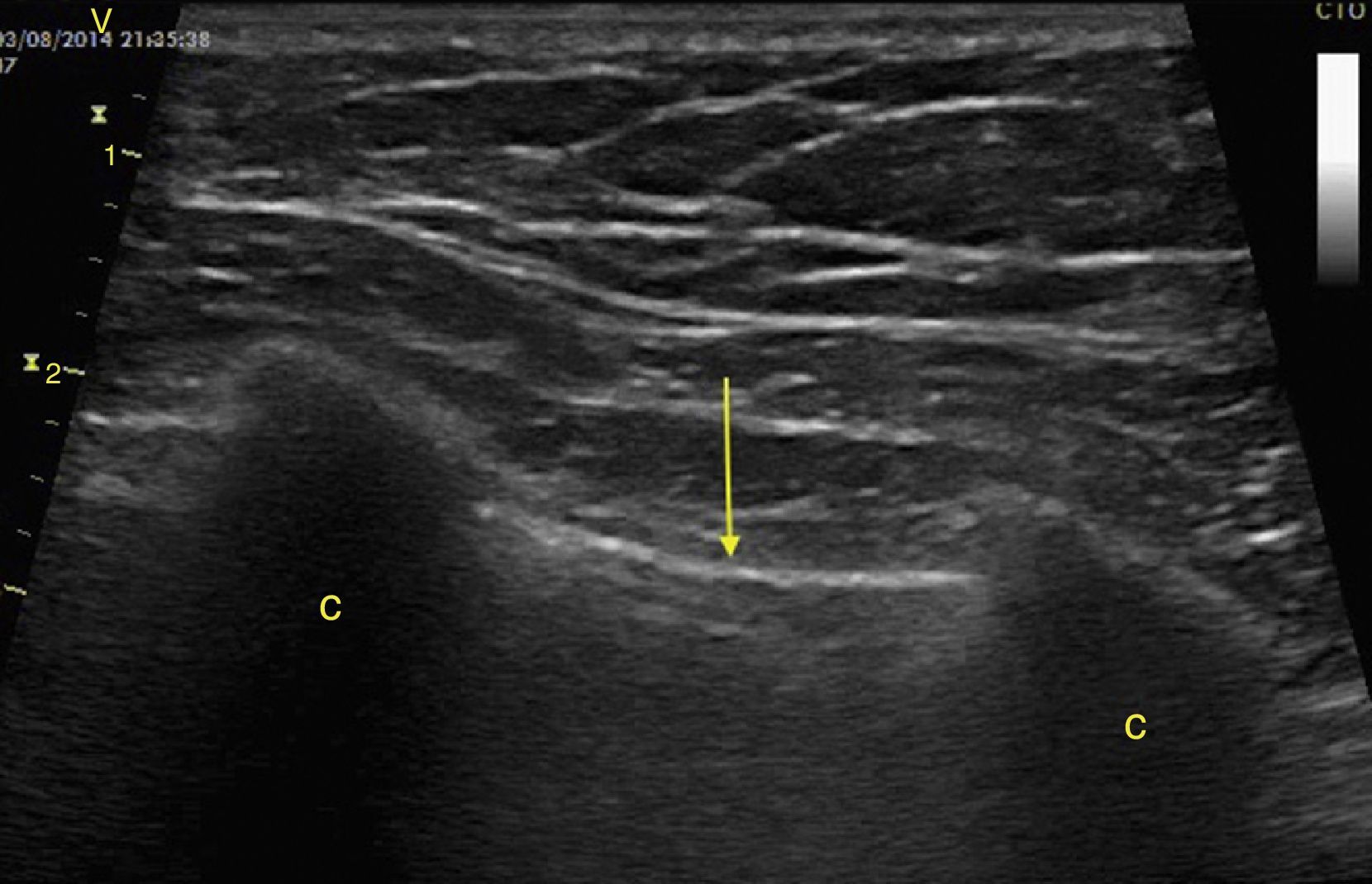
Pleural effusion should be searched for in the perisplenic and perihepatic quadrants of EFAST, moving two intercostal spaces in the direction of the head to locate the diaphragm.24 Normally, in the direction of the head from the diaphragmatic cupola there is a reflection of the spleen or the liver (Fig. 1C) while, in the presence of pleural effusion, a hypoechoic image can be observed and the lung compresses giving it the appearance of a solid organ (hepatization) (Fig. 16).24
ConclusionUltrasonography is an important tool for diagnosis and managing critical events, for reducing morbidity and mortality and predicting survival in cases of trauma and PEA, reducing complications from invasive procedures, and improving decision-making in cases of cardiac arrest and acute respiratory failure. Ultrasounds performed by non-experts with a minimum of training focused on recognizing specific situations have an adequate correlation with those performed by experts. The variables of evaluation that are most relevant in critical situations in the operating room are left ventricular function, volume responsiveness, cardiac tamponade, and pulmonary evaluation.
FundingThe authors did not receive sponsorship to undertake this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pérez-Coronado JD, Franco-Gruntorad GA. Utilidad del Ultrasonido en Reanimación. Rev Colomb Anestesiol. 2015;43:321–330.



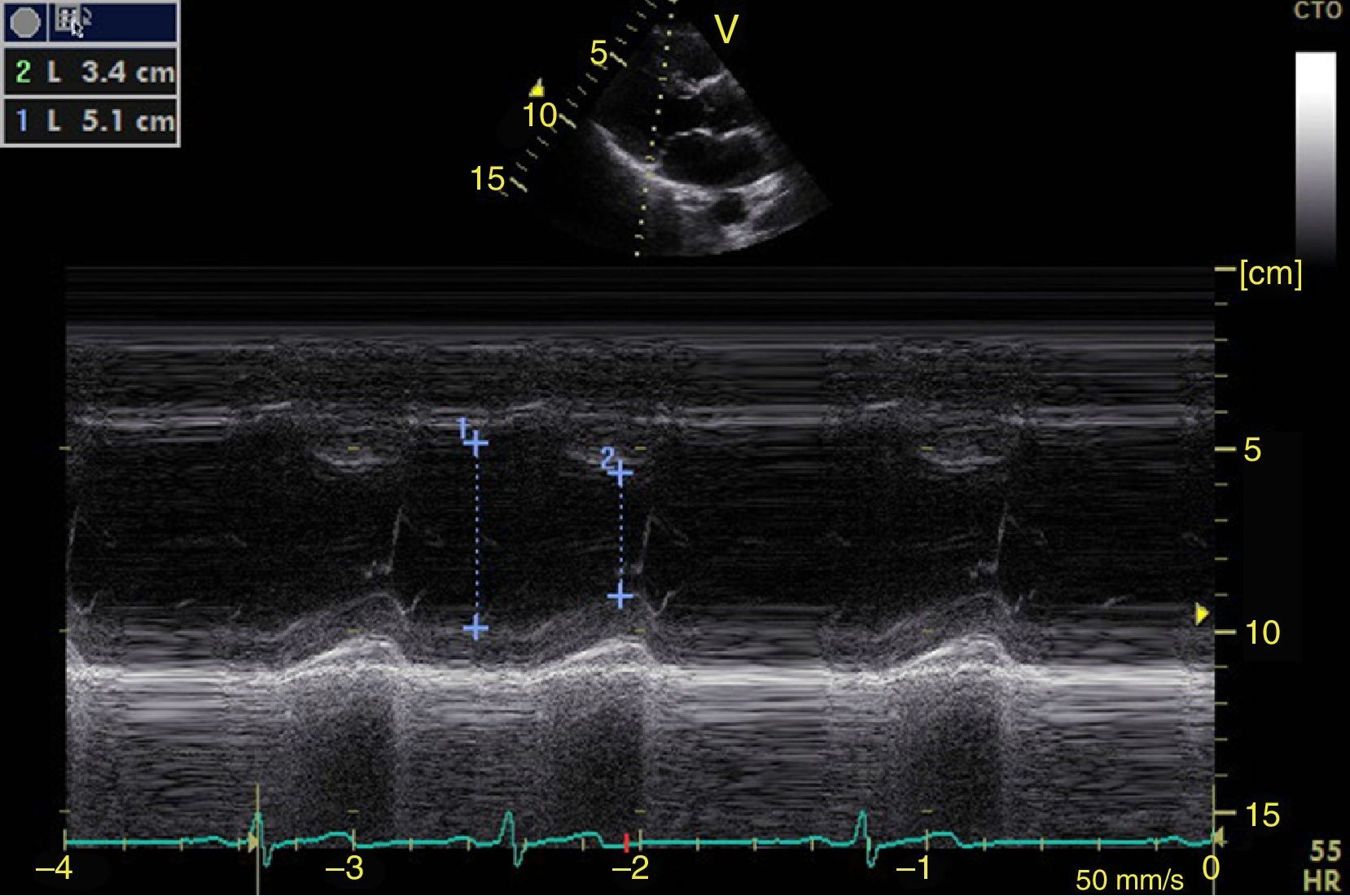
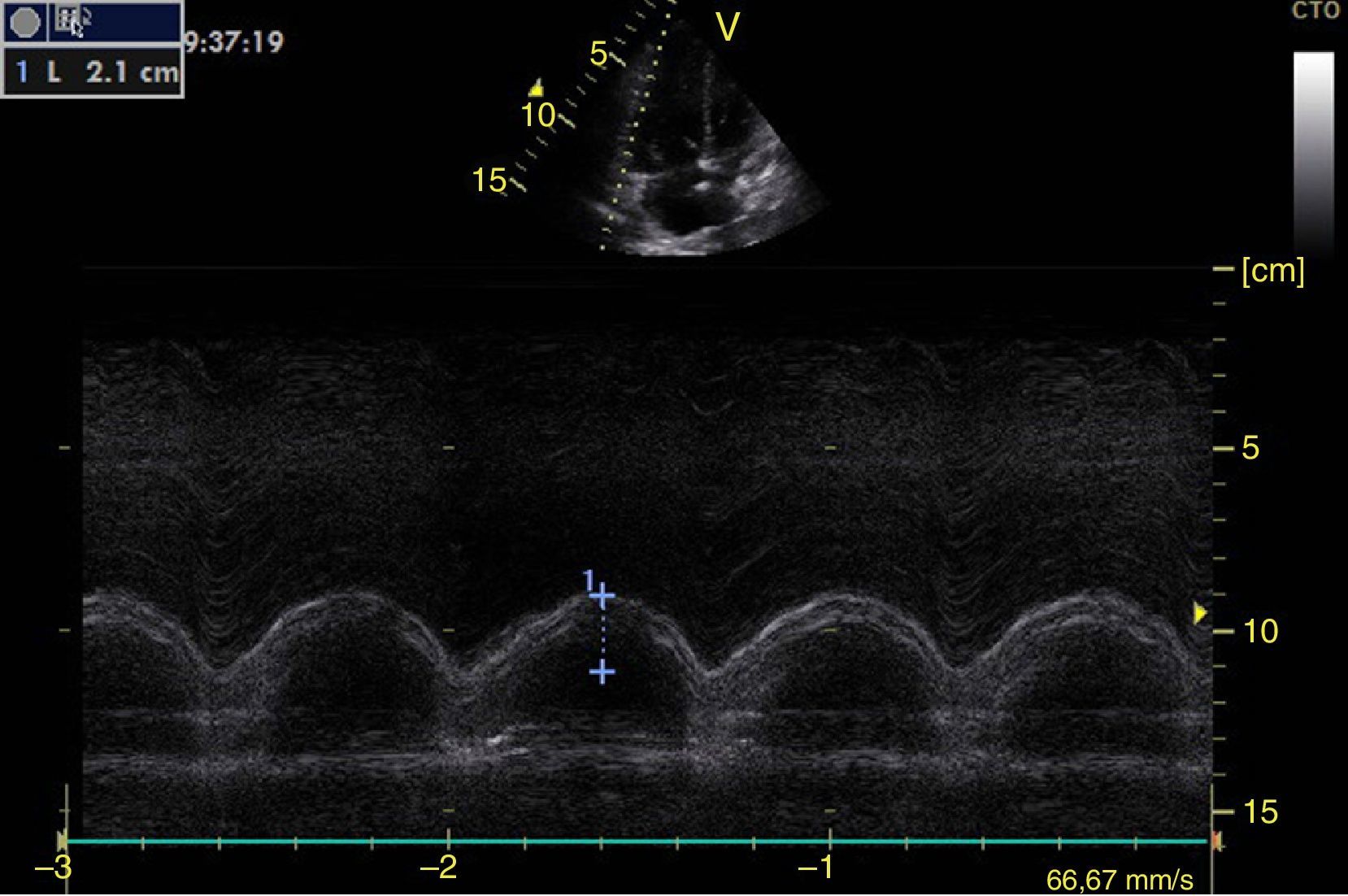
![Parasternal long axis window in M-mode. Shortening fraction=[DM−Dm/DM]×100. Normal value greater than 25%. DM: greatest diameter (blue line 1), DM: Smallest Diameter (blue line 2). Parasternal long axis window in M-mode. Shortening fraction=[DM−Dm/DM]×100. Normal value greater than 25%. DM: greatest diameter (blue line 1), DM: Smallest Diameter (blue line 2).](https://static.elsevier.es/multimedia/22562087/0000004300000004/v1_201510250024/S2256208715000371/v1_201510250024/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)